Assessment of Retinal Pigment Epithelium Alterations and Chorioretinal Vascular Network Analyses in Patients under Treatment with BRAF/MEK Inhibitor for Different Malignancies: A Pilot Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Characteristics and Study Design
2.2. Baseline Characteristics
2.3. Image Acquisition and Evaluation
2.4. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sarcognato, S.; García-Lezana, T.; Villanueva, A. Mechanisms of Action of Drugs Effective in Hepatocellular Carcinoma. Clin. Liver Dis. 2019, 14, 62–65. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, A.S.; Hagan, S.; Rath, O.; Kolch, W. MAP kinase signalling pathways in cancer. Oncogene 2007, 26, 3279–3290. [Google Scholar] [CrossRef] [PubMed]
- Ascierto, P.; McArthur, G.A.; Dréno, B. Cobimetinib combined with vemurafenib in advanced BRAF(V600)-mutant melanoma (coBRIM): Updated efficacy results from a randomised, double-blind, phase 3 trial. Lancet Oncol. 2016, 17, 1248–1260. [Google Scholar] [CrossRef] [PubMed]
- Robert, C.; Grob, J.J.; Stroyakovskiy, D. Five-Year Outcomes with Dabrafenib plus Trametinib in Metastatic Melanoma. N. Engl. J. Med. 2019, 381, 626–636. [Google Scholar] [CrossRef] [PubMed]
- Dummer, R.; Ascierto, P.A.; Gogas, H.J. Encorafenib plus binimetinib versus vemurafenib or encorafenib in patients with BRAF-mutant melanoma (COLUMBUS): A multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2018, 19, 603–615. [Google Scholar] [CrossRef] [PubMed]
- Fabrocini, G.; Panariello, L.; Caro, G. Acneiform Rash Induced by EGFR Inhibitors: Review of the Literature and New Insights. Ski. Appendage Disord. 2015, 1, 31–37. [Google Scholar] [CrossRef]
