Large-Scale Genetic Correlation Analysis between Spondyloarthritis and Human Blood Metabolites
Abstract
:1. Introduction
2. Materials and Methods
2.1. The GWAS Summary Data of SpA
2.2. The GWAS Summary Data of Human Blood Metabolite
2.3. LDSC Regression
2.4. MR Analysis
3. Results
3.1. Genetic Correlation Analysis
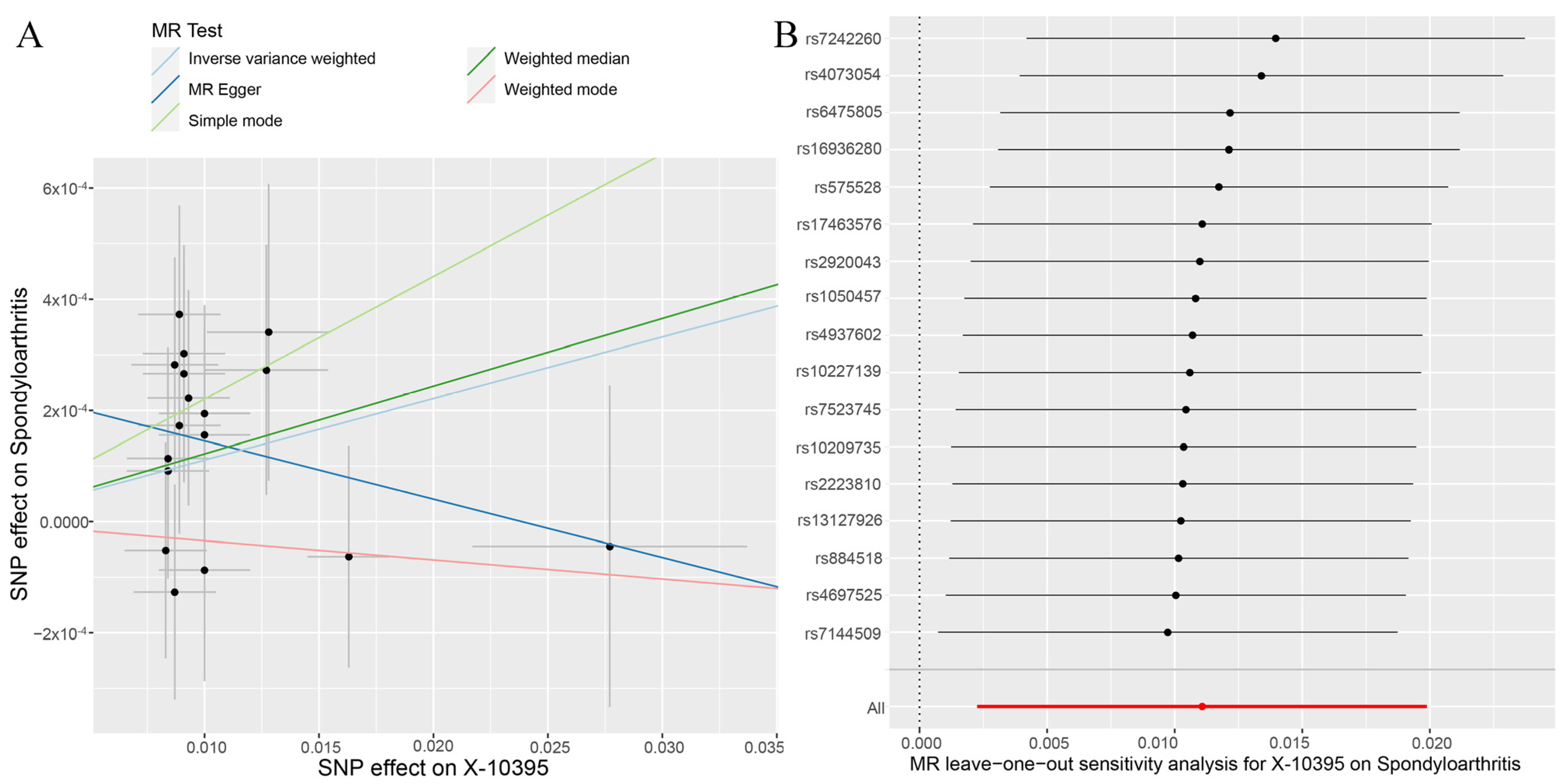
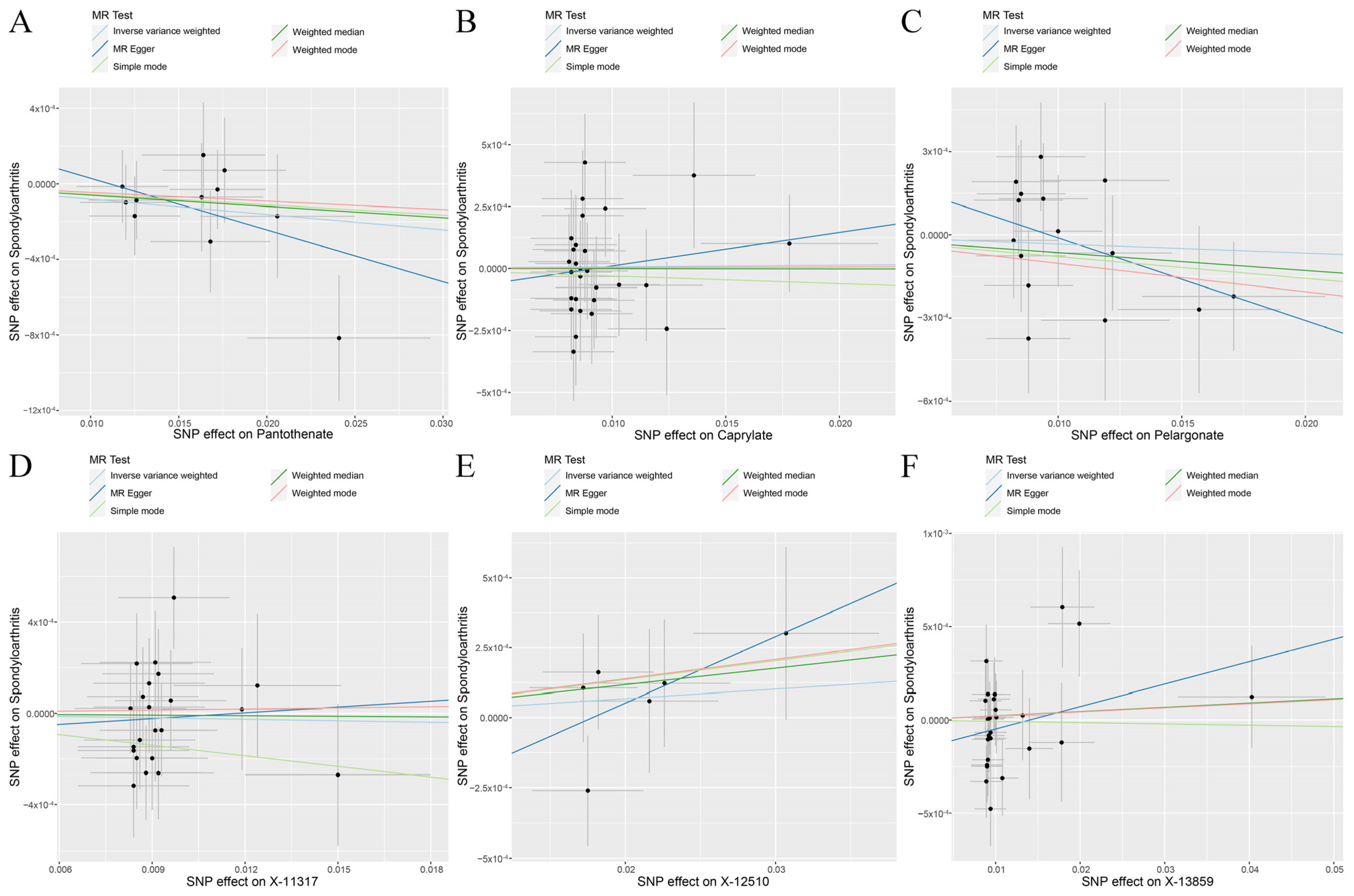
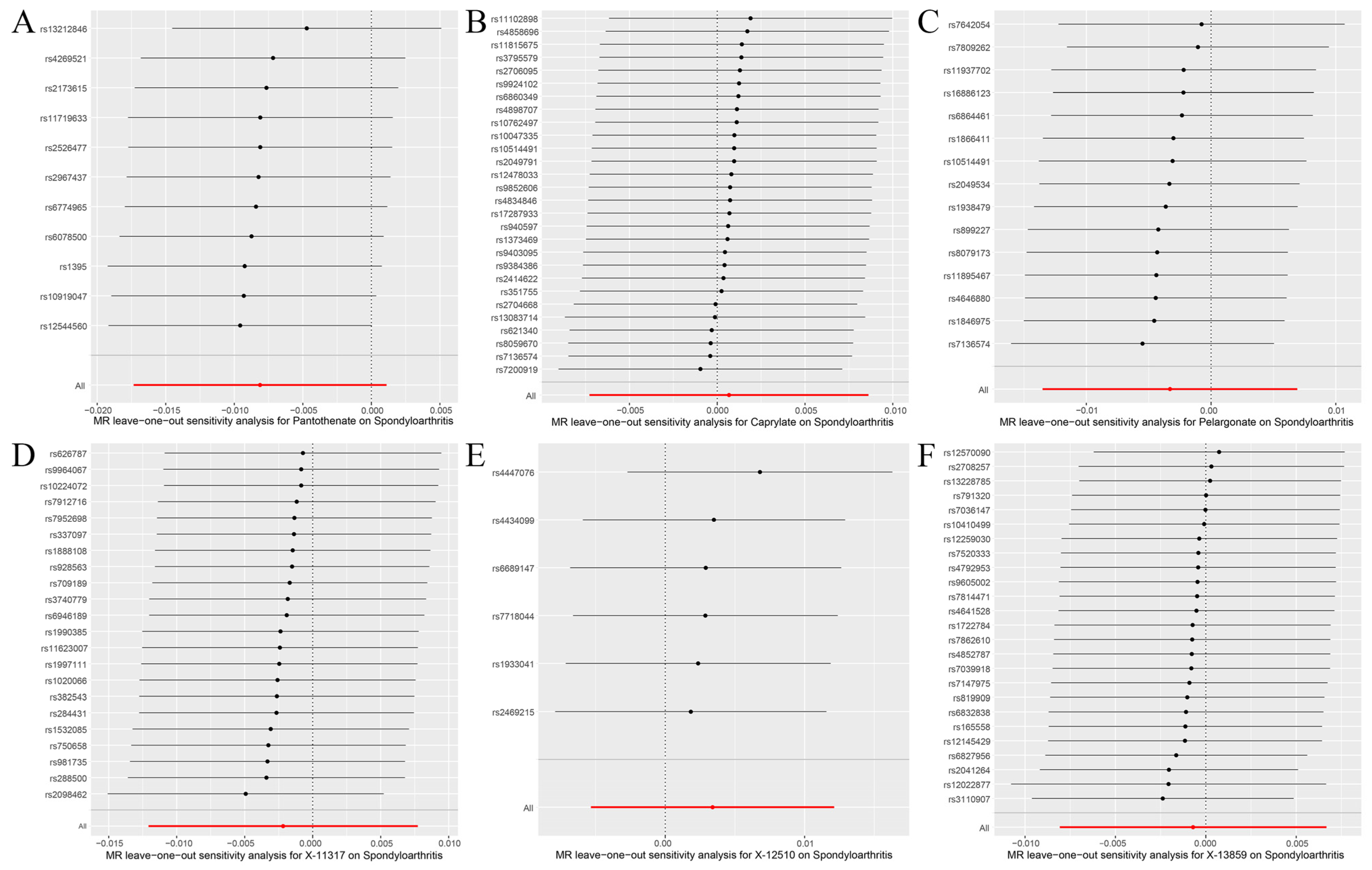
3.2. Genetic Causal Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dougados, M.; Baeten, D. Spondyloarthritis. Lancet 2011, 377, 2127–2137. [Google Scholar] [CrossRef] [PubMed]
- Stolwijk, C.; Boonen, A.; Van Tubergen, A.; Reveille, J.D. Epidemiology of spondyloarthritis. Rheum. Dis. Clin. N. Am. 2012, 38, 441–476. [Google Scholar] [CrossRef] [PubMed]
- McGonagle, D.; Wakefield, R.J.; Tan, A.L.; D’Agostino, M.A.; Toumi, H.; Hayashi, K.; Emery, P.; Benjamin, M. Distinct topography of erosion and new bone formation in achilles tendon enthesitis: Implications for understanding the link between inflammation and bone formation in spondylarthritis. Arthritis Rheum. 2008, 58, 2694–2699. [Google Scholar] [CrossRef] [PubMed]
- Saraux, A.; Guillemin, F.; Guggenbuhl, P.; Roux, C.H.; Fardellone, P.; Le Bihan, E.; Cantagrel, A.; Chary-Valckenaere, I.; Euller-Ziegler, L.; Flipo, R.M.; et al. Prevalence of spondyloarthropathies in France: 2001. Ann. Rheum. Dis. 2005, 64, 1431–1435. [Google Scholar] [CrossRef] [PubMed]
- Akkoc, N.; Khan, M.A. Overestimation of the prevalence of ankylosing spondylitis in the Berlin study: Comment on the article by Braun et al. Arthritis Rheum. 2005, 52, 4048–4049. [Google Scholar] [CrossRef]
- Ng, S.C.; Liao, Z.; Yu, D.T.; Chan, E.S.; Zhao, L.; Gu, J. Epidemiology of spondyloarthritis in the People’s Republic of China: Review of the literature and commentary. Semin. Arthritis Rheum. 2007, 37, 39–47. [Google Scholar] [CrossRef]
