Electric Cardioversion vs. Pharmacological with or without Electric Cardioversion for Stable New-Onset Atrial Fibrillation: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Criteria for Considering Studies for This Review
2.1.1. Type of Studies
2.1.2. Type of Participants
2.1.3. Types of Interventions
2.1.4. Types of Outcome Measures
2.2. Outcomes
2.3. Search Methods for Identification of Studies
Electronic Searches
2.4. Data Collection and Analysis
2.5. Selection of Studies
2.6. Data Extraction and Management
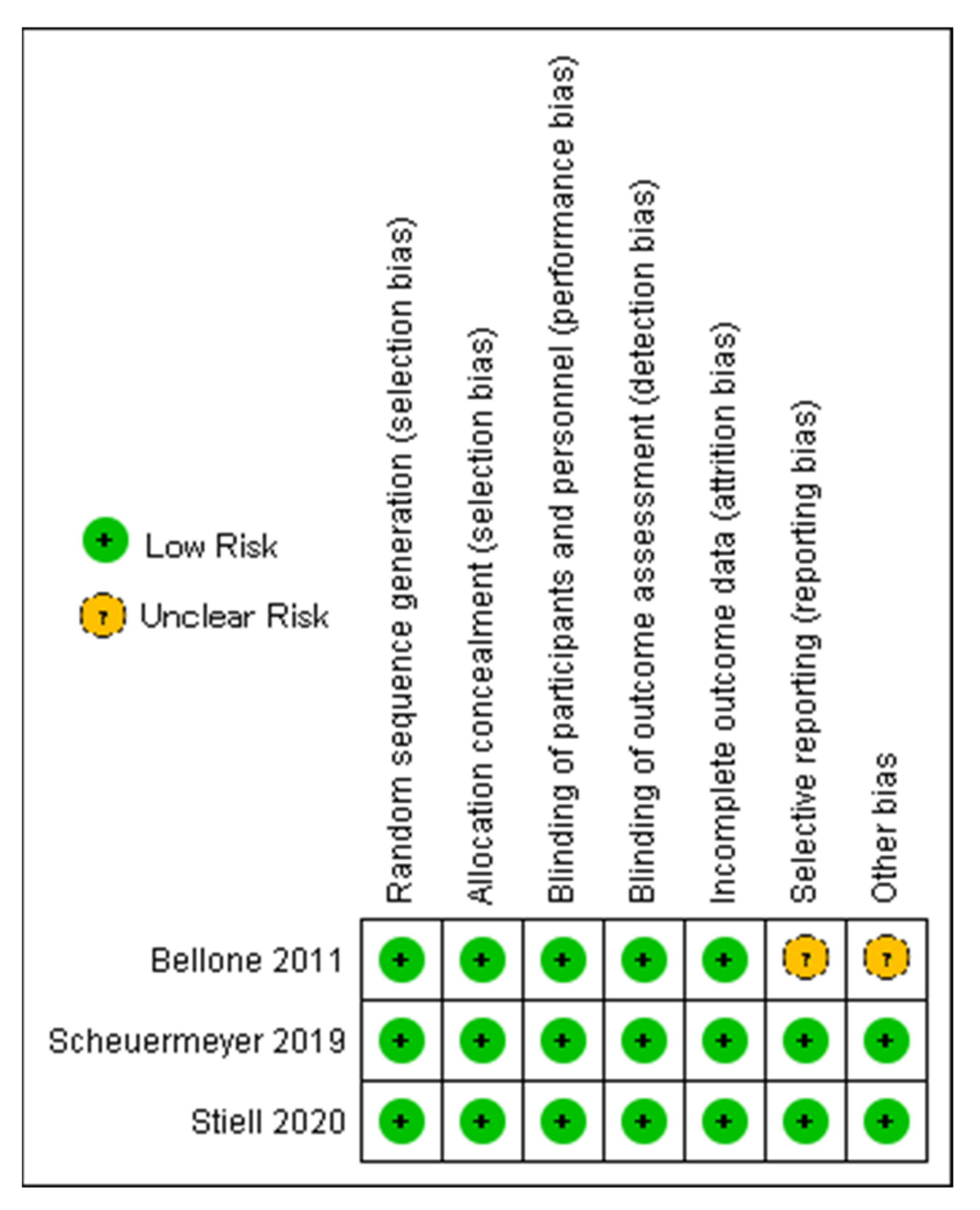
2.7. Assessment of Risk of Bias in Included Studies
2.8. Assessment of Heterogeneity
2.9. Assessment of Reporting Biases
2.10. Data Synthesis
2.11. Investigation of Heterogeneity
3. Results
3.1. Study Characteristics
3.2. Qualitative Summary
3.3. Quantitative Analysis
3.3.1. Successful Cardioversion
3.3.2. Emergency Department (ED) Visit
3.3.3. Readmission
3.3.4. Length of Hospital Stay
3.3.5. Overall, AE
3.3.6. Hypotension, AE
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hindricks, G.; Potpara, T.; Dagres, N.; Bax, J.J.; Boriani, G.; Dan, G.A.; Fauchier, L.; Kalman, J.M.; Lane, D.A.; Lettino, M.; et al. 2020 ESC Guidelines for the Diagnosis and Management of Atrial Fibrillation Developed in Collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2021, 42, 373–498. [Google Scholar] [PubMed]
- Stiell, I.G.; Sivilotti, M.L.A.; Taljaard, M.; Birnie, D.; Vadeboncoeur, A.; Hohl, C.M.; Mcrae, A.D.; Rowe, B.H.; Brison, R.J.; Thiruganasambandamoorthy, V.; et al. Electrical versus Pharmacological Cardioversion for Emergency Department Patients with Acute Atrial Fibrillation (RAFF2): A Partial Factorial Randomised Trial. Lancet 2020, 395, 339–349. [Google Scholar] [CrossRef] [PubMed]
- Dankner, R.; Shahar, A.; Novikov, I.; Agmon, U.; Ziv, A.; Hod, H. Treatment of Stable Atrial Fibrillation in the Emergency Department: A Population-Based Comparison of Electrical Direct-Current versus Pharmacological Cardioversion or Conservative Management. Cardiology 2009, 112, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. Int. J. Surg. 2021, 88, 105906. [Google Scholar] [CrossRef] [PubMed]
- PROSPERO. Available online: https://www.crd.york.ac.uk/prospero/#recordDetails (accessed on 26 December 2022).
- Prasai, P.; Shrestha, D.; Sedhai, Y.R.; Trongtorsak, A.; Adhikari, A.; Saad, E.A. Pharmacologic Cardioversion Followed by Electrical Cardioversion, If Needed, versus Electrical Cardioversion Alone in Atrial Fibrillation: A Systematic Review and Meta-Analysis. Available online: https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=315427 (accessed on 26 December 2022).
- RevMan for Non-Cochrane Reviews. Cochrane Training. Available online: https://training.cochrane.org/online-learning/core-software-cochrane-reviews/revman/revman-non-cochrane-reviews (accessed on 26 December 2022).
