Safety and Efficacy of Surgery for Metastatic Tumor to the Pancreas: A Single-Center Experience
Abstract
:1. Introduction
2. Materials and Methods
2.1. Surgical Approach
2.2. Postoperative Follow-Up
2.3. Statistical Analysis
3. Results
3.1. Demographics and Clinical Presentation
3.2. Diagnostic Work-Up
3.3. Surgical Approach and Postoperative Outcomes
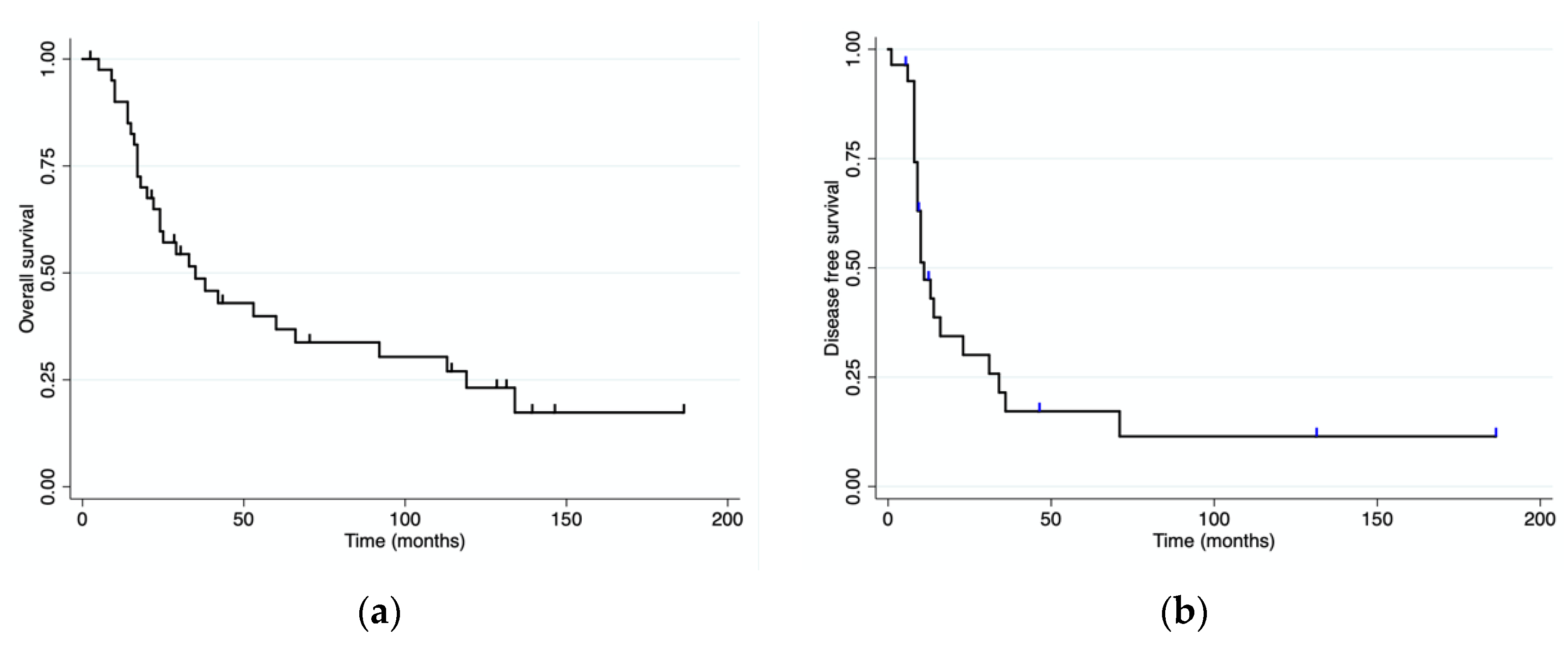
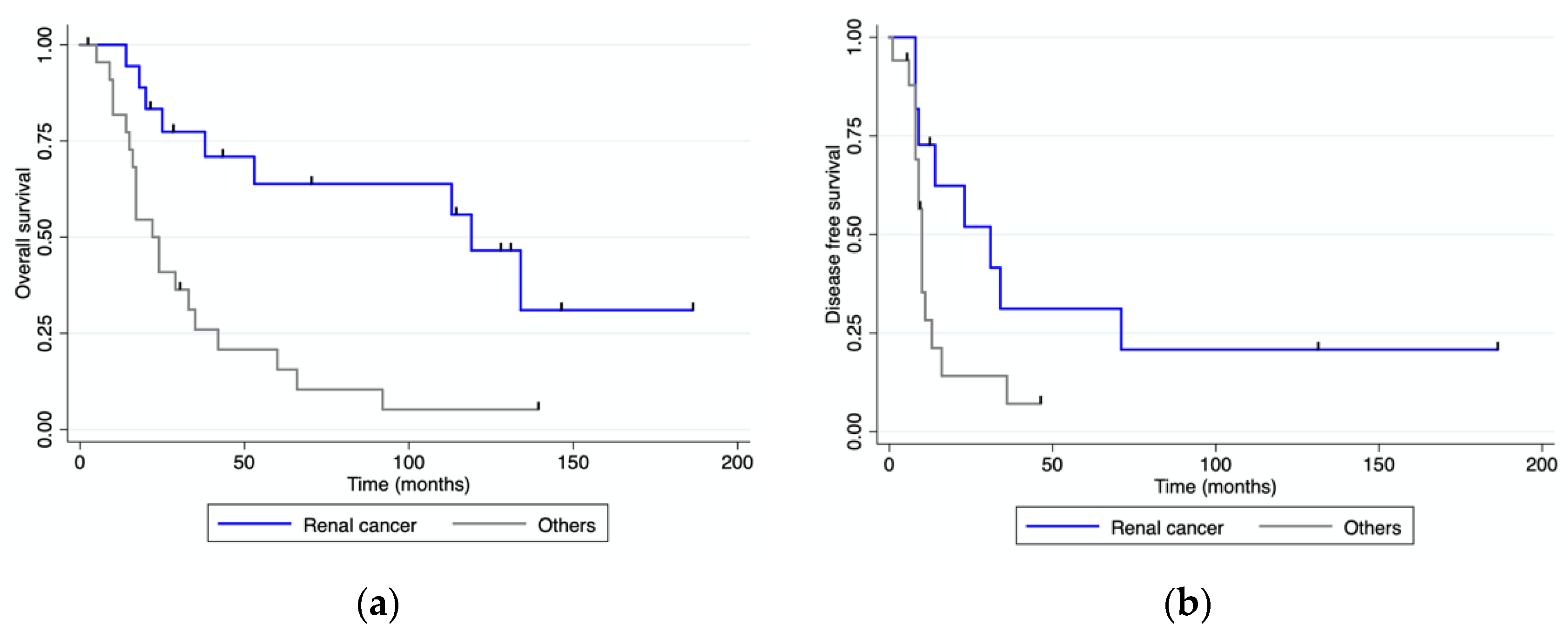
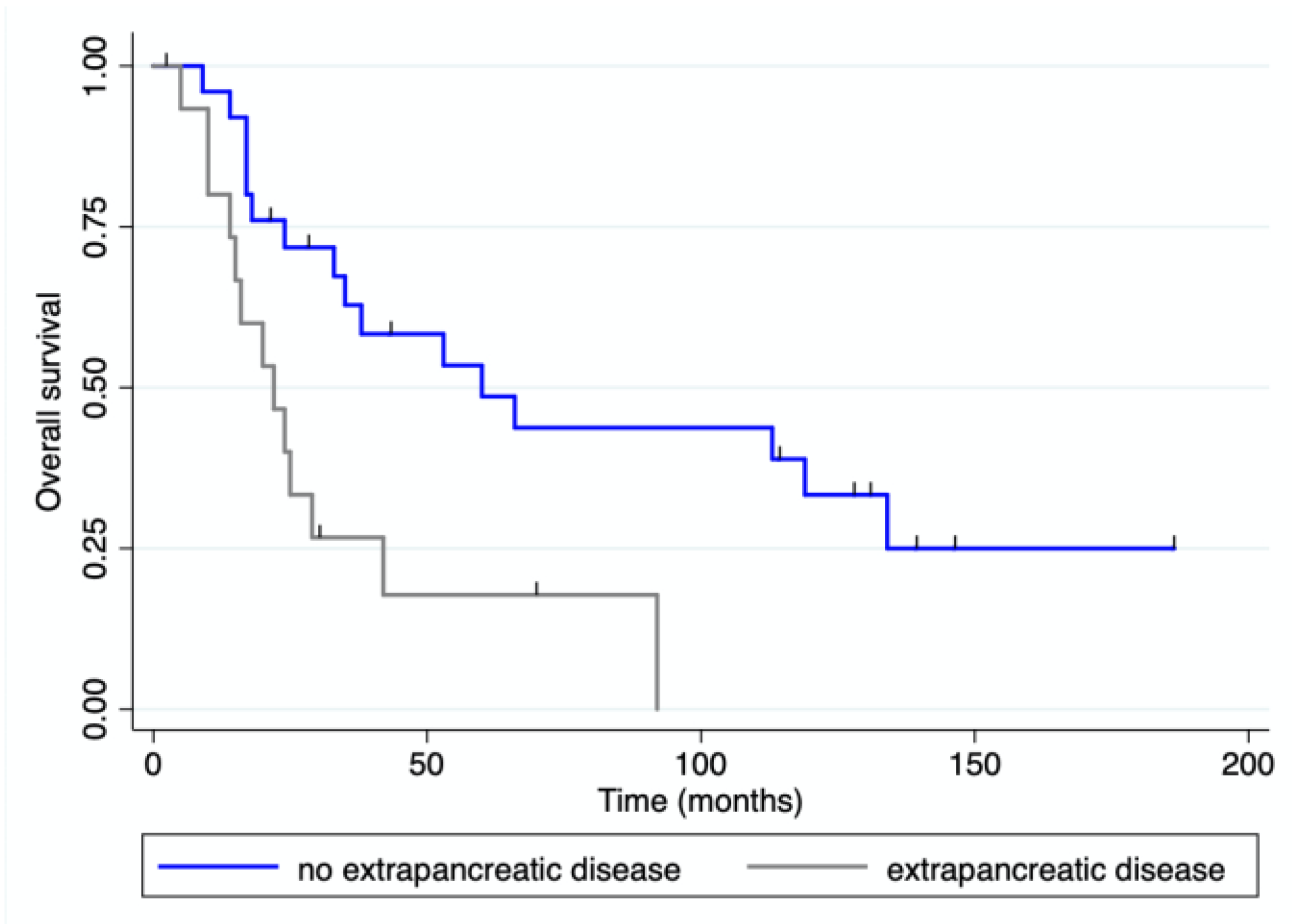
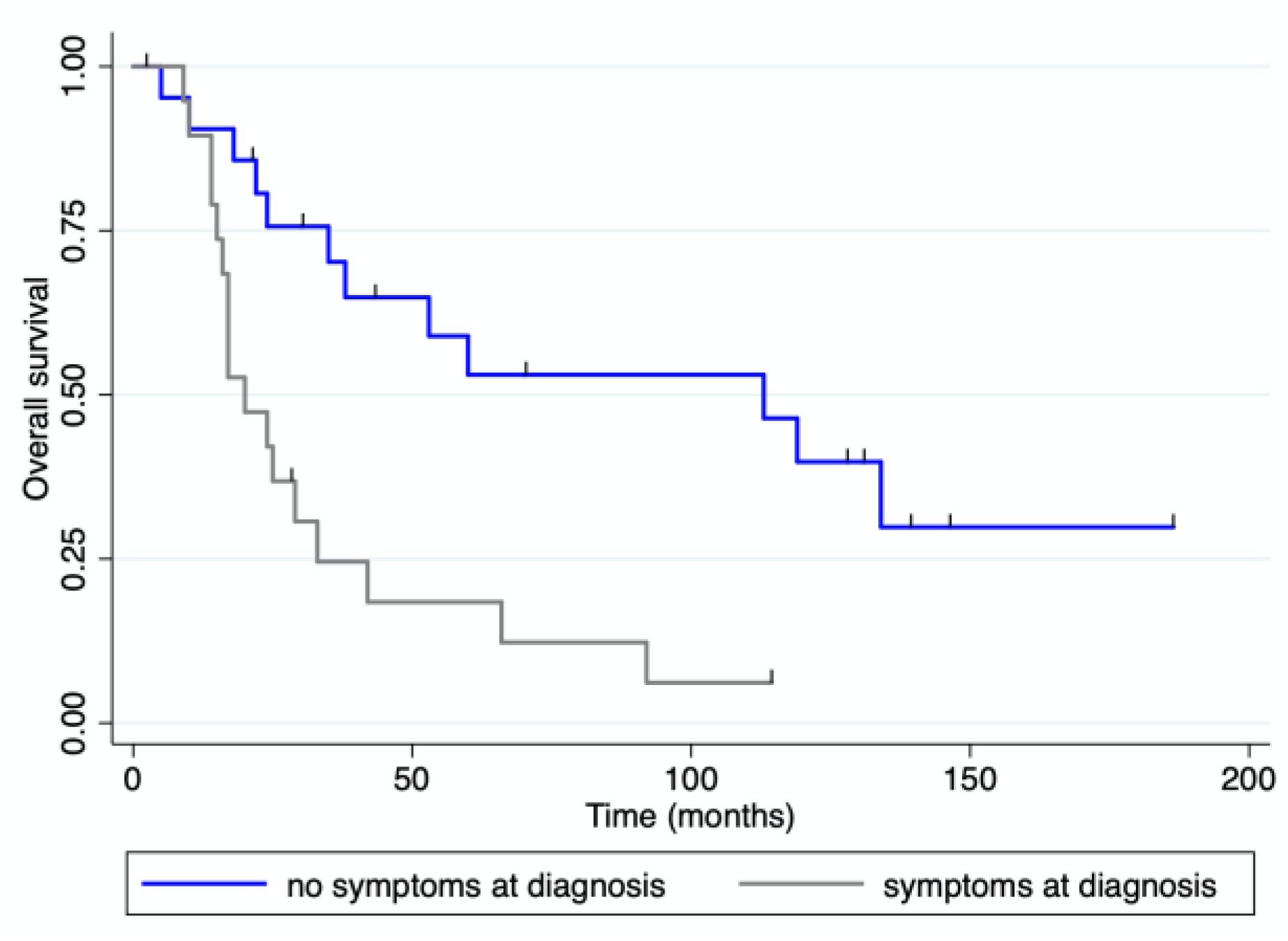
3.4. Survival Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Roland, C.F.; Van Heerden, J.A. Nonpancreatic primary tumors with metastasis to the pancreas. Surg. Gynecol. Obstet. 1989, 168, 345–347. [Google Scholar]
- Z’Graggen, K.; Castillo, C.F.-D.; Rattner, D.W.; Sigala, H.; Warshaw, A.L. Metastases to the Pancreas and Their Surgical Extirpation. Arch. Surg. 1998, 133, 413–418. [Google Scholar] [CrossRef]
- Faure, J.-P.; Tuech, J.-J.; Richer, J.-P.; Pessaux, P.; Arnaud, J.-P.; Carretier, M. Pancreatic metastasis of renal cell carcinoma: Presentation, treatment and survival. J. Urol. 2001, 165, 20–22. [Google Scholar] [CrossRef]
- Brady, L.W.; O’Neill, E.A.; Farber, S.H. Unusual sites of metastases. Semin. Oncol. 1977, 4, 59–64. [Google Scholar]
- Reddy, S.; Edil, B.H.; Cameron, J.L.; Pawlik, T.M.; Herman, J.M.; Gilson, M.M.; Campbell, K.A.; Schulick, R.D.; Ahuja, N.; Wolfgang, C.L. Pancreatic Resection of Isolated Metastases from Nonpancreatic Primary Cancers. Ann. Surg. Oncol. 2008, 15, 3199–3206. [Google Scholar] [CrossRef]
