Do Individualized Patient-Specific Situations Predict the Progression Rate and Fate of Knee Osteoarthritis? Prediction of Knee Osteoarthritis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Classification
2.2. Affecting Factor Analysis
2.3. Statistical Analysis
| Total (Knees) | Time < 5 Years (Fast) | 5 years ≤ Time < 10 Years (Usual) | Time ≥ 10 Years (Slow) | p-Value |
|---|---|---|---|---|
| 100% (2152) | 36.0% (774) | 33.5% (721) | 30.5% (657) | |
| Age (Mean) | 62.2 ± 8.8 | 58.6 ± 9.2 | 58.9 ± 11.8 | <0.001 * |
| Age < 55 (553) | 24.2% (134) | 38.5% (213) | 37.3% (206) | <0.001 * |
| 55 ≤ Age < 65 (894) | 36.4% (325) | 36.7% (328) | 27.0% (241) | |
| Age ≥ 65 (705) | 44.7% (315) | 25.5% (180) | 29.8% (210) | |
| SEX (Male/Female) | 12.7%/87.3% (98/676) | 18.6%/81.4% (134/587) | 19.6%/80.4% (129/528) | |
| BMI | 23.8 ± 3.4 | 25.0 ± 3.2 | 25.4 ± 3.3 | <0.001 * |
| BMD | <0.001 * | |||
| Normal (134) | 39.6% (53) | 32.1% (43) | 28.4% (38) | |
| Osteopenia or Osteoporosis (553) | 43.6% (241) | 32.2% (178) | 24.2% (134) | |
| Total performed (687) | 42.8% (294) | 32.2% (221) | 25.0% (172) | |
| Initial K–L grade | <0.001 * | |||
| K–L grade 0 (1542) | 29.4% (453) | 37.7% (581) | 32.9% (508) | |
| K–L grade 1 (296) | 38.5% (114) | 27.4% (81) | 34.1% (101) | |
| K–L grade 2 (314) | 65.9% (207) | 18.8% (59) | 15.3% (48) | |
| Physical demand for occupation | <0.001 * | |||
| Low demand (719) | 32.0% (230) | 26.7% (192) | 41.3% (297) | |
| Mild demand (914) | 32.1% (293) | 38.7% (354) | 29.2% (267) | |
| High demand (519) | 48.4% (251) | 33.7% (175) | 17.9% (93) | |
| Metabolic disorders | ||||
| HTN (1091) | 40.8% (445) | 31.7% (346) | 27.5% (300) | <0.001 * |
| DM (405) | 39.8% (161) | 31.9% (129) | 28.4% (115) | 0.208 |
| Other disorders (one or more) (517) | 35.4% (183) | 35.0% (181) | 29.6% (153) | 0.699 |
| (Knees) | Time < 5 Years | Time ≥ 5 Years (Max: 15 Years) | Conservative Treatment | p-Value |
|---|---|---|---|---|
| Age (Mean) | 63.5 ± 7.0 | 59.6 ± 9.3 | 56.9 ± 11.9 | <0.001 * |
| SEX (Male/Female) | 13.5%/86.5% (42/270) | 12.8%/87.2% (56/383) | 21.0%/79.0% (366/1375) | <0.001 * |
| BMI | 23.9 ± 3.4 | 24.0 ± 3.2 | 25.2 ± 3.4 | <0.001 * |
| BMD | <0.001 * | |||
| Normal (159) | 9.4% (14) | 14.0% (25) | 27.8% (120) | |