- Méndez-Martínez, S.; Calvo, P.; Ruiz-Morena, O. Ocular Adverse Events Associated with Mek Inhibitors. Retina 2019, 39, 1435–1450. [Google Scholar] [CrossRef]
- Huang, W.; Yang, A.H.; Matsumoto, D.; Collette, W.; Marroquin, L.; Ko, M. PD0325901, a mitogen-activated protein kinase kinase inhibitor, produces ocular toxicity in a rabbit animal model of retinal vein occlusion. J. Ocul. Pharmacol. Ther. 2009, 25, 519–530. [Google Scholar] [CrossRef]
- Van Dijk, E.H.; van Herpen, C.M.; Marinkovic, M.; Haanen, J.B.; Amundson, D.; Luyten, G.P.; Jager, M.J.; Kapiteijn, E.H.; Keunen, J.E.; Adamus, G.; et al. Serous Retinopathy Associated with Mitogen-Activated Protein Kinase Kinase Inhibition (Binimetinib) for Metastatic Cutaneous and Uveal Melanoma. Ophthalmology 2015, 122, 1907–1916. [Google Scholar] [CrossRef]
- Kashani, A.H.; Chen, C.L.; Gahm, J.K.; Zheng, F.; Richter, G.M.; Rosenfeld, P.J.; Shi, Y.; Wang, R.K. Optical Coherence Tomography Angiography: A Comprehensive Review of Current Methods and Clinical Applications. Prog. Retin. Eye Res. 2017, 60, 66–100. [Google Scholar] [CrossRef]
- Al-Sheikh, M.; Phasukkijwatana, N.; Dolz-Marco, R. Quantitative OCT angiography of the retinal microvasculature and the choriocapillaris in myopic eyes. Investig. Ophthalmol. Vis. Sci. 2017, 58, 2063. [Google Scholar] [CrossRef] [PubMed]
- Marmor, M.F.; Brigell, M.G.; McCulloch, D.L. ISCEV standard for clinical electro-oculography (2010 update). Doc. Ophthalmol. 2011, 122, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Camino, A.; Zhang, M.; Gao, S.S. Evaluation of artifact reduction in optical coherence tomography angiography with real-time tracking and motion correction technology. Biomed. Opt. Express. 2016, 7, 3905. [Google Scholar] [CrossRef]