- Ramos, M.; Alvarez, I.; Sesma, L.; Logean, A.; Rognan, D.; Lopez de Castro, J.A. Molecular mimicry of an HLA-B27-derived ligand of arthritis-linked subtypes with chlamydial proteins. J. Biol. Chem. 2002, 277, 37573–37581. [Google Scholar] [CrossRef]
- Said-Nahal, R.; Miceli-Richard, C.; D’agostino, M.-A.; Dernis-Labous, E. Phenotypic Diversity Is Not Determined by Independent Genetic Factors in Familial Spondylarthropathy. Arthritis Care Res. 2001, 45, 478–484. [Google Scholar] [CrossRef]
- Breban, M. Genetics of spondyloarthritis. Best Pr. Res. Clin. Rheumatol. 2006, 20, 593–599. [Google Scholar] [CrossRef]
- Said-Nahal, R.; Miceli-Richard, C.; Berthelot, J.-M. The familial form of spondylarthropathy. Arthritis Rheum. 2000, 43, 1356–1365. [Google Scholar] [CrossRef]
- Breban, M.; Said-Nahal, R.; Hugot, J.-P.; Miceli-Richard, C. Familial and genetic aspects of spondyloarthropathy. Rheum. Dis. Clin. N. Am. 2003, 29, 575–594. [Google Scholar] [CrossRef] [PubMed]
- Tautog, B.J.D.; Richardson, J.A.; Croft, J.T.; Simmons, W.A. The Germfree State Prevents Development of Gut and Joint Inflammatory Disease in HLA-B27 Transgenic Rats. J. Exp. Med. 1994, 180, 2359–2364. [Google Scholar]
- Tran, T.M.; Dorris, M.L.; Satumtira, N.; Richardson, J.A.; Hammer, R.E.; Shang, J.; Taurog, J.D. Additional human beta2-microglobulin curbs HLA-B27 misfolding and promotes arthritis and spondylitis without colitis in male HLA-B27-transgenic rats. Arthritis Rheum. 2006, 54, 1317–1327. [Google Scholar] [CrossRef] [PubMed]
- Durham, L.E.; Kirkham, B.W.; Taams, L.S. Contribution of the IL-17 Pathway to Psoriasis and Psoriatic Arthritis. Curr. Rheumatol. Rep. 2015, 17, 55. [Google Scholar] [CrossRef]
- Vitulano, C.; Tedeschi, V.; Paladini, F.; Sorrentino, R.; Fiorillo, M.T. The interplay between HLA-B27 and ERAP1/ERAP2 aminopeptidases: From anti-viral protection to spondyloarthritis. Clin. Exp. Immunol. 2017, 190, 281–290. [Google Scholar] [CrossRef]