| Osteopenia or osteoporosis (600) | 90.6% (135) | 86.0% (154) | 72.2% (311) | |
| Total performed (759) | 100% (149) | 100% (179) | 100% (431) | |
| Initial K–L grade | <0.001 * | |||
| K–L grade 0 (1850) | 27.5% (86) | 43.3% (190) | 86.6% (1507) | |
| K–L grade 1 (303) | 23.5% (73) | 18.9% (83) | 10.2% (178) | |
| K–L grade 2 (339) | 49.0% (153) | 37.8% (166) | 3.2% (56) | |
| Total (2492) | 100% (312) | 100% (439) | 100% (1741) | |
| Physical demand for occupation | <0.001 * | |||
| Low demand (813) | 32.1% (100) | 23.2% (102) | 35.1% (611) | |
| Mild demand (1109) | 26.6% (83) | 41.7% (183) | 48.4% (843) | |
| High demand (570) | 41.3% (129) | 35.1% (154) | 16.5% (287) | |
| Total (2492) | 100% (312) | 100% (439) | 100% (1741) | |
| Rate of metabolic disorders | ||||
| HTN (1204) | 61.5% (192) | 57.2% (251) | 43.7% (761) | <0.001 * |
| DM (443) | 25.3% (79) | 18.7% (82) | 16.2% (282) | <0.001 * |
| Other disorders (one or more) (575) | 16.5% (95) | 19.7% (113) | 63.8% (367) | 0.001 * |
3. Results
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
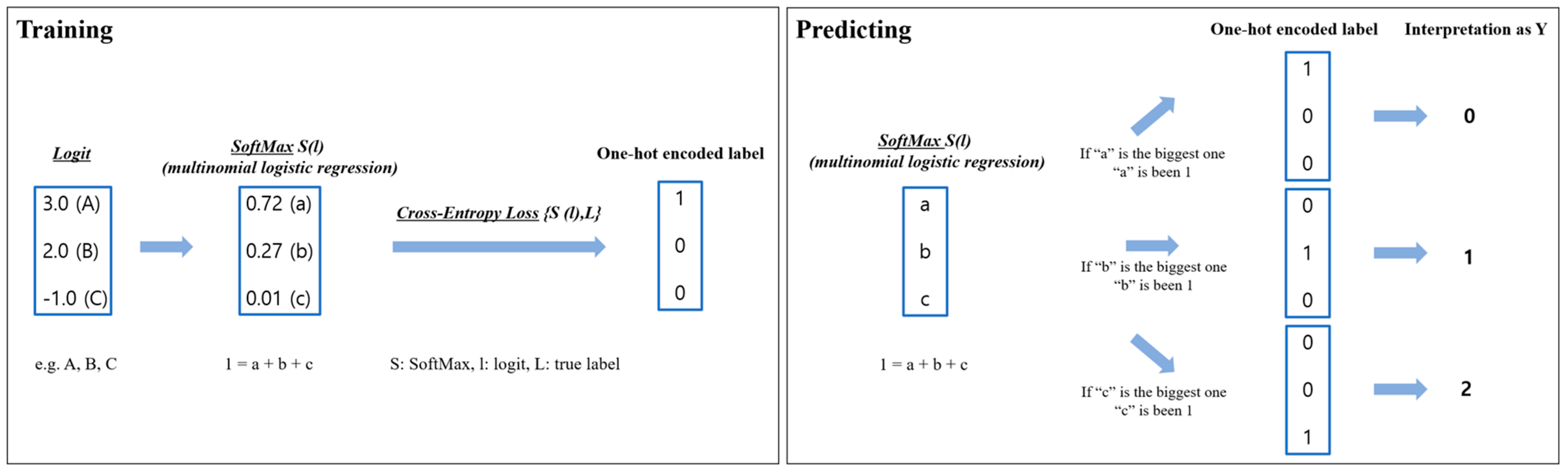
Appendix A. A Detailed Explanation of Algorithms and Models
Appendix A.1. Model Explanation
- ⮚
- Logistic regression
- ⮚
- SoftMax (S)
- ⮚
- Cross-Entropy Loss (CE)
References
- Cross, M.; Smith, E.; Hoy, D.; Nolte, S.; Ackerman, I.; Fransen, M.; Bridgett, L.; Williams, S.; Guillemin, F.; Hill, C.L.; et al. The global burden of hip and knee osteoarthritis: Estimates from the global burden of disease 2010 study. Ann. Rheum. Dis. 2014, 73, 1323–1330. [Google Scholar] [CrossRef] [PubMed]
- Eyre, D.R. Collagens and cartilage matrix homeostasis. Clin. Orthop. Relat. Res. 2004, 427, S118–S122. [Google Scholar] [CrossRef] [PubMed]
- Guccione, A.A.; Felson, D.T.; Anderson, J.J.; Anthony, J.M.; Zhang, Y.; Wilson, P.W.; Kelly-Hayes, M.; Wolf, P.A.; Kreger, B.E.; Kannel, W.B. The effects of specific medical conditions on the functional limitations of elders in the Framingham Study. Am. J. Public Health 1994, 84, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Felson, D.T.; Zhang, Y. An update on the epidemiology of knee and hip osteoarthritis with a view to prevention. Arthritis Rheum. 1998, 41, 1343–1355. [Google Scholar] [CrossRef]