- Nelis, P.; Alten, F.; Clemens, C.R. Quantification of changes in foveal capillary architecture caused by idiopathic epiretinal membrane using OCT angiography. Graefes. Arch. Clin. Exp. Ophthalmol. 2017, 255, 1319–1324. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Nelis, P.; Clemens, C.R.; Alten, F.; Eter, N. OCT angiography reveals changes in foveal vessel architecture and foveal flow in central serous chorioretinopathy. Acta Ophthalmol. 2017, 95, e802–e803. [Google Scholar] [CrossRef]
- Phansalkar, N.; More, S.; Sabale, A.; Joshi, M. Adaptive local thresholding for detection of nuclei in diversity stained cytology images. In Proceedings of the 2011 International Conference on Communications and Signal Processing, Kerala, India, 21–22 July 2011; pp. 218–220. [Google Scholar]
- Nelis, P.; Kleffner, I.; Burg, M.C. OCT-Angiography reveals reduced vessel density in the deep retinal plexus of CADASIL patients. Sci. Rep. 2018, 8, 8148. [Google Scholar] [CrossRef]
- Matet, A.; Daruich, A.; Hardy, S.; Behar-Cohen, F. Patterns of choriocapillaris flow signal voids in central serous chorioretinopathy: An optical coherence tomography angiography study. Retina 2019, 39, 2178–2188. [Google Scholar] [CrossRef] [PubMed]
- Spaide, R.F. Choriocapillaris flow features follow a power law distribution: Implications for characterization and mechanisms of disease progression. Am. J. Ophthalmol. 2016, 170, 58–67. [Google Scholar] [CrossRef]
- Fasolino, G.; Awada, G.; Koulalis, J.S.; Neyns, B.; Van Elderen, P.; Kuijpers, R.W.; Nelis, P.; Ten Tusscher, M. Choriocapillaris Assessment in Patients Under Mek-Inhibitor Therapy For Cutaneous Melanoma: An Optical Coherence Tomography Angiography Study. Semin. Ophthalmol. 2021, 36, 765–771. [Google Scholar] [CrossRef]