- Zerbi, A.; Ortolano, E.; Balzano, G.; Borri, A.; Beneduce, A.A.; Di Carlo, V. Pancreatic Metastasis from Renal Cell Carcinoma: Which Patients Benefit from Surgical Resection? Ann. Surg. Oncol. 2008, 15, 1161–1168. [Google Scholar] [CrossRef]
- Curley, S.A.; Izzo, F.; Abdalla, E.; Vauthey, J.N. Surgical treatment of colorectal cancer metastasis. Cancer Metastasis Rev. 2004, 23, 165–182. [Google Scholar] [CrossRef]
- Internullo, E.; Cassivi, S.D.; Van Raemdonck, D.; Friedel, G.; Treasure, T. Pulmonary Metastasectomy: A Survey of Current Practice Amongst Members of the European Society of Thoracic Surgeons. J. Thorac. Oncol. 2008, 3, 1257–1266. [Google Scholar] [CrossRef]
- Charlson, M.; Szatrowski, T.P.; Peterson, J.; Gold, J. Validation of a combined comorbidity index. J. Clin. Epidemiol. 1994, 47, 1245–1251. [Google Scholar] [CrossRef] [PubMed]
- Bassi, C.; Marchegiani, G.; Dervenis, C.; Sarr, M.; Hilal, M.A.; Adham, M.; Allen, P.; Andersson, R.; Asbun, H.J.; Besselink, M.G.; et al. The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 Years After. Surgery 2017, 161, 584–591. [Google Scholar] [CrossRef]
- Wente, M.N.; Veit, J.A.; Bassi, C.; Dervenis, C.; Fingerhut, A.; Gouma, D.J.; Izbicki, J.R.; Neoptolemos, J.P.; Padbury, R.T.; Sarr, M.G.; et al. Postpancreatectomy hemorrhage (PPH)–An International Study Group of Pancreatic Surgery (ISGPS) definition. Surgery 2007, 142, 20–25. [Google Scholar] [CrossRef]