- Cho, H.J.; Morey, V.; Kang, J.Y.; Kim, K.W.; Kim, T.K. Prevalence and Risk Factors of Spine, Shoulder, Hand, Hip, and Knee Osteoarthritis in Community-dwelling Koreans Older Than Age 65 Years. Clin. Orthop. Relat. Res. 2015, 473, 3307–3314. [Google Scholar] [CrossRef]
- Gardiner, B.S.; Woodhouse, F.G.; Besier, T.F.; Grodzinsky, A.J.; Lloyd, D.G.; Zhang, L.; Smith, D.W. Predicting Knee Osteoarthritis. Ann. Biomed. Eng. 2016, 44, 222–233. [Google Scholar] [CrossRef]
- Lee, S.H.; Lee, O.S.; Kim, S.T.; Lee, Y.S. Revisiting Arthroscopic Partial Meniscectomy for Degenerative Tears in Knees With Mild or No Osteoarthritis: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Clin. J. Sport. Med. 2020, 30, 195–202. [Google Scholar] [CrossRef]
- Zhang, Y.M.; Wang, J.; Liu, X.G. Association between hypertension and risk of knee osteoarthritis: A meta-analysis of observational studies. Medicine 2017, 96, e7584. [Google Scholar] [CrossRef]
- Louati, K.; Vidal, C.; Berenbaum, F.; Sellam, J. Association between diabetes mellitus and osteoarthritis: Systematic literature review and meta-analysis. RMD Open 2015, 1, e000077. [Google Scholar] [CrossRef]
- Srikanth, V.K.; Fryer, J.L.; Zhai, G.; Winzenberg, T.M.; Hosmer, D.; Jones, G. A meta-analysis of sex differences prevalence, incidence and severity of osteoarthritis. Osteoarthr. Cartil. 2005, 13, 769–781. [Google Scholar] [CrossRef] [Green Version]
- Cho, H.J.; Chang, C.B.; Kim, K.W.; Park, J.H.; Yoo, J.H.; Koh, I.J.; Kim, T.K. Gender and prevalence of knee osteoarthritis types in elderly Koreans. J Arthroplast. 2011, 26, 994–999. [Google Scholar] [CrossRef]
- Heidari, B. Knee osteoarthritis prevalence, risk factors, pathogenesis and features: Part I. Casp. J. Intern. Med. 2011, 2, 205–212. [Google Scholar]
- Moghimi, N.; Rahmani, K.; Delpisheh, A.; Saidi, A.; Azadi, N.A.; Afkhamzadeh, A. Risk factors of knee osteoarthritis: A case-control study. Pak. J. Med. Sci. 2019, 35, 636–640. [Google Scholar] [CrossRef]
- Reid, C.R.; Bush, P.M.; Cummings, N.H.; McMullin, D.L.; Durrani, S.K. A review of occupational knee disorders. J. Occup. Rehabil. 2010, 20, 489–501. [Google Scholar] [CrossRef]
- Tiulpin, A.; Klein, S.; Bierma-Zeinstra, S.M.A.; Thevenot, J.; Rahtu, E.; Meurs, J.V.; Oei, E.H.G.; Saarakkala, S. Multimodal Machine Learning-based Knee Osteoarthritis Progression Prediction from Plain Radiographs and Clinical Data. Sci. Rep. 2019, 9, 20038. [Google Scholar] [CrossRef]