- Costantino, F.; Breban, M.; Garchon, H.J. Genetics and Functional Genomics of Spondyloarthritis. Front Immunol. 2018, 9, 2933. [Google Scholar] [CrossRef]
- Wishart, D.S.; Feunang, Y.D.; Marcu, A.; Guo, A.C.; Liang, K.; Vazquez-Fresno, R.; Sajed, T.; Johnson, D.; Li, C.; Karu, N.; et al. HMDB 4.0: The human metabolome database for 2018. Nucleic Acids Res. 2018, 46, D608–D617. [Google Scholar] [CrossRef]
- Hagenbeek, F.A.; Pool, R.; Van Dongen, J.; Draisma, H.H.M.; Jan Hottenga, J.; Willemsen, G.; Abdellaoui, A.; Fedko, I.O.; Den Braber, A.; Visser, P.J.; et al. Heritability estimates for 361 blood metabolites across 40 genome-wide association studies. Nat. Commun. 2020, 11, 39. [Google Scholar] [CrossRef]
- Kastenmuller, G.; Raffler, J.; Gieger, C.; Suhre, K. Genetics of human metabolism: An update. Hum. Mol. Genet. 2015, 24, R93–R101. [Google Scholar] [CrossRef] [PubMed]
- Fischer, R.; Trudgian, D.C.; Wright, C.; Thomas, G.; Bradbury, L.A.; Brown, M.A.; Bowness, P.; Kessler, B.M. Discovery of candidate serum proteomic and metabolomic biomarkers in ankylosing spondylitis. Mol. Cell Proteom. 2012, 11, M111.013904. [Google Scholar] [CrossRef]
- Looby, N.; Roszkowska, A.; Reyes-Garces, N.; Yu, M.; Baczek, T.; Kulasingam, V.; Pawliszyn, J.; Chandran, V. Serum metabolic fingerprinting of psoriasis and psoriatic arthritis patients using solid-phase microextraction-liquid chromatography-high-resolution mass spectrometry. Metabolomics 2021, 17, 59. [Google Scholar] [CrossRef] [PubMed]
- Bogunia-Kubik, K.; Wojtowicz, W.; Swierkot, J.; Mielko, K.A.; Qasem, B.; Wielinska, J.; Sokolik, R.; Pruss, L.; Mlynarz, P. Disease Differentiation and Monitoring of Anti-TNF Treatment in Rheumatoid Arthritis and Spondyloarthropathies. Int. J. Mol. Sci. 2021, 22, 7389. [Google Scholar] [CrossRef] [PubMed]