- Covidence Systematic Review Software, Veritas Health Innovation, Melbourne, Australia. Available online: www.covidence.org (accessed on 26 December 2022).
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A Revised Tool for Assessing Risk of Bias in Randomised Trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed]
- Critical-Appraisal-Tools-Critical Appraisal Tools—JBI. Joanna Briggs Institute. Available online: https://jbi.global/critical-appraisal-tools (accessed on 26 December 2022).
- Scheuermeyer, F.X.; Andolfatto, G.; Christenson, J.; Villa-Roel, C.; Rowe, B. A Multicenter Randomized Trial to Evaluate a Chemical-First or Electrical-First Cardioversion Strategy for Patients with Uncomplicated Acute Atrial Fibrillation. Acad. Emerg. Med. 2019, 26, 969–981. [Google Scholar] [CrossRef] [PubMed]
- Bellone, A.; Etteri, M.; Vettorello, M.; Bonetti, C.; Clerici, D.; Gini, G.; Maino, C.; Mariani, M.; Natalizi, A.; Nessi, I.; et al. Cardioversion of Acute Atrial Fibrillation in the Emergency Department: A Prospective Randomised Trial. Emerg. Med. J. 2012, 29, 188–191. [Google Scholar] [CrossRef] [PubMed]
- Cumpston, M.; Li, T.; Page, M.J.; Chandler, J.; Welch, V.A.; Higgins, J.P.; Thomas, J. Updated Guidance for Trusted Systematic Reviews: A New Edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst. Rev. 2019, 10, ED000142. [Google Scholar] [CrossRef] [PubMed]
- MacKenzie, M.; Stewart, D.; Hall, C. Electrical or Pharmacologic Cardioversion for Atrial Fibrillation in the ED? Can. J. Emerg. Med. 2021, 23, 32–33. [Google Scholar] [CrossRef] [PubMed]
- de Paola, A.A.V.; Figueiredo, E.; Sesso, R.; Veloso, H.H.; Nascimento, L.O.T. Effectiveness and Costs of Chemical versus Electrical Cardioversion of Atrial Fibrillation. Int. J. Cardiol. 2003, 88, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Pluymaekers, N.A.H.A.; Dudink, E.A.M.P.; Luermans, J.G.L.M.; Meeder, J.G.; Lenderink, T.; Widdershoven, J.; Bucx, J.J.J.; Rienstra, M.; Kamp, O.; van Opstal, J.M.; et al. Early or Delayed Cardioversion in Recent-Onset Atrial Fibrillation. N. Engl. J. Med. 2019, 380, 1499–1508. [Google Scholar] [CrossRef] [PubMed]
- Scheuermeyer, F.X.; Grafstein, E.; Stenstrom, R.; Innes, G.; Poureslami, I.; Sighary, M. Thirty-Day Outcomes of Emergency Department Patients Undergoing Electrical Cardioversion for Atrial Fibrillation or Flutter. Acad. Emerg. Med. 2010, 17, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Roy, D.; Pratt, C.M.; Torp-Pedersen, C.; Wyse, D.G.; Toft, E.; Juul-Moller, S.; Nielsen, T.; Rasmussen, S.L.; Stiell, I.G.; Coutu, B.; et al. Vernakalant Hydrochloride for Rapid Conversion of Atrial Fibrillation. Circulation 2008, 117, 1518–1525. [Google Scholar] [CrossRef] [PubMed]