- Palmer, K.T. Occupational activities and osteoarthritis of the knee. Br. Med. Bull. 2012, 102, 147–170. [Google Scholar] [CrossRef]
- Losina, E.; Daigle, M.E.; Suter, L.G.; Hunter, D.J.; Solomon, D.H.; Walensky, R.P.; Jordan, J.M.; Burbine, S.A.; Paltiel, A.D.; Katz, J.N. Disease-modifying drugs for knee osteoarthritis: Can they be cost-effective? Osteoarthr. Cartil. 2013, 21, 655–667. [Google Scholar] [CrossRef]
- Vaishya, R.; Pariyo, G.B.; Agarwal, A.K.; Vijay, V. Non-operative management of osteoarthritis of the knee joint. J. Clin. Orthop. Trauma 2016, 7, 170–176. [Google Scholar] [CrossRef]
- Nevitt, M.C.; Zhang, Y.; Javaid, M.K.; Neogi, T.; Curtis, J.R.; Niu, J.; McCulloch, C.E.; Segal, N.A.; Felson, D.T. High systemic bone mineral density increases the risk of incident knee OA and joint space narrowing, but not radiographic progression of existing knee OA: The MOST study. Ann. Rheum. Dis. 2010, 69, 163–168. [Google Scholar] [CrossRef]
- Im, G.I.; Kim, M.K. The relationship between osteoarthritis and osteoporosis. J. Bone Min. Metab. 2014, 32, 101–109. [Google Scholar] [CrossRef]
- Lee, J.Y.; Harvey, W.F.; Price, L.L.; Paulus, J.K.; Dawson-Hughes, B.; McAlindon, T.E. Relationship of bone mineral density to progression of knee osteoarthritis. Arthritis Rheum. 2013, 65, 1541–1546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hardcastle, S.A.; Dieppe, P.; Gregson, C.L.; Davey Smith, G.; Tobias, J.H. Osteoarthritis and bone mineral density: Are strong bones bad for joints? Bonekey Rep. 2015, 4, 624. [Google Scholar] [CrossRef] [PubMed]
- Makiev, K.G.; Vasios, I.S.; Georgoulas, P.; Tilkeridis, K.; Drosos, G.; Ververidis, A. Clinical significance and management of meniscal extrusion in different knee pathologies: A comprehensive review of the literature and treatment algorithm. Knee Surg. Relat. Res. 2022, 34, 35. [Google Scholar] [CrossRef]
- Dequeker, J.; Aerssens, J.; Luyten, F.P. Osteoarthritis and osteoporosis: Clinical and research evidence of inverse relationship. Aging Clin. Exp. Res. 2003, 15, 426–439. [Google Scholar] [CrossRef] [PubMed]
- Do, S.; Lee, C.G.; Kim, D.H.; Lee, G.; Kim, K.Y.; Ryu, S.Y.; Song, H. Factors related to femoral bowing among Korean female farmers: A cross-sectional study. Ann. Occup. Envrion. Med. 2020, 32, e23. [Google Scholar] [CrossRef]