- Wente, M.N.; Bassi, C.; Dervenis, C.; Fingerhut, A.; Gouma, D.J.; Izbicki, J.R.; Neoptolemos, J.P.; Padbury, R.T.; Sarr, M.G.; Traverso, L.W.; et al. Delayed gastric emptying (DGE) after pancreatic surgery: A suggested definition by the International Study Group of Pancreatic Surgery (ISGPS). Surgery 2007, 142, 761–768. [Google Scholar] [CrossRef]
- Chikhladze, S.; Lederer, A.-K.; Kühlbrey, C.M.; Hipp, J.; Sick, O.; Fichtner-Feigl, S.; Wittel, U.A. Curative-intent pancreas resection for pancreatic metastases: Surgical and oncological results. Clin. Exp. Metastasis 2020, 37, 313–324. [Google Scholar] [CrossRef]
- Huang, Q.; Zhou, H.; Liu, C.; Jin, K.; Fan, K.; Cheng, H.; Fan, Z.; Yang, C.; Liu, L.; Long, J.; et al. Surgical Resection for Metastatic Tumors in the Pancreas: A Single-Center Experience and Systematic Review. Ann. Surg. Oncol. 2019, 26, 1649–1656. [Google Scholar] [CrossRef] [PubMed]
- Moletta, L.; Milanetto, A.C.; Vincenzi, V.; Alaggio, R.; Pedrazzoli, S.; Pasquali, C. Pancreatic Secondary Lesions from Renal Cell Carcinoma. World J. Surg. 2014, 38, 3002–3006. [Google Scholar] [CrossRef]
- Fullarton, G.; Burgoyne, M. Gallbladder and pancreatic metastasesfrom bilateral renal carcinoma presenting with hematobilia and anemia. Urology 1991, 38, 184–186. [Google Scholar] [CrossRef] [PubMed]
- Vincenzi, M.; Pasquotti, G.; Polverosi, R.; Pasquali, C.; Pomerri, F. Imaging of pancreatic metastases from renal cell carcinoma. Cancer Imaging 2014, 14, 5. [Google Scholar] [CrossRef]
- Nadal, E.; Burra, P.; Mescoli, C.; Albertoni, L.; Sperti, C.; Sturniolo, G.C.; Fantin, A. Pancreatic melanoma metastasis diagnosed by endoscopic ultrasound-guided SharkCore biopsy. Endoscopy 2016, 48, E208–E209. [Google Scholar] [CrossRef]