- Bok, D. The retinal pigment epithelium: A versatile partner in vision. J. Cell Sci. 1993, 1993 (Suppl. S17), 189–195. [Google Scholar] [CrossRef] [PubMed]
- Strauss, O. The retinal pigment epithelium in visual function. Physiol. Rev. 2005, 85, 845–881. [Google Scholar] [CrossRef] [PubMed]
- Wimmers, S.; Karl, M.O.; Strauss, O. Ion channels in the RPE. Prog. Retin. Eye Res. 2007, 26, 263–301. [Google Scholar] [CrossRef] [PubMed]
- Gallemore, R.P.; Steinberg, R.H. Light-evoked modulation of basolateral membrane Cl– conductance in chick retinal pigment epithelium: The light peak and fast oscillation. J. Neurophysiol. 1993, 70, 1669–1680. [Google Scholar] [CrossRef] [PubMed]
- Gallemore, R.P.; Griff, E.R.; Steinberg, R.H. Evidence in support of a photoreceptoral origin for the “light-peak substance”. Investig. Ophthalmol. Vis. Sci. 1988, 29, 566–571. [Google Scholar]
- Gallemore, R.P.; Hernandez, E.; Tayyanipour, R.; Fujii, S.; Steinberg, R.H. Basolateral membrane Cl– and K+ conductances of the dark-adapted chick retinal pigment epithelium. J. Neurophysiol. 1993, 70, 1656–1668. [Google Scholar] [CrossRef]
- McCannel, T.A.; Chmielowski, B.; Finn, R.S. Bilateral subfoveal neurosensory retinal detachment associated with MEK inhibitor use for metastatic cancer. JAMA Ophthalmol. 2014, 132, 1005–1009. [Google Scholar] [CrossRef]
- Urner-Bloch, U.; Urner, M.; Stieger, P.; Galliker, N.; Winterton, N.; Zubel, A.; Parseval, L.M.-D.; Dummer, R.; Goldinger, S.M. Transient MEK inhibitor-associated retinopathy in metastatic melanoma. Ann. Oncol. 2014, 25, 1437–1441. [Google Scholar] [CrossRef]