- Georgiev, T.; Angelov, A.K. Modifiable risk factors in knee osteoarthritis: Treatment implications. Rheumatol. Int. 2019, 39, 1145–1157. [Google Scholar] [CrossRef]
- Primeau, C.A.; Birmingham, T.B.; Leitch, K.M.; Appleton, C.T.; Giffin, J.R. Degenerative Meniscal Tears and High Tibial Osteotomy: Do Current Treatment Algorithms Need to Be Realigned? Clin. Sport. Med. 2019, 38, 471–482. [Google Scholar] [CrossRef]
- Gelber, P.E.; Barenius, B.; Perelli, S. Role of Alignment and Osteotomy in Meniscal Injuries. Clin. Sport. Med. 2020, 39, 211–221. [Google Scholar] [CrossRef]
- Kraus, V.B.; Blanco, F.J.; Englund, M.; Karsdal, M.A.; Lohmander, L.S. Call for standardized definitions of osteoarthritis and risk stratification for clinical trials and clinical use. Osteoarthr. Cartil. 2015, 23, 1233–1241. [Google Scholar] [CrossRef]
- Zhang, Y.; Hunter, D.J.; Nevitt, M.C.; Xu, L.; Niu, J.; Lui, L.Y.; Yu, W.; Aliabadi, P.; Felson, D.T. Association of squatting with increased prevalence of radiographic tibiofemoral knee osteoarthritis: The Beijing Osteoarthritis Study. Arthritis Rheum. 2004, 50, 1187–1192. [Google Scholar] [CrossRef]
- Xie, D.X.; Wei, J.; Zeng, C.; Yang, T.; Li, H.; Wang, Y.L.; Long, H.Z.; Wu, Z.Y.; Qian, Y.X.; Li, K.H.; et al. Association between metabolic syndrome and knee osteoarthritis: A cross-sectional study. BMC Musculoskelet. Disord. 2017, 18, 533. [Google Scholar] [CrossRef] [Green Version]
- Lo, K.; Au, M.; Ni, J.; Wen, C. Association between hypertension and osteoarthritis: A systematic review and meta-analysis of observational studies. J. Orthop. Transl. 2022, 32, 12–20. [Google Scholar] [CrossRef]
- Sanchez-Santos, M.T.; Judge, A.; Gulati, M.; Spector, T.D.; Hart, D.J.; Newton, J.L.; Arden, N.K.; Kluzek, S. Association of metabolic syndrome with knee and hand osteoarthritis: A community-based study of women. Semin. Arthritis Rheum. 2019, 48, 791–798. [Google Scholar] [CrossRef]
- Shi, X.; Schlenk, E.A. Association of Hypertension with Knee Pain Severity Among People with Knee Osteoarthritis. Pain Manag. Nurs. 2022, 23, 135–141. [Google Scholar] [CrossRef]
- Khor, A.; Ma, C.A.; Hong, C.; Hui, L.L.; Leung, Y.Y. Diabetes mellitus is not a risk factor for osteoarthritis. RMD Open 2020, 6, e001030. [Google Scholar] [CrossRef]