- Gupta, L.; Guleria, A.; Rawat, A.; Kumar, D.; Aggarwal, A. NMR-based clinical metabolomics revealed distinctive serum metabolic profiles in patients with spondyloarthritis. Magn. Reson. Chem. 2021, 59, 85–98. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wang, S.; Wen, Y.; Li, P.; Cheng, S.; Ma, M.; Zhang, L.; Cheng, B.; Qi, X.; Liang, C.; et al. Assessing the genetic relationships between osteoarthritis and human plasma proteins: A large scale genetic correlation scan. Ann. Transl. Med. 2020, 8, 677. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.C.; Demontis, D.; Thorgeirsson, T.E.; Walters, R.K.; Polimanti, R.; Hatoum, A.S.; Sanchez-Roige, S.; Paul, S.E.; Wendt, F.R.; Clarke, T.-K.; et al. A large-scale genome-wide association study meta-analysis of cannabis use disorder. Lancet Psychiatry 2020, 7, 1032–1045. [Google Scholar] [CrossRef]
- Ference, B.A.; Ray, K.K.; Catapano, A.L.; Ference, T.B.; Burgess, S.; Neff, D.R.; Oliver-Williams, C.; Wood, A.M.; Butterworth, A.S.; Di Angelantonio, E.; et al. Mendelian Randomization Study of ACLY and Cardiovascular Disease. N. Engl. J. Med. 2019, 380, 1033–1042. [Google Scholar] [CrossRef]
- Burgess, S.; Daniel, R.M.; Butterworth, A.S.; Thompson, S.G.; Consortium, E.P.-I. Network Mendelian randomization: Using genetic variants as instrumental variables to investigate mediation in causal pathways. Int. J. Epidemiol. 2015, 44, 484–495. [Google Scholar] [CrossRef]
- Consortium, T.G.P. An integrated map of genetic variation from 1092 human genomes. Nature 2012, 491, 56–65. [Google Scholar] [CrossRef]
- McCarthy, S.; Das, S.; Kretzschmar, W.; Delaneau, O.; Wood, A.R.; Teumer, A.; Kang, H.M.; Fuchsberger, C.; Danecek, P.; Sharp, K.; et al. A reference panel of 64,976 haplotypes for genotype imputation. Nat. Genet. 2016, 48, 1279–1283. [Google Scholar]
- Canela-Xandri, O.; Rawlik, K.; Tenesa, A. An atlas of genetic associations in UK Biobank. Nat. Genet. 2018, 50, 1593–1599. [Google Scholar] [CrossRef]
