Machine Learning Predictive Models for Evaluating Risk Factors Affecting Sperm Count: Predictions Based on Health Screening Indicators
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Material
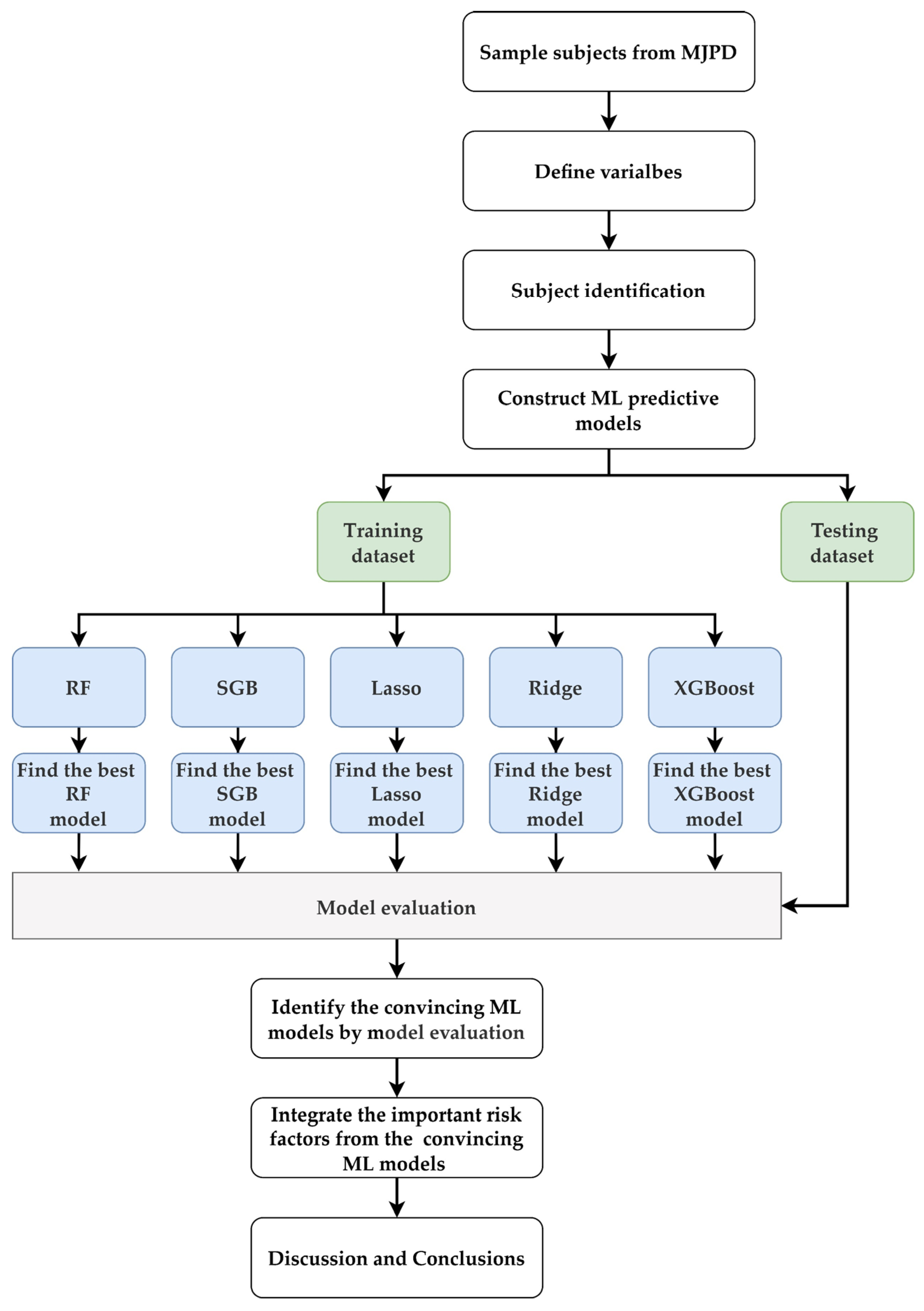
2.2. Proposed Framework
3. Results
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Central Intelligence Agency. The World Factbook—Central Intelligence Agency: East and Southeast Asia: Taiwan. Available online: https://www.cia.gov/the-world-factbook/countries/taiwan/ (accessed on 14 July 2022).
- Plachot, M.; Belaisch-Allart, J.; Mayenga, J.M.; Chouraqui, A.; Tesquier, L.; Serkine, A.M. Outcome of conventional IVF and ICSI on sibling oocytes in mild male fator infertility. Hum. Reprod. 2002, 17, 362–369. [Google Scholar] [CrossRef]
- Carlsen, E.; Giwercman, A.; Keiding, N.; Skakkebaek, N.E. Evidence for decreasing quality of semen during past 50 years. BMJ 1992, 305, 609–613. [Google Scholar] [CrossRef] [PubMed]
- Tiegs, A.W.; Landis, J.; Garrido, N.; Scott, R.T., Jr.; Hotaling, J.M. Total Motile Sperm Count Trend Over Time: Evaluation of Semen Analyses From 119,972 Men From Subfertile Couples. Urology 2019, 132, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Krausz, C.; Cioppi, F.; Riera-Escamilla, A. Testing for genetic contributions to infertility: Potential clinical impact. Expert Rev. Mol. Diagn. 2018, 18, 331–346. [Google Scholar] [CrossRef]
- Sharpe, R.M.; Fisher, J.S.; Millar, M.M.; Jobling, S.; Sumpter, J.P. Gestational and lactational exposure of rats to xenoestrogens results in reduced testicular size and sperm production. Environ. Health Perspect. 1995, 103, 1136–1143. [Google Scholar] [CrossRef] [PubMed]
- Jurewicz, J.; Hanke, W.; Radwan, M.; Bonde, J.P. Environmental factors and semen quality. Int. J. Occup. Med. Environ. Health 2009, 22, 305–329. [Google Scholar] [CrossRef]
- Martins, A.D.; Majzoub, A.; Agawal, A. Metabolic Syndrome and Male Fertility. World J. Men’s Health 2019, 37, 113–127. [Google Scholar] [CrossRef]
- Palmer, N.O.; Bakos, H.W.; Fullston, T.; Lane, M. Impact of obesity on male fertility, sperm function and molecular composition. Spermatogenesis 2012, 2, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Chan, C