- Rogers-Soeder, T.S.; Lane, N.E.; Walimbe, M.; Schwartz, A.V.; Tolstykh, I.; Felson, D.T.; Lewis, C.E.; Segal, N.A.; Nevitt, M.C. Association of Diabetes Mellitus and Biomarkers of Abnormal Glucose Metabolism With Incident Radiographic Knee Osteoarthritis. Arthritis Care Res. 2020, 72, 98–106. [Google Scholar] [CrossRef]
- Kim, D.H.; Lee, K.J.; Choi, D.; Lee, J.I.; Choi, H.G.; Lee, Y.S. Can Additional Patient Information Improve the Diagnostic Performance of Deep Learning for the Interpretation of Knee Osteoarthritis Severity. J. Clin. Med. 2020, 9, 3341. [Google Scholar] [CrossRef]
- Lee, D.H.; Kim, S.J.; Kim, S.A.; Ju, G.-i. Past, present, and future of cartilage restoration: From localized defect to arthritis. Knee Surg. Relat. Res. 2022, 34, 1. [Google Scholar] [CrossRef]
- Boutefnouchet, T.; Puranik, G.; Holmes, E.; Bell, K.M. Hylan GF-20 Viscosupplementation in the Treatment of Symptomatic Osteoarthritis of the Knee: Clinical Effect Survivorship at 5 Years. Knee Surg. Relat. Res. 2017, 29, 129–136. [Google Scholar] [CrossRef] [Green Version]

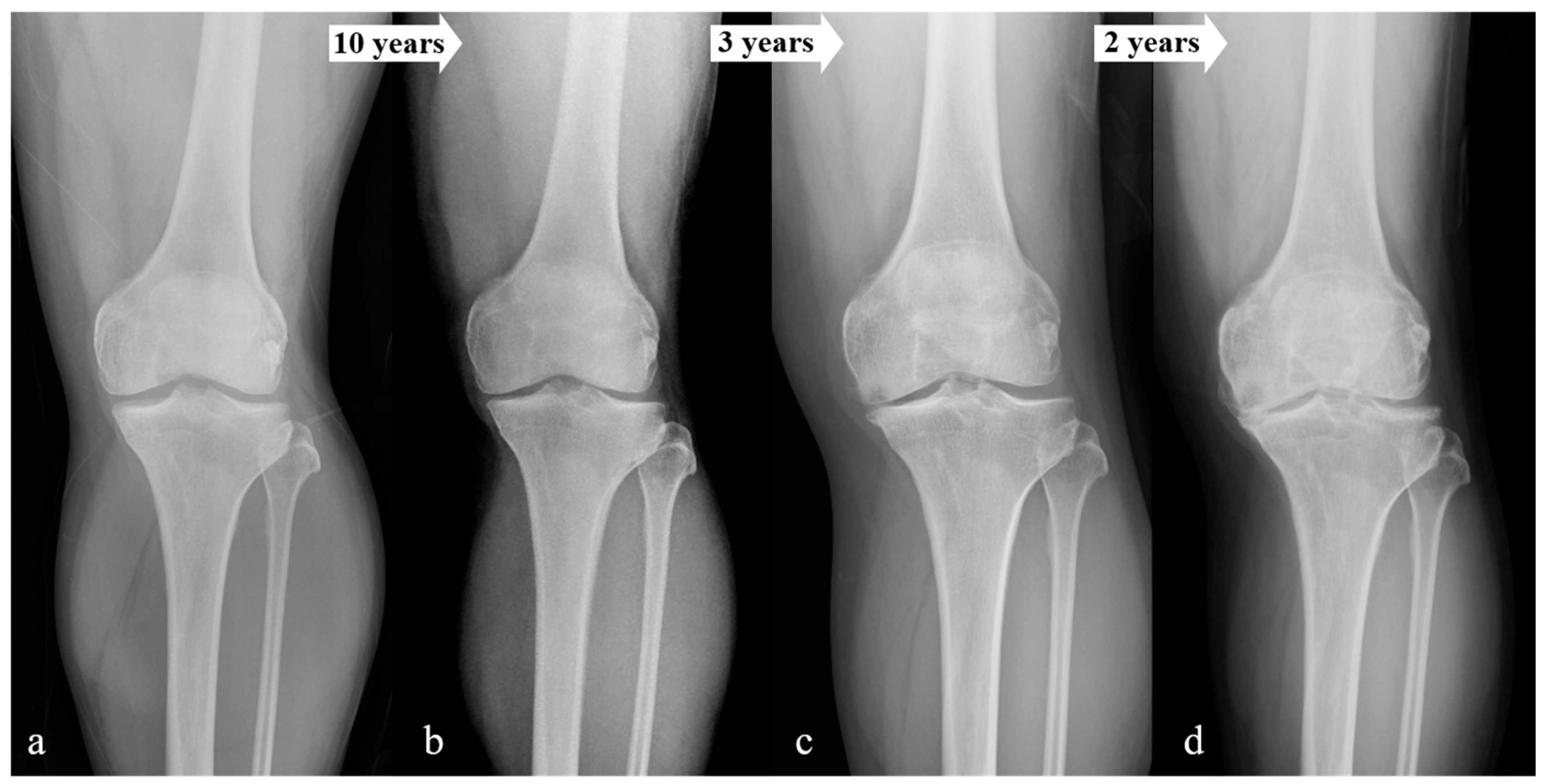

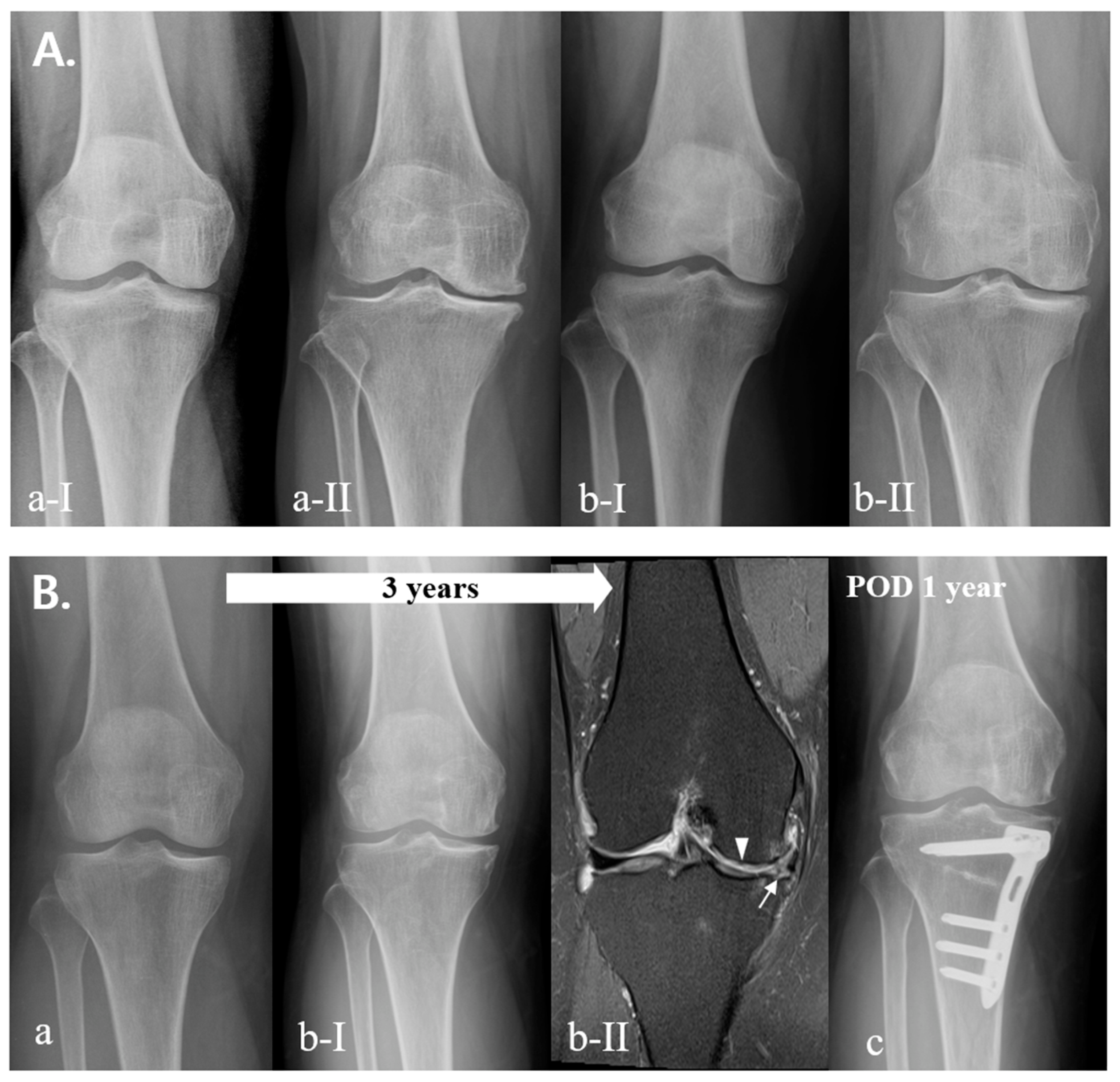
| (Knees) | K–L Grade 0 | K–L Grade 1 | K–L Grade 2 | p-Value |
|---|---|---|---|---|
| Age (Mean) | 57.6 ± 11.9 | 59.7 ± 9.1 | 60.6 ± 8.1 | <0.001 * |
| Age < 55 | 38.6% (714) | 21.9% (66) | 14.7% (50) | <0.001 * |
| 55 ≤ Age < 65 | 31.9% (591) | 48.0% (145) | 60.2% (204) | |
| Age ≥ 65 | 29.5% (545) | 28.1% (85) | 27.1% (92) | |
| Total (2492) | 100% (1850) | 100% (303) | 100% (339) | |
| SEX (Male/Female) | 21.2%/78.8% (392/1458) | 14.5%/85.5% (44/259) | 8.3%/91.7% (28/311) | <0.001 * |
| BMI | 25.3 ± 3.4 | 24.2 ± 3.2 | 23.0 ± 2.8 | <0.001 * |
| BMD | 0.001 | |||
| Normal (159) | 22.9% (116) | 18.4% (21) | 15.8% (22) | |
| Osteopenia or Osteoporosis (600) | 77.1% (390) | 81.6% (93) | 85.2% (117) | |
| Total performed (759) | 100% (506) | 100% (114) | 100% (139) | |
| Physical demand for occupation | <0.001 * | |||
| Low demand (813) | 34.8% (643) | 36.6% (111) | 17.4% (59) | |
| Mild demand (1109) | 51.5% (952) | 23.4% (71) | 25.4% (86) | |
| High demand (570) | 13.8% (255) | 39.9% (121) | 57.2% (194) | |
| Total (2492) | 100% (1850) | 100% (303) | 100% (339) | |