- Shin, S.Y.; Fauman, E.B.; Petersen, A.K.; Krumsiek, J.; Santos, R.; Huang, J.; Arnold, M.; Erte, I.; Forgetta, V.; Yang, T.P.; et al. An atlas of genetic influences on human blood metabolites. Nat. Genet. 2014, 46, 543–550. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.J.; McGue, M.; Iacono, W.G.; Chow, C.C. The accuracy of LD Score regression as an estimator of confounding and genetic correlations in genome-wide association studies. Genet. Epidemiol. 2018, 42, 783–795. [Google Scholar] [CrossRef] [PubMed]
- Ni, G.; Moser, G.; Wray, N.R.; Lee, S.H. Estimation of Genetic Correlation via Linkage Disequilibrium Score Regression and Genomic Restricted Maximum Likelihood. Am. J. Hum. Genet. 2018, 102, 1185–1194. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Luo, P.; Zhang, F.; Xu, K.; Feng, R.; Xu, P. Large-scale correlation analysis of deep venous thrombosis and gut microbiota. Front. Cardiovasc. Med. 2022, 9, 1025918. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Li, P.; Wen, Y.; Liang, X.; Liu, L.; Cheng, B.; Ding, M.; Zhao, Y.; Ma, M.; Zhang, L.; et al. Evaluating the Correlations Between Osteoporosis and Lifestyle-Related Factors Using Transcriptome-Wide Association Study. Calcif. Tissue Int. 2020, 106, 256–263. [Google Scholar] [CrossRef]
- Ni, J.J.; Xu, Q.; Yan, S.S.; Han, B.X.; Zhang, H.; Wei, X.T.; Feng, G.J.; Zhao, M.; Pei, Y.F.; Zhang, L. Gut Microbiota and Psychiatric Disorders: A Two-Sample Mendelian Randomization Study. Front. Microbiol. 2021, 12, 737197. [Google Scholar] [CrossRef]
- Shu, M.J.; Li, J.R.; Zhu, Y.C.; Shen, H. Migraine and Ischemic Stroke: A Mendelian Randomization Study. Neurol. Ther. 2022, 11, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, J.; Nieto-Gonzalez, J.C.; Curbelo Rodriguez, R.; Castaneda, S.; Carmona, L. Prevalence and risk factors for osteoporosis and fractures in axial spondyloarthritis: A systematic review and meta-analysis. Semin. Arthritis Rheum. 2018, 48, 44–52. [Google Scholar] [CrossRef]