- Smith, A.L.; Odronic, S.I.; Springer, B.S.; Reynolds, J.P. Solid tumor metastases to the pancreas diagnosed by FNA: A single-institution experience and review of the literature. Cancer Cytopathol. 2015, 123, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-R.; Gemenetzis, G.; Cooper, M.; Javed, A.A.; Cameron, J.L.; Wolfgang, C.L.; Eckhauser, F.E.; He, J.; Weiss, M.J. Long-Term Outcomes of 98 Surgically Resected Metastatic Tumors in the Pancreas. Ann. Surg. Oncol. 2016, 24, 801–807. [Google Scholar] [CrossRef] [PubMed]
- Pedrazzoli, S. Pancreatoduodenectomy (PD) and postoperative pancreatic fistula (POPF). Medicine 2017, 96, e6858. [Google Scholar] [CrossRef]
- Bassi, C.; Butturini, G.; Falconi, M.; Sargenti, M.; Mantovani, W.; Pederzoli, P. High recurrence rate after atypical resection for pancreatic metastases from renal cell carcinoma. Br. J. Surg. 2003, 90, 555–559. [Google Scholar] [CrossRef]
- Law, C.H.L.; Wei, A.C.; Hanna, S.S.; Al-Zahrani, M.; Taylor, B.R.; Greig, P.D.; Langer, B.; Gallinger, S. Pancreatic resection for metastatic renal cell carcinoma: Presentation, treatment, and outcome. Ann. Surg. Oncol. 2003, 10, 922–926. [Google Scholar] [CrossRef] [PubMed]
- Sperti, C.; Pozza, G.; Brazzale, A.R.; Buratin, A.; Moletta, L.; Beltrame, V.; Valmasoni, M. Metastatic tumors to the pancreas: A systematic review and meta-analysis. Int. J. Clin. Rev. 2016, 71, 337–344. [Google Scholar]