| Rate of metabolic disorders | ||||
| HTN (1204) | 45.3% (838) | 56.1% (170) | 57.8% (196) | <0.001 * |
| DM (443) | 17.5% (324) | 21.1% (64) | 16.2% (55) | 0.227 |
| Other disorders (one or more) (575) | 21.8% (403) | 26.1% (79) | 27.4% (93) | 0.032 * |
| Fate | ||||
| Final K–L grade (Mean) | 2.3 ± 1.0 | 2.9 ± 0.9 | 3.8 ± 0.4 | <0.001 * |
| Surgical intervention | <0.001 * | |||
| Time < 5 years (312) | 8.3% (153) | 13.9% (42) | 34.5% (117) | |
| Time ≥ 5 years (439) (Max: 15 years) | 10.3% (190) | 27.4% (83) | 49.0% (166) | |
| Conservative treatment (1741) | 81.5% (1507) | 58.7% (178) | 16.5% (56) | |
| Total (2492) | 100% (1850) | 100% (303) | 100% (339) |
| Accuracy | Precision | Recall | F1-Score | Specificity | Error Rate | |
|---|---|---|---|---|---|---|
| Progression rate of OA | ||||||
| Time < 5 years (Fast) | 0.632 | 0.488 | 0.509 | 0.498 | 0.700 | 0.368 |
| 5 years ≤ Time < 10 years (Usual) | 0.616 | 0.432 | 0.452 | 0.441 | 0.699 | 0.384 |
| Time ≥ 10 years (Slow) | 0.644 | 0.407 | 0.365 | 0.385 | 0.766 | 0.356 |
| Fate of OA | ||||||
| 5 years > surgical intervention | 0.874 | 0.500 | 0.255 | 0.338 | 0.963 | 0.126 |
| 5 years ≤ surgical intervention (Max: 15 years) | 0.803 | 0.459 | 0.629 | 0.530 | 0.841 | 0.197 |
| Conservative | 0.876 | 0.913 | 0.908 | 0.911 | 0.801 | 0.124 |
| Coefficient (95% Confidence Interval) | Initial K–L Grade | Age | Sex | BMI | BMD | Physical Demand for Occupation | HTN | DM | Other Disorders (One or More) |
|---|---|---|---|---|---|---|---|---|---|
| Progression rate of OA (contribution) | Major * | Minor | Minor | Minor | Major * | Major * | Minor | Minor | Minor |
| Time < 5 years (Fast) | 0.610 (0.609 ~ 0.610) | 0.039 (0.039 ~ 0.039) | 0.262 (0.262 ~ 0.262) | −0.099 (−0.099 ~ −0.098) | 0.295 (0.294 ~ 0.295) | 0.405 (0.404 ~ 0.405) | 0.196 (0.196 ~ 0.197) | 0.079 (0.079 ~ 0.079) | 0.135 (0.135 ~ 0.136) |
| 5 years ≤ Time < 10 years (Usual) | −0.125 (−0.126 ~ −0.125) | −0.012 (−0.013 ~ −0.012) | −0.092 (−0.092 ~ −0.092) | 0.010 (0.010 ~ 0.011) | 0.145 (0.145 ~ 0.145) | 0.218 (0.217 ~ 0.218) | −0.075 (−0.075 ~ −0.075) | 0.127 (0.127 ~ 0.128) | 0.127 (0.126 ~ 0.127) |
| Time ≥ 10 years (Slow) | −0.667 (−0.668 ~ −0.667) | −0.027 (−0.027 ~ −0.027) | −0.178 (−0.179 ~ −0.178) | 0.074 (0.074 ~ 0.075) | −0.459 (−0.460 ~ −0.459) | −0.669 (−0.669 ~ −0.669) | −0.087 (−0.088 ~ −0.087) | −0.078 (−0.079 ~ −0.078) | −0.088 (−0.088 ~ −0.088) |