- Hemani, G.; Zheng, J.; Elsworth, B.; Wade, K.H.; Haberland, V.; Baird, D.; Laurin, C.; Burgess, S.; Bowden, J.; Langdon, R.; et al. The MR-Base platform supports systematic causal inference across the human phenome. Elife 2018, 7, e34408. [Google Scholar] [CrossRef]
- Lee, Y.H. Causal association between smoking behavior and the decreased risk of osteoarthritis: A Mendelian randomization. Z. Rheumatol. 2019, 78, 461–466. [Google Scholar] [CrossRef]
- Tahiliani, A.G.; Beinlich, C.J. Pantothenic Acid in Health and Disease. Vitam. Horm. 1991, 46, 165–228. [Google Scholar]
- He, W.; Hu, S.; Du, X.; Wen, Q.; Zhong, X.P.; Zhou, X.; Zhou, C.; Xiong, W.; Gao, Y.; Zhang, S.; et al. Vitamin B5 Reduces Bacterial Growth via Regulating Innate Immunity and Adaptive Immunity in Mice Infected with Mycobacterium tuberculosis. Front. Immunol. 2018, 9, 365. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.; Kim, M.K.; Choi, B.Y. The long-term relationship between dietary pantothenic acid (vitamin B5) intake and C-reactive protein concentration in adults aged 40 years and older. Nutr. Metab. Cardiovasc. Dis. 2017, 27, 806–816. [Google Scholar] [CrossRef] [PubMed]
- Gominak, S.C. Vitamin D deficiency changes the intestinal microbiome reducing B vitamin production in the gut. The resulting lack of pantothenic acid adversely affects the immune system, producing a “pro-inflammatory” state associated with atherosclerosis and autoimmunity. Med. Hypotheses 2016, 94, 103–107. [Google Scholar] [PubMed]
- Ehmedah, A.; Nedeljkovic, P.; Dacic, S.; Repac, J.; Draskovic Pavlovic, B.; Vucevic, D.; Pekovic, S.; Bozic Nedeljkovic, B. Vitamin B Complex Treatment Attenuates Local Inflammation after Peripheral Nerve Injury. Molecules 2019, 24, 4615. [Google Scholar] [CrossRef]
- Haslock, B.D.I.; Wright, A.V. Pantothenic acid in the treatment of osteoarthrosis. Rheumatol. Phys. Med. 1971, 11, 10–13. [Google Scholar] [CrossRef]
- Annand, J.C. Pantothenic acid and osteoarthrosis. Lancet 1963, 2, 1168. [Google Scholar] [CrossRef]