- Milanetto, A.C.; Morelli, L.; Di Franco, G.; David, A.; Campra, D.; De Paolis, P.; Pasquali, C. A Plea for Surgery in Pancreatic Metastases from Renal Cell Carcinoma: Indications and Outcome from a Multicenter Surgical Experience. J. Clin. Med. 2020, 9, 3278. [Google Scholar] [CrossRef]
- Tanis, P.J.; van der Gaag, N.A.; Busch, O.R.C.; van Gulik, T.M.; Gouma, D.J. Systematic review of pancreatic surgery for metastatic renal cell carcinoma. Br. J. Surg. 2009, 96, 579–592. [Google Scholar] [CrossRef]
- Sellner, F.; Tykalsky, N.; De Santis, M.; Pont, J.; Klimpfinger, M. Solitary and Multiple Isolated Metastases of Clear Cell Renal Carcinoma to the Pancreas: An Indication for Pancreatic Surgery. Ann. Surg. Oncol. 2006, 13, 75–85. [Google Scholar] [CrossRef]
- Ravaud, A.; Wallerand, H.; Culine, S.; Bernhard, J.-C.; Fergelot, P.; Bensalah, K.; Patard, J.-J. Update on the Medical Treatment of Metastatic Renal Cell Carcinoma. Eur. Urol. 2008, 54, 315–325. [Google Scholar] [CrossRef]
- Santoni, M.; Conti, A.; Partelli, S.; Porta, C.; Sternberg, C.N.; Procopio, G.; Bracarda, S.; Basso, U.; De Giorgi, U.; DeRosa, L.; et al. Surgical Resection Does Not Improve Survival in Patients with Renal Metastases to the Pancreas in the Era of Tyrosine Kinase Inhibitors. Ann. Surg. Oncol. 2014, 22, 2094–2100. [Google Scholar] [CrossRef]
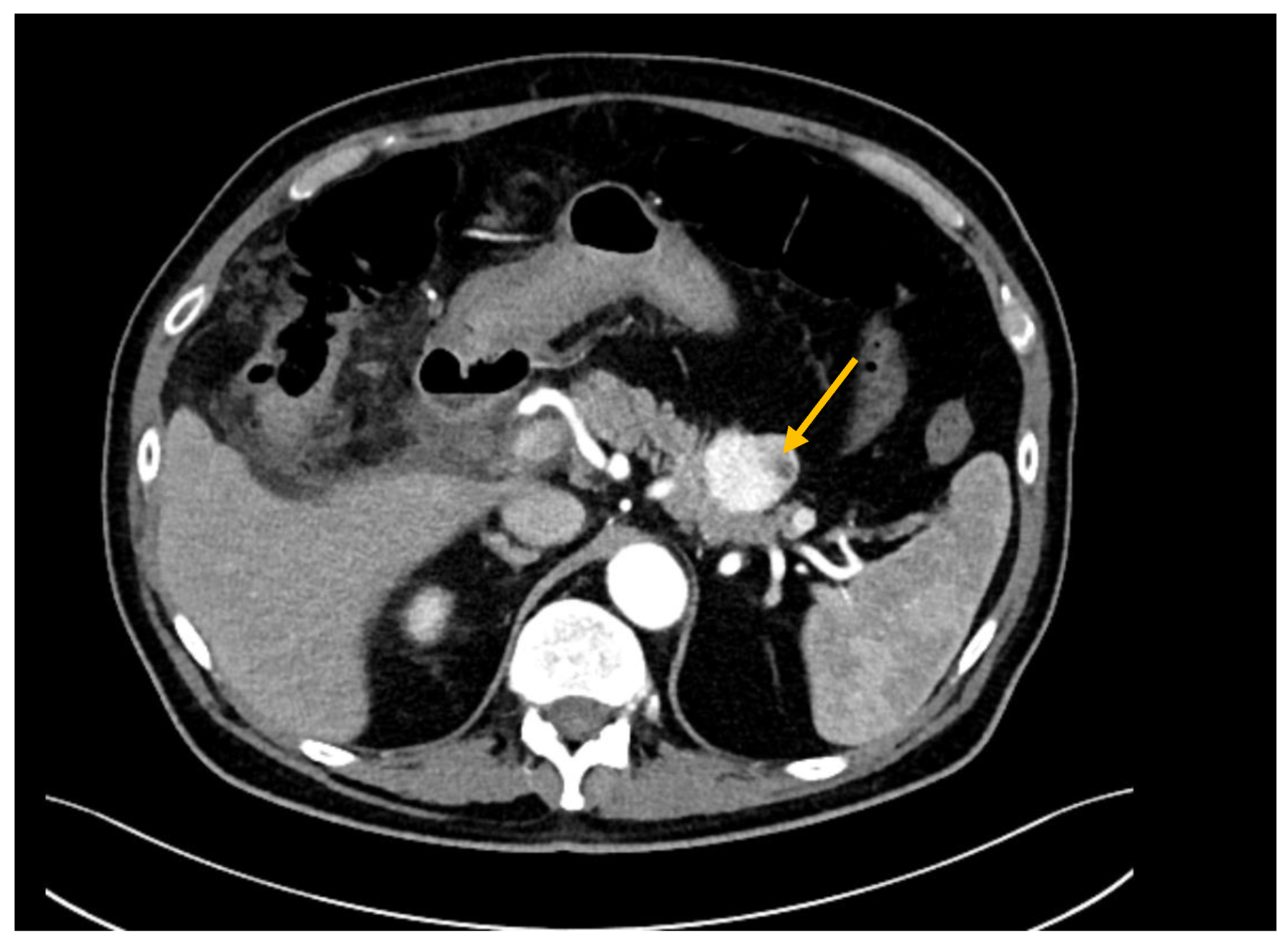
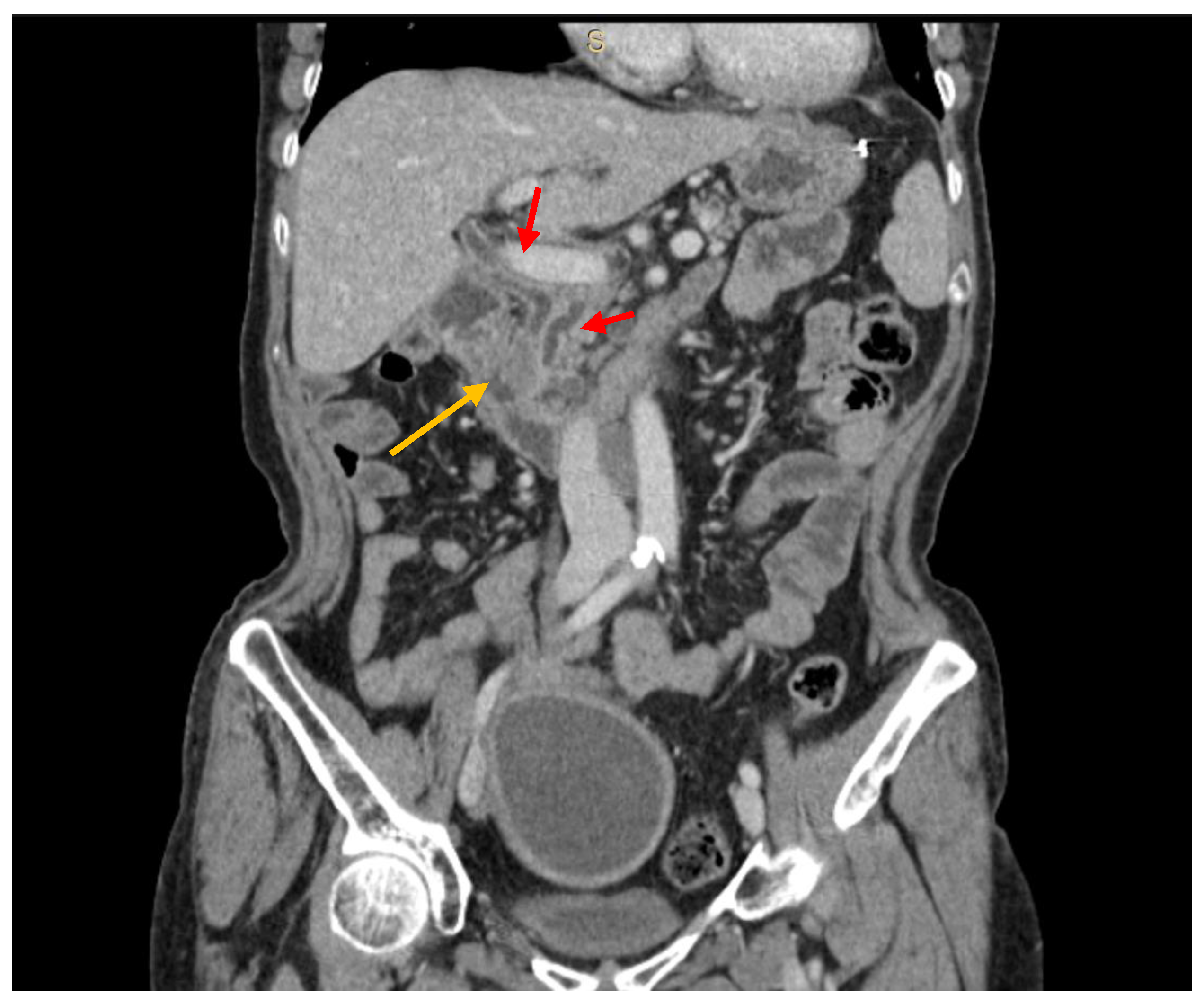
| All (n = 44) | RCC (n = 19) | Other Tumors (n = 25) | p | |||||
| n | % | n | % | n | % | |||
| Sex | M | 22 | 50 | 11 | 57.9 | 11 | 44.0 | 0.27 |
| F | 22 | 50 | 8 | 42.1 | 14 | 56.0 | ||
| Age mean ± SD, years | 63.7 ± 10 | 66.4 ± 8.1 | 61.7 ± 10.7 | 0.06 | ||||