- Rochepeau, C.; Kodjikian, L.; Garcia, M.-A. Optical coherence tomography angiography quantitative assessment of choriocapillaris blood flow in central serous chorioretinopathy. Am. J. Ophthalmol. 2018, 194, 26–34. [Google Scholar] [CrossRef]
- Stamer, W.D.; Bok, D.; Hu, J.; Jaffe, G.J.; McKay, B.S. Aquaporin-1 Channels in Human Retinal Pigment Epithelium: Role in Transepithelial Water Movement. Investig. Ophthalmol. Vis. Sci. 2003, 44, 2803–2808. [Google Scholar] [CrossRef]
- Jiang, Q.; Cao, C.; Lu, S.; Kivlin, R.; Wallin, B.; Chu, W. MEK/ERK pathway mediates UVB-induced AQP1 downregulation and water permeability impairment in human retinal pigment epithelial cells. Int. J. Mol. Med. 2009, 23, 771–777. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mettler, C.; Monnet, D.; Kramkimel, N.; Tréluyer, J.M.; Mouthon, L.; Brézin, A.; Dupin, N.; Valnet-Rabier, M.B.; Chouchana, L.; Terrier, B. Ocular Safety Profile of BRAF and MEK Inhibitors: Data from the World Health Organization Pharmacovigilance Database. Ophthalmology 2021, 128, 1748–1755. [Google Scholar] [CrossRef] [PubMed]
- Weeraratna, R.A. RAF around the edges the paradox of BRAF inhibitors. N. Engl. J. Med. 2012, 366, 271–273. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, P.; Mehtani, A.; Gandhi, H.C.; Bhalla, J.S.; Tapariya, S. Paraneoplastic ocular syndrome: A pandora’s box of underlying malignancies. Eye 2022, 36, 1355–1367. [Google Scholar] [CrossRef] [PubMed]
- Sawyer, R.A.; Selhorst, J.B.; Zimmerman, L.E.; Hoyt, W.F. Blindness caused by photoreceptor degeneration as a remote effect of cancer. Am. J. Ophthalmol. 1976, 81, 606–613. [Google Scholar] [CrossRef]
- Chan, J.W. Paraneoplastic retinopathies and optic neuropathies. Surv. Ophthalmol. 2003, 48, 12–38. [Google Scholar] [CrossRef]