- Camm, A.J.; Capucci, A.; Hohnloser, S.H.; Torp-Pedersen, C.; van Gelder, I.C.; Mangal, B.; Beatch, G. A Randomized Active-Controlled Study Comparing the Efficacy and Safety of Vernakalant to Amiodarone in Recent-Onset Atrial Fibrillation. J. Am. Coll. Cardiol. 2011, 57, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Markey, G.C.; Salter, N.; Ryan, J. Intravenous Flecainide for Emergency Department Management of Acute Atrial Fibrillation. J. Emerg. Med. 2018, 54, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Marcos, F.J.; García-Garmendia, J.L.; Ortega-Carpio, A.; Fernández-Gómez, J.M.; Santos, J.M.; Camacho, C. Comparison of Intravenous Flecainide, Propafenone, and Amiodarone for Conversion of Acute Atrial Fibrillation to Sinus Rhythm. Am. J. Cardiol. 2000, 86, 950–953. [Google Scholar] [CrossRef] [PubMed]





| Checklist | Danker, 2009 [3] |
|---|---|
| 1. Were the two groups similar and recruited from the same population? | Yes |
| 2. Were the exposures measured similarly to assign people to both exposed and unexposed groups? | Unclear |
| 3. Was the exposure measured in a valid and reliable way? | Yes |
| 4. Were confounding factors identified? | Yes |
| 5. Were strategies to deal with confounding factors stated? | Unclear |
| 6. Were the groups/participants free of the outcome at the start of the study (or at the moment of exposure)? | Yes |
| 7. Were the outcomes measured in a valid and reliable way? | Yes |
| 8. Was the follow up time reported and sufficient to be long enough for outcomes to occur? | No |
| 9. Was follow up complete, and if not, were the reasons to loss to follow up described and explored? | Yes |
| 10. Were strategies to address incomplete follow up utilized? | Unclear |
| 11. Was appropriate statistical analysis used? | Yes |
| Author, Year | No. of Patient Population | Age, Years | Gender | CHADS₂ Score | Co-Morbidities | Medications | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M | F | 0 | 1 | ≥2 | Diabetes | HTN | VHD | AC | AAD | Antiplatelets | Other Cardiac Medications | ||||
| Stiell 2020 [2] | n = 396 | Drug–shock (n = 204) | 60 | 134 | 70 | 65 | 45 | 94 | 1 | 10 | 17 | 66 | 13 | 59 | 101 |
| Shock-only (n = 192) | 60.1 | 126 | 66 | 57 | 54 | 81 | 2 | 14 | 14 | 66 | 13 | 48 | 83 | ||
| Scheuermeyer 2019 [11] | n = 86 | Chemical-first (n = 41) | 58 | 26 | 15 | 29 | 12 | 0 | 2 | 8 | 18 | 5 | |||
| Electrical-first (n = 43) | 60 | 26 | 17 | 25 | 15 | 3 | 1 | 8 | 19 | 3 | |||||
| Bellone 2011 [12] | n = 247 | Propafenone group (n = 126) | 67 ± 14 | 65 | 61 | 25 | 65 | 43 | 161 | ||||||
| Electrical group (n = 121) | 68 ± 13 | 65 | 56 | 22 | 67 | 50 | 145 | ||||||||
| Dankner 2009 [3] | DCC n = 85 | 44 | 41 | 6 | 34 | 13 | 19 | 30 | 28 | ||||||
| Pharmacological n = 56 | 19 | 37 | 6 | 29 | 5 | 15 | 19 | 12 | |||||||
| wait and watch n = 233 | 106 | 127 | 34 | 124 | 23 | 40 | 92 | 69 | |||||||