| Preoperative diabetes | 6 | 13.6 | 4 | 21.0 | 2 | 8.0 | 0.21 | |
| ECOG > 1 | 4 | 9.1 | 2 | 10.5 | 2 | 8.0 | 0.59 | |
| Symptoms at diagnosis | 21 | 47.7 | 6 | 31.6 | 15 | 60.0 | 0.6 | |
| Interval from primary tumor to PM median (range), months | 48 (0–260) | 111 (0–260) | 29 (0–115) | 0.009 | ||||
| Previous metastases | 6 | 13.6 | 2 | 10.5 | 4 | 16.0 | 0.48 | |
| Synchronous | 11 | 25 | 3 | 15.8 | 8 | 32.0 | 0.19 | |
| Multiple pancreatic lesions | 15 | 34.1 | 12 | 63.1 | 3 | 12.0 | 0.001 | |
| Extrapancreatic disease | 15 | 34.1 | 3 | 15.8 | 12 | 48.0 | 0.001 | |
| Location of PM | ||||||||
| Head | 13 | 29.5 | 3 | 15.8 | 11 | 44.0 | 0.04 | |
| Body | 7 | 15.9 | 2 | 10.5 | 4 | 16.0 | 0.48 | |
| Tail | 9 | 20.4 | 2 | 10.5 | 7 | 15.9 | 0.15 | |
| Multiple locations | 15 | 34.1 | 12 | 63.1 | 3 | 12.0 | 0.001 | |
| Size of largest PM Median (range), mm | 30.0 (7.0–100.0) | 25.0 (7.0–40.0) | 30.0 (20.0–100.0) | 0.0124 | ||||
| Variable | n (%) |
|---|---|
| Side | Right kidney: 8 (42.1) Left kidney: 10 (52.7) Bilateral: 1 (5.2) |
| Histology | Clear cell carcinoma: 19 (100) |
| Fuhrman nuclear grade | G1: 2 (10.5) G2: 14 (73.7) G3: 3 (15.8) |
| TNM | Stage 1: 4 (21.1) Stage 2: 5 (26.3) Stage 3: 8 (42.1) Stage 4: 2 (10.5) |
| Type of intervention | Unilateral radical nephrectomy 18 (94.7) Left radical nephrectomy + right partial nephrectomy 1 (5.3) |
| Timing of PM from RCC, n | Synchronous: 3 (15.8) |
| Metachronous: 16 (84.2) | |
| Diagnostic work-up | |
| 18-FDG-PET | Performed in 7, positive in 5 |
| Octreoscan | Performed in 4, positive in 2 |