| Fate of OA (contribution) | Major * | Minor | Minor | Minor | Minor | Major * | Major * | Minor | Minor |
| 5 years > surgical intervention | 0.462 (0.462 ~ 0.463) | 0.131 (0.131 ~ 0.131) | 0.271 (0.270 ~ 0.271) | −0.103 (−0.104 ~ −0.103) | 0.225 (0.224 ~ 0.225) | 0.496 (0.496 ~ 0.496) | 0.290 (0.289 ~ 0.290) | 0.239 (0.239 ~ 0.239) | −0.092 (−0.093 ~ 0.091) |
| 5 years ≤ surgical intervention (Max: 15 years) | 0.111 (0.110 ~ 0.111) | 0.108 (0.108 ~ 0.108) | 0.016 (0.015 ~ 0.017) | −0.030 (−0.031 ~ −0.030) | 0.080 (0.079 ~ 0.080) | −0.156 (−0.156 ~ −0.156) | 0.038 (0.038 ~ 0.038) | 0.029 (0.029 ~ 0.029) | −0.085 (−0.086 ~ −0.085) |
| Conservative | −0.643 (−0.643 ~ −0.643) | −0.060 (−0.061 ~ −0.060) | −0.330 (−0.331 ~ −0.330) | 0.071 (0.071 ~ 0.072) | −0.324 (−0.324 ~ −0.324) | −0.592 (−0.593 ~ −0.592) | −0.379 (−0.380 ~ −0.379) | −0.310 (−0.311 ~ −0.310) | 0.339 (0.339 ~ 0.339) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoo, H.J.; Jeong, H.W.; Park, S.B.; Shim, S.J.; Nam, H.S.; Lee, Y.S. Do Individualized Patient-Specific Situations Predict the Progression Rate and Fate of Knee Osteoarthritis? Prediction of Knee Osteoarthritis. J. Clin. Med. 2023, 12, 1204. https://doi.org/10.3390/jcm12031204
Yoo HJ, Jeong HW, Park SB, Shim SJ, Nam HS, Lee YS. Do Individualized Patient-Specific Situations Predict the Progression Rate and Fate of Knee Osteoarthritis? Prediction of Knee Osteoarthritis. Journal of Clinical Medicine. 2023; 12(3):1204. https://doi.org/10.3390/jcm12031204
Chicago/Turabian StyleYoo, Hyun Jin, Ho Won Jeong, Sung Bae Park, Seung Jae Shim, Hee Seung Nam, and Yong Seuk Lee. 2023. "Do Individualized Patient-Specific Situations Predict the Progression Rate and Fate of Knee Osteoarthritis? Prediction of Knee Osteoarthritis" Journal of Clinical Medicine 12, no. 3: 1204. https://doi.org/10.3390/jcm12031204
APA StyleYoo, H. J., Jeong, H. W., Park, S. B., Shim, S. J., Nam, H. S., & Lee, Y. S. (2023). Do Individualized Patient-Specific Situations Predict the Progression Rate and Fate of Knee Osteoarthritis? Prediction of Knee Osteoarthritis. Journal of Clinical Medicine, 12(3), 1204. https://doi.org/10.3390/jcm12031204