- Berson, E.L.; Lessell, S. Paraneoplastic night blindness with malignant melanoma. Am. J. Ophthalmol. 1988, 106, 307–311. [Google Scholar] [CrossRef]
- Lu, Y.; Jia, L.; He, S.; Hurley, M.C.; Leys, M.J.; Jayasundera, T.; Heckenlively, J.R. Melanoma-associated retinopathy: A paraneoplastic autoimmune complication. Arch Ophthalmol. 2009, 127, 1572–1580. [Google Scholar] [CrossRef]
- Keltner, J.L.; Thirkill, C.E.; Yip, P.T. Clinical and immunologic characteristics of melanoma-associated retinopathy syndrome: Eleven new cases and a review of 51 previously published cases. J. Neuro-Ophthalmol. 2001, 21, 173–187. [Google Scholar] [CrossRef]
- Nieuwendijk, T.J.; Hooymans, J.M. Paraneoplastic vitelliform retinopathy associated with metastatic choroidal melanoma. Eye 2007, 21, 1436–1437. [Google Scholar] [CrossRef]
- Boon, C.J.; Klevering, B.J.; Leroy, B.P.; Hoyng, C.B.; Keunen, J.E.; den Hollander, A.I. The spectrum of ocular phenotypes caused by mutations in the BEST1 gene. Prog. Retin. Eye Res. 2009, 28, 187–205. [Google Scholar] [CrossRef] [PubMed]
- Adamus, G. Autoantibody targets and their cancer relationship in the pathogenicity of paraneoplastic retinopathy. Autoimmun. Rev. 2009, 8, 410–414. [Google Scholar] [CrossRef] [PubMed]
- Ten Berge, J.C.; van Rosmalen, J.; Vermeer, J.; Hellström, C.; Lindskog, C.; Nilsson, P.; Qundos, U.; Rothova, A.; Schreurs, M.W. Serum Autoantibody Profiling of Patients with Paraneoplastic and Non-Paraneoplastic Autoimmune Retinopathy. PLoS ONE 2016, 11, e0167909. [Google Scholar] [CrossRef] [PubMed] [Green Version]
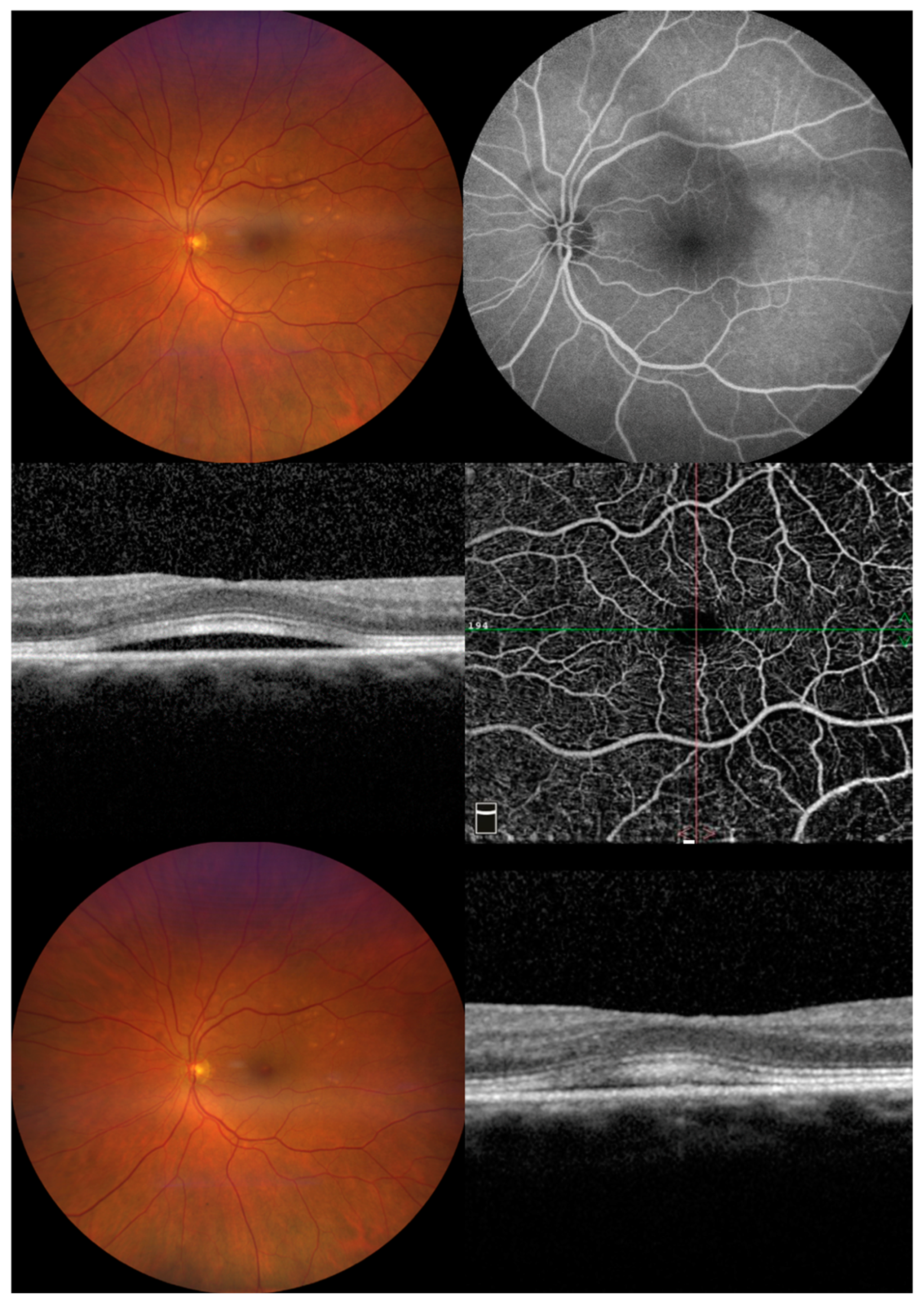
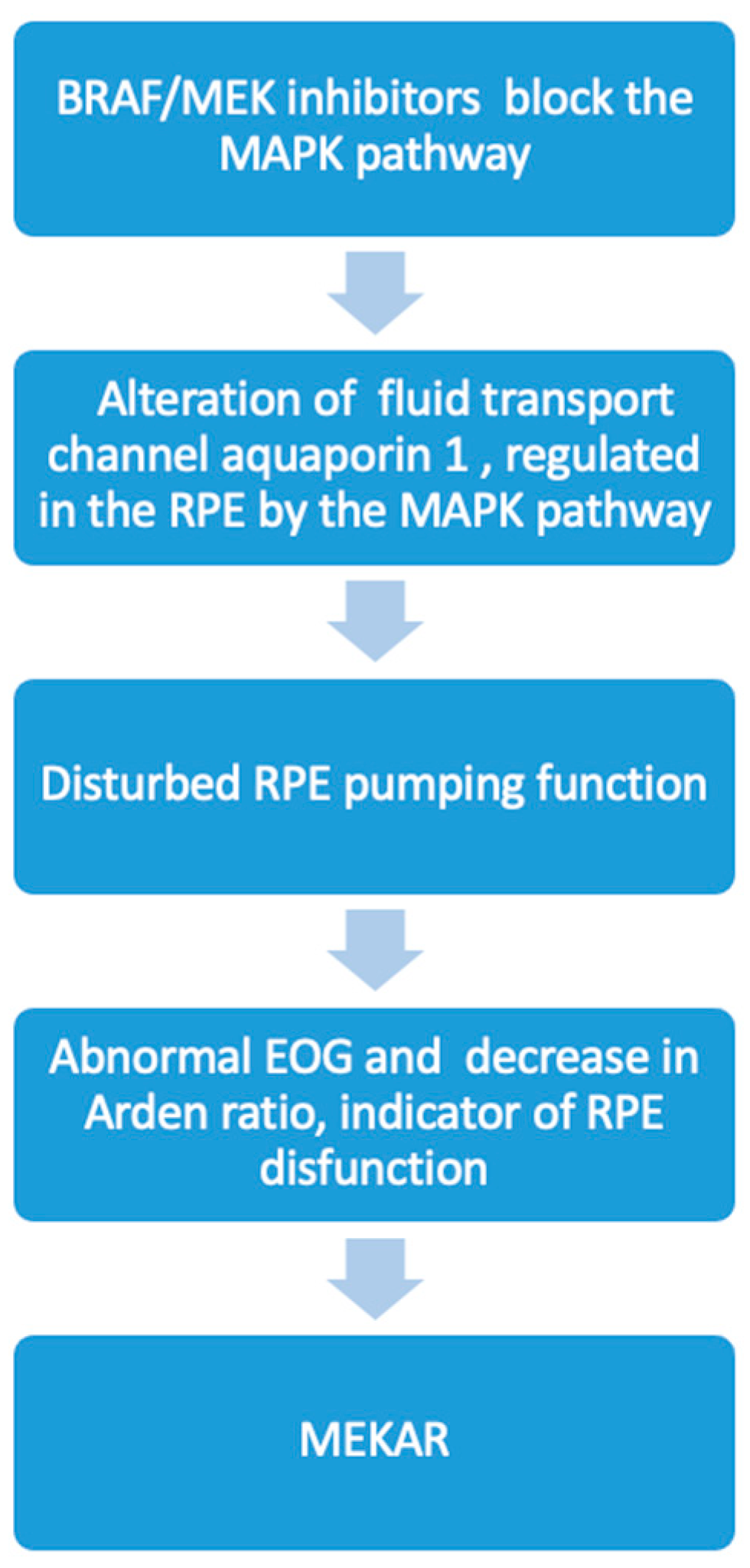
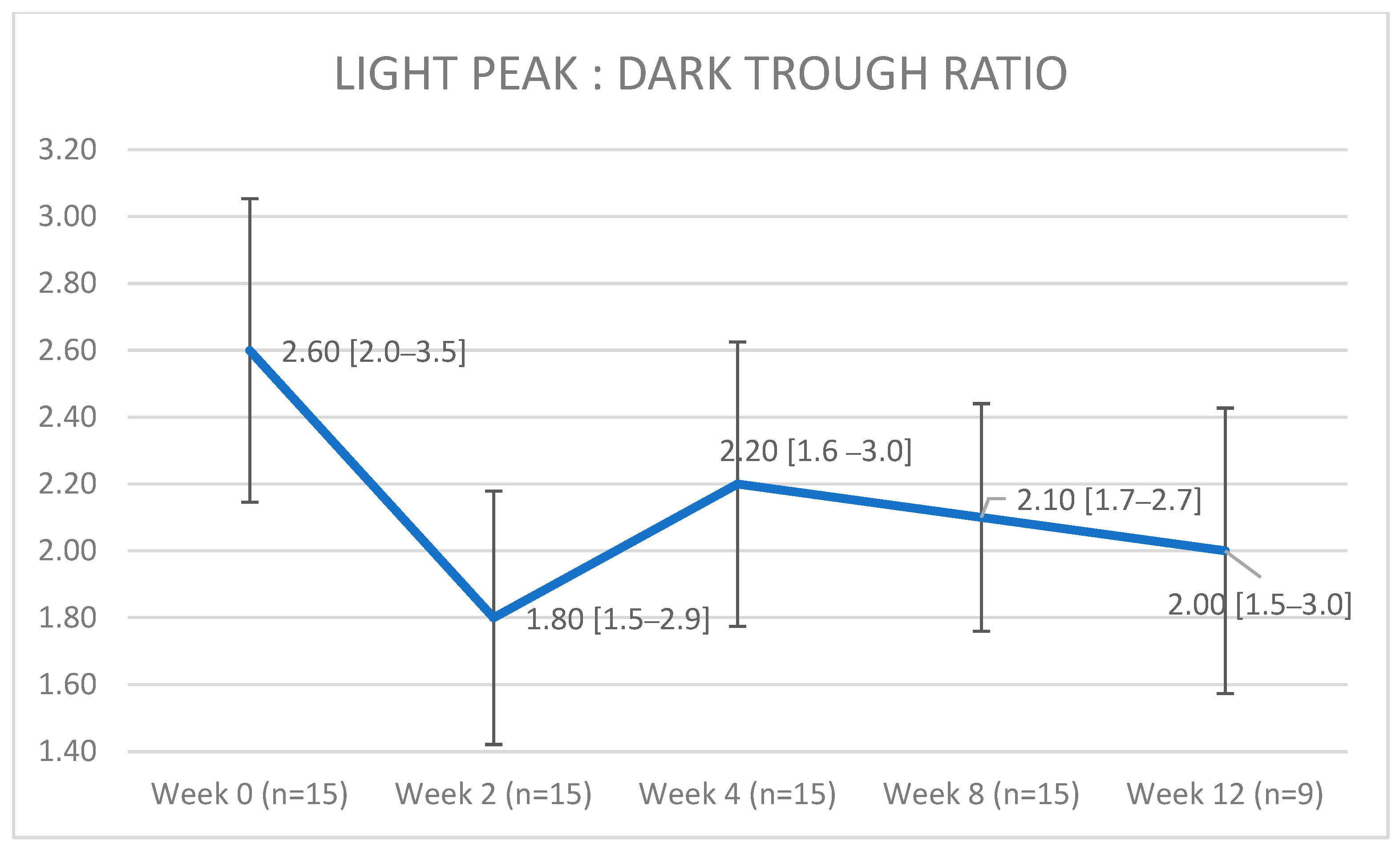

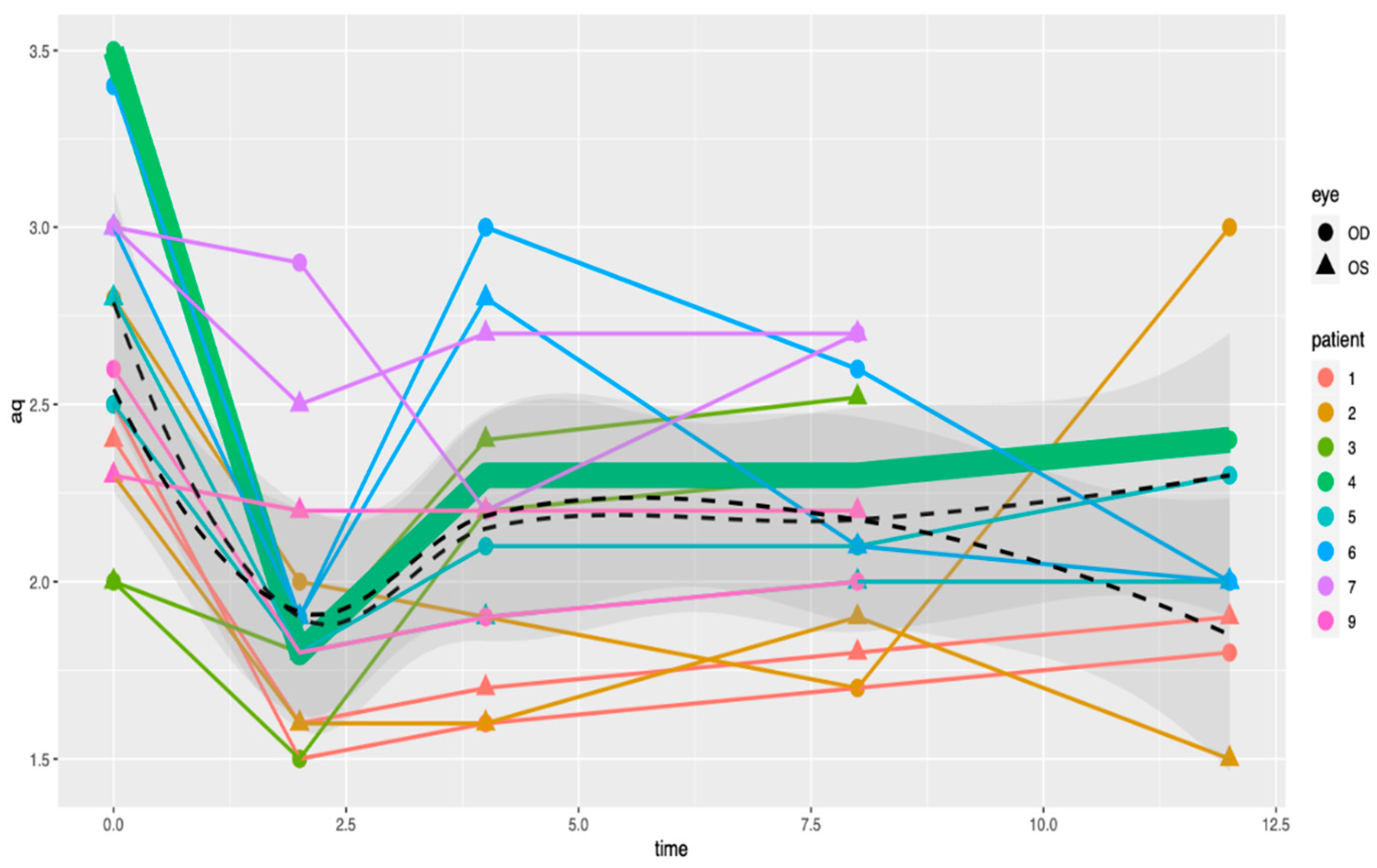