| CT scan | Performed in all patients, all positive |
| Variables | Total | RCC | Other Tumors | p |
|---|---|---|---|---|
| Surgical procedures n (%) | 37 | 16 | 21 | |
| PD | 13 (35.1) | 1 (6.2) | 12 (57.1) | 0.001 |
| DP | 18 (48.6) | 9 (56.2) | 9 (42.9) | 0.32 |
| CP | 2 (5.4) | 2 (12.6) | 0 | 0.18 |
| DPPHR | 1 (2.7) | 1 (6.2) | 0 | 0.43 |
| TP | 1 (2.7) | 1 (6.2) | 0 | 0.43 |
| Exploratory/palliative | 2 (5.4) | 2 (12.6) | 0 | 0.18 |
| Operating time min, median (range) | 208.0 (115.0–470.0) | 242.5 (115.0–395.0) | 300.0 (165.0–470.0) | 0.05 |
| Associated procedures n (%) | 16 (43.2) | 2 (12.5) | 14 (66.7) | 0.001 |
| R0 resection n (%) | 29 (78.4) | 12 (75) | 17 (80.9) | 0.48 |
| Lymph node metastases n (%) | 13 (35.1) | 0 * (0) | 13 (61.9) | <0.001 |
| LOS median (range) | 13 (7–89) | 10 (7–66) | 17 (7–89) | 0.09 |
| Major postoperative complications n (%) | 15 (40.5) | 7 (43.7) | 8 (38.1) | 0.50 |
| Pancreatic fistula n (%) | ||||
| B | 8 (21.6) | 6 (37.5) | 2 (9.5) | 0.05 |
| C | 0 (0) | 0 (0) | 0 (0) | 1.00 |
| In-hospital mortality n (%) | 2 (5.4) | 1 (6.2) | 1 (4.7) | 0.69 |
| Postoperative diabetes n (%) | 5 (13.5) | 3 (18.7) | 2 (9.5) | 0.37 |
| Adjuvant treatment n (%) | 18 (48.6) | 7 (43.7) | 11 (52.4) | 0.43 |
| Disease recurrence n (%) | 25 (67.6) | 9 (56.2) | 16 (76.2) | 0.18 |
| Disease-free survival median (range) | 11 mo (0–186) | 31 mo (0–186) | 10 mo (0–46) | 0.04 |
| Overall survival median (range) | 38 mo (0–186) | 119 mo (0–186) | 24 mo (0–139) | <0.001 |
| Variables | Univariate | Multivariate | ||
|---|---|---|---|---|