- Ma, Q.; Liang, M.; Tang, X.; Luo, F.; Dou, C. Vitamin B5 inhibit RANKL induced osteoclastogenesis and ovariectomy induced osteoporosis by scavenging ROS generation. Am. J. Transl. Res. 2019, 11, 5008–5018. [Google Scholar]
- Calvo-Gutierrez, J.; Garrido-Castro, J.L.; Gil-Cabezas, J. Is Spinal Mobility in Patients with Spondylitis Determined by Age, Structural Damage, and Inflammation? Arthritis Care Res. 2015, 67, 74–79. [Google Scholar] [CrossRef]
- Vinker Shuster, M.; Gendelman, O.; Tiosano, S.; Comaneshter, D.; Cohen, A.D.; Amital, H. Ischemic heart disease and ankylosing spondylitis-assessing the role of inflammation. Clin. Rheumatol. 2018, 37, 1053–1058. [Google Scholar] [CrossRef]
- Pryshchep, O.; Ma-Krupa, W.; Younge, B.R.; Goronzy, J.J.; Weyand, C.M. Vessel-specific Toll-like receptor profiles in human medium and large arteries. Circulation 2008, 118, 1276–1284. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Wang, L.; Zhao, Q.; Wu, Z.; Kong, L. MicroRNA93 inhibits chondrocyte apoptosis and inflammation in osteoarthritis by targeting the TLR4/NFkappaB signaling pathway. Int. J. Mol. Med. 2019, 43, 779–790. [Google Scholar] [PubMed]
- Zhang, X.; Xue, C.; Xu, Q.; Zhang, Y.; Li, H.; Li, F.; Liu, Y.; Guo, C. Caprylic acid suppresses inflammation via TLR4/NF-kappaB signaling and improves atherosclerosis in ApoE-deficient mice. Nutr. Metab. 2019, 16, 40. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xu, R.; Wu, L.; Jiang, J. Expression and function of Tolllike receptors in peripheral blood mononuclear cells in patients with ankylosing spondylitis. Mol. Med. Rep. 2019, 20, 3565–3572. [Google Scholar] [PubMed]
- Yamatani, H.; Takahashi, K.; Yoshida, T.; Soga, T.; Kurachi, H. Differences in the fatty acid metabolism of visceral adipose tissue in postmenopausal women. Menopause 2014, 21, 170–176. [Google Scholar] [CrossRef]