| Patient (Eyes) | Sex | Age | Cancer Type | Drug (Dosing) | Relevant Medical History |
|---|---|---|---|---|---|
| 1 (2) | Male | 56 | MEL | Trametinib (2 mg QD) plus dabrafenib (50 mg BID) | None |
| 2 (2) | Male | 58 | MEL | Trametinib (2 mg QD) plus dabrafenib (150 mg BID) | None |
| 3 (2) | Male | 59 | MEL | Trametinib (2 mg QD) plus dabrafenib (50 mg BID) | None |
| 4 (2) | Male | 58 | MEL | Trametinib (2 mg QD) plus dabrafenib (150 mg BID) | Arterial hypertension |
| 5 (2) | Male | 55 | GBM | Trametinib (2 mg QD) plus dabrafenib (50 mg BID) | None |
| 6 (1) | Female | 65 | MEL | Trametinib (2 mg QD) plus dabrafenib (50 mg BID) | Enucleation due to MEL |
| 7 (2) | Female | 45 | MEL | Trametinib (2 mg QD) plus dabrafenib (50 mg BID) | None |
| 8 (2) | Female | 83 | GLOM | Trametinib (1 mg OD QD) plus dabrafenib (150 mg BID) | Heart disease |
| 9 (2) | Female | 75 | MEL | Trametinib (2 mg QD) plus dabrafenib (50 mg BID) | Hypertension |
| Week 0 (n = 15) | Week 2 (n = 15) | Week 4 (n = 11) | Week 8 (n = 9) | Week 12 (n = 6) | |
|---|---|---|---|---|---|
| Flow void number | 72.07 ± 17.68 | 71.67 ± 17.94 | 75.82 ± 12.88 | 72.44 ± 13.87 | 82.50 ± 4.46 |
| Total flow void area (mm2) | 2.46 ± 0.77 | 2.39 ± 0.89 | 2.10 ± 0.53 | 2.27 ± 0.75 | 1.93 ± 0.16 |
| Total flow void area (%) | 27.37 ± 8.63 | 26.55 ± 9.90 | 23.29 ± 5.92 | 25.19 ± 8.30 | 21.47 ± 1.83 |
| Pre-MEK/BRAF Inhibitor | During Follow-Up * | p-Value | |
|---|---|---|---|
| Flow void number 1 | 72.07 ± 17.68 | 72.33 ± 14.80 | 0.798 |
| Total flow void area 2 (mm2) | 2.46 ± 0.77 | 2.34 ± 0.76 | 0.308 |
| Total flow void area 2 (%) | 27.37 ± 8.63 | 26.03 ± 8.40 | 0.308 |
| Week 0 (n = 15) | Week 2 (n = 15) | Week 4 (n = 11) | Week 8 (n = 9) | Week 12 (n = 6) | |
|---|---|---|---|---|---|