| n (%) | p | OR (95% CI) | p | |
| Synchronous PM | 11 (25) | ns | - | - |
| Renal cell carcinoma | 19 (43.2) | 0.001 | 2.48 (1.00–6.14) | 0.050 |
| Symptoms | 21 (47.7) | 0.003 | 2.08 (0.86–5.00) | 0.101 |
| Postoperative complications | 15 (40.5) | ns | - | - |
| Adjuvant treatment | 18 (48.6) | ns | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moletta, L.; Friziero, A.; Serafini, S.; Grillo, V.; Pierobon, E.S.; Capovilla, G.; Valmasoni, M.; Sperti, C. Safety and Efficacy of Surgery for Metastatic Tumor to the Pancreas: A Single-Center Experience. J. Clin. Med. 2023, 12, 1171. https://doi.org/10.3390/jcm12031171
Moletta L, Friziero A, Serafini S, Grillo V, Pierobon ES, Capovilla G, Valmasoni M, Sperti C. Safety and Efficacy of Surgery for Metastatic Tumor to the Pancreas: A Single-Center Experience. Journal of Clinical Medicine. 2023; 12(3):1171. https://doi.org/10.3390/jcm12031171
Chicago/Turabian StyleMoletta, Lucia, Alberto Friziero, Simone Serafini, Valeria Grillo, Elisa Sefora Pierobon, Giovanni Capovilla, Michele Valmasoni, and Cosimo Sperti. 2023. "Safety and Efficacy of Surgery for Metastatic Tumor to the Pancreas: A Single-Center Experience" Journal of Clinical Medicine 12, no. 3: 1171. https://doi.org/10.3390/jcm12031171
APA StyleMoletta, L., Friziero, A., Serafini, S., Grillo, V., Pierobon, E. S., Capovilla, G., Valmasoni, M., & Sperti, C. (2023). Safety and Efficacy of Surgery for Metastatic Tumor to the Pancreas: A Single-Center Experience. Journal of Clinical Medicine, 12(3), 1171. https://doi.org/10.3390/jcm12031171