| Author, Year | Cardioversion Intervention | Cardioversion Control | LoHS Intervention | loHS Control | Re Admission/ Re-Hospitalization Intervention | Re Admission/ Re-Hospitalization Control | Mortality Intervention | Mortality Control | Thromboembolic Events Intervention | Thromboembolic Events Control | AE Intervention | AE Control |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Stiell 2020 [2] | Electrical only: 176/192 Chemical: 106/204, 14 days SR: 149/192 | Chemical followed by electrical: 196/20, 14 days SR 141/204 | 7.6 (5.4) h | 7.1 (5.5) h | ED visit 14 days: 21/192, Outpatient Visit: 68/192, Hospital admission: 3/192 | ED visit first 14 days: 21/204, Outpatient visit: 66/204, Hospital admission: 3/204 | 0/192 | 1/204 | 0 | 0 | Total adverse effects: 5/192 Hypotension: 4/192 | Total adverse effects: 53/204 Hypotension: 38/204 |
| Scheuermeyer 2019 [11] | Electrical only: 38/43 | Chemical followed by electrical: 41/41 | 3.5 (2.8–4.8) h | 5.1 (3.5–6.3) h | 30 days ED revisit: 3/43 Hospital admssion:0/43 | 30 days ED revisit: 9/41 Hospital admission: 2/41 | 0 | 0 | Stroke: 0/43 | 0/41 | Total adverse effects: 11/43 Hypotension: 0/43 | Total adverse effects: 10/41 Hypotension: 2/41 |
| Bellone 2011 [12] | 108/121 patients | 93/126 patients | Stay in ED: 180 min (120–900) | Stay in ED:420 min (120–400) | Recurrence of AF during 2 months of follow-up in EC group: 24/91 | Recurrence of AF during 2 months of follow-up in PC group: 21/74 | 0 | 0 | Not reported as secondary outcome | Not reported as secondary outcome | Total adverse effects: 1/121 Hypoten: 0/121 | Total adverse effects: 6/126 Hypotension: 2/126 |
| Dankner 2009 [3] | 69/85 patients | 34/56 patients | Not reported as secondary outcome | Not reported as secondary outcome | ED visit (7 days): 0/85 Hospital readmission (14 days): 6/85 | ED visit: 2/56 Hospital admission (14 days): 8/56 | 0 | 0 | Not reported as secondary outcome | Not reported as secondary outcome |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prasai, P.; Shrestha, D.B.; Saad, E.; Trongtorsak, A.; Adhikari, A.; Gaire, S.; Oli, P.R.; Shtembari, J.; Adhikari, P.; Sedhai, Y.R.; et al. Electric Cardioversion vs. Pharmacological with or without Electric Cardioversion for Stable New-Onset Atrial Fibrillation: A Systematic Review and Meta-Analysis. J. Clin. Med. 2023, 12, 1165. https://doi.org/10.3390/jcm12031165
Prasai P, Shrestha DB, Saad E, Trongtorsak A, Adhikari A, Gaire S, Oli PR, Shtembari J, Adhikari P, Sedhai YR, et al. Electric Cardioversion vs. Pharmacological with or without Electric Cardioversion for Stable New-Onset Atrial Fibrillation: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2023; 12(3):1165. https://doi.org/10.3390/jcm12031165
Chicago/Turabian StylePrasai, Paritosh, Dhan Bahadur Shrestha, Eltaib Saad, Angkawipa Trongtorsak, Aarya Adhikari, Suman Gaire, Prakash Raj Oli, Jurgen Shtembari, Pabitra Adhikari, Yub Raj Sedhai, and et al. 2023. "Electric Cardioversion vs. Pharmacological with or without Electric Cardioversion for Stable New-Onset Atrial Fibrillation: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 12, no. 3: 1165. https://doi.org/10.3390/jcm12031165