| SCP VAD (%) | 37.10 ± 3.64 | 36.29 ± 4.29 | 36.51 ± 4.14 | 36.56 ± 4.17 | 38.22 ± 2.05 |
| DCP VAD (%) | 36.96 ± 7.47 | 36.73 ± 7.61 | 36.79 ± 7.96 | 36.84 ± 6.75 | 42.93 ± 4.17 |
| Pre-MEK/BRAF Inhibitor | During Follow Up * | p-Value | |
|---|---|---|---|
| SCP VAD (%) 1 | 37.10 ± 3.64 | 36.37 ± 3.77 | 0.293 |
| DCP VAD (%) 1 | 36.96 ± 7.47 | 36.36 ± 6.87 | 0.582 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fasolino, G.; Awada, G.; Moschetta, L.; Koulalis, J.S.; Neyns, B.; Verhelst, B.; Van Elderen, P.; Nelis, P.; de Lichtbuer, P.C.; Cools, W.; et al. Assessment of Retinal Pigment Epithelium Alterations and Chorioretinal Vascular Network Analyses in Patients under Treatment with BRAF/MEK Inhibitor for Different Malignancies: A Pilot Study. J. Clin. Med. 2023, 12, 1214. https://doi.org/10.3390/jcm12031214
Fasolino G, Awada G, Moschetta L, Koulalis JS, Neyns B, Verhelst B, Van Elderen P, Nelis P, de Lichtbuer PC, Cools W, et al. Assessment of Retinal Pigment Epithelium Alterations and Chorioretinal Vascular Network Analyses in Patients under Treatment with BRAF/MEK Inhibitor for Different Malignancies: A Pilot Study. Journal of Clinical Medicine. 2023; 12(3):1214. https://doi.org/10.3390/jcm12031214
Chicago/Turabian StyleFasolino, Giuseppe, Gil Awada, Laura Moschetta, Jorgos Socrates Koulalis, Bart Neyns, Bert Verhelst, Peter Van Elderen, Pieter Nelis, Paul Cardon de Lichtbuer, Wilfried Cools, and et al. 2023. "Assessment of Retinal Pigment Epithelium Alterations and Chorioretinal Vascular Network Analyses in Patients under Treatment with BRAF/MEK Inhibitor for Different Malignancies: A Pilot Study" Journal of Clinical Medicine 12, no. 3: 1214. https://doi.org/10.3390/jcm12031214