- Shi, H.; Kokoeva, M.V.; Inouye, K.; Tzameli, I.; Yin, H.; Flier, J.S. TLR4 links innate immunity and fatty acid-induced insulin resistance. J. Clin. Investig. 2006, 116, 3015–3025. [Google Scholar] [CrossRef] [Green Version]

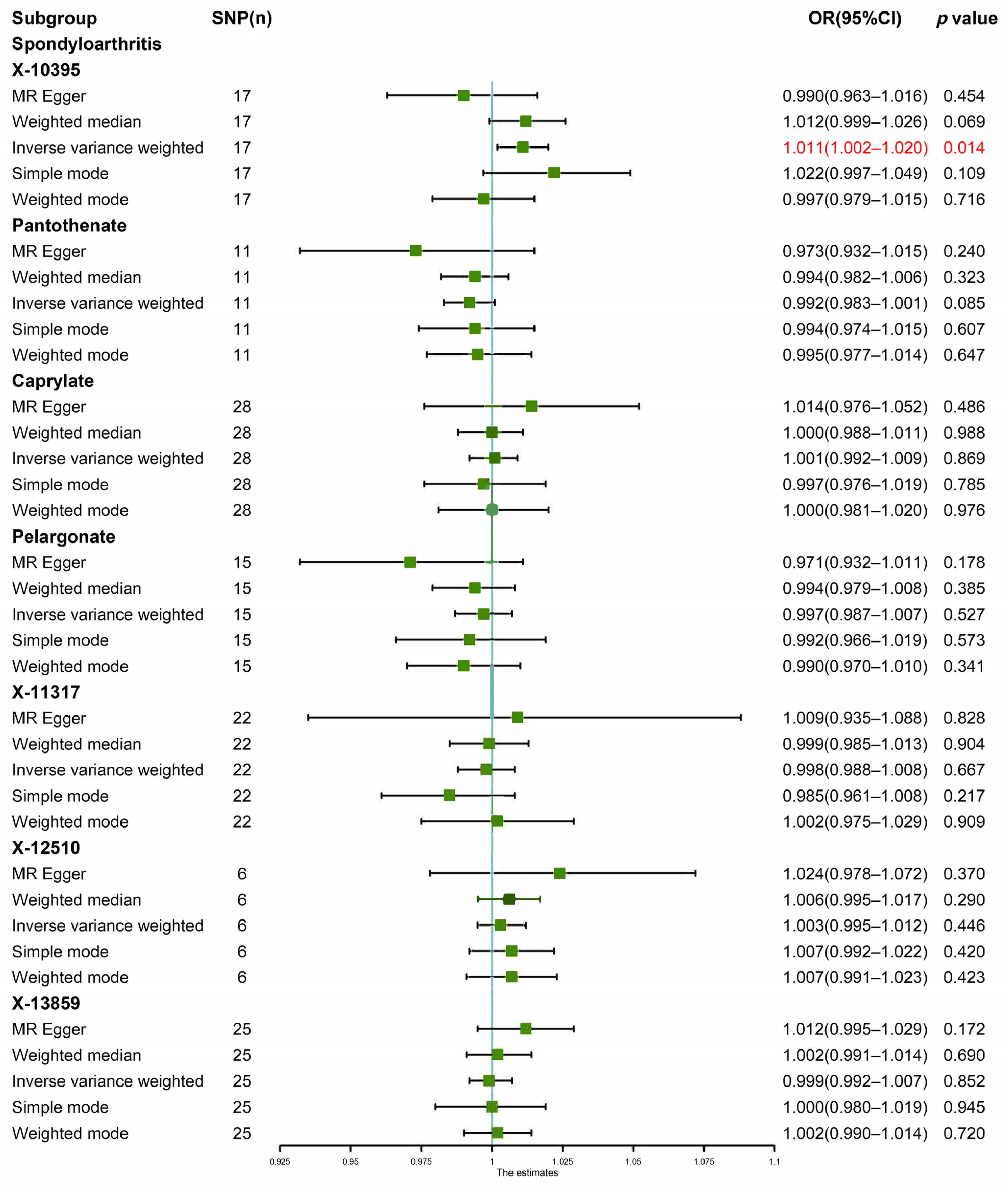
| Blood Metabolites | Correlation Coefficient | p-Values | |
|---|---|---|---|
| X-10395 | SpA | −0.546 | 0.025 |
| Pantothenate | −0.565 | 0.038 | |
| Caprylate | −0.333 | 0.037 | |
| Pelargonate | −0.339 | 0.047 | |
| X-11317 | −0.350 | 0.038 | |
| X-12510 | −0.399 | 0.034 | |
| X-13859 | −0.458 | 0.015 |
| Exposure | Outcome | Heterogeneity Test | Pleiotropy Test | MR-PRESSO | ||
|---|---|---|---|---|---|---|
| Cochran’s Q Test (p-Value) | Rucker’s Q Test (p-Value) | Egger Intercept (p-Value) | Distortion Test | Global Test | ||
| IVW | MR-Egger | MR-Egger | Outliers | p-Value | ||
| X-10395 | SpA | 0.733 | 0.858 | 0.115 | 0 | 0.741 |
| Pantothenate | 0.802 | 0.804 | 0.388 | 0 | 0.251 | |
| Caprylate | 0.753 | 0.731 | 0.498 | 0 | 0.798 | |
| Pelargonate | 0.578 | 0.641 | 0.213 | 0 | 0.670 | |
| X-11317 | 0.674 | 0.619 | 0.783 | 0 | 0.404 | |
| X-12510 | 0.639 | 0.623 | 0.428 | 0 | 0.659 | |
| X-13859 | 0.264 | 0.352 | 0.113 | 0 | 0.084 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, M.; Xu, J.; Zhang, F.; Luo, P.; Xu, K.; Feng, R.; Xu, P. Large-Scale Genetic Correlation Analysis between Spondyloarthritis and Human Blood Metabolites. J. Clin. Med. 2023, 12, 1201. https://doi.org/10.3390/jcm12031201
Yang M, Xu J, Zhang F, Luo P, Xu K, Feng R, Xu P. Large-Scale Genetic Correlation Analysis between Spondyloarthritis and Human Blood Metabolites. Journal of Clinical Medicine. 2023; 12(3):1201. https://doi.org/10.3390/jcm12031201
Chicago/Turabian StyleYang, Mingyi, Jiawen Xu, Feng Zhang, Pan Luo, Ke Xu, Ruoyang Feng, and Peng Xu. 2023. "Large-Scale Genetic Correlation Analysis between Spondyloarthritis and Human Blood Metabolites" Journal of Clinical Medicine 12, no. 3: 1201. https://doi.org/10.3390/jcm12031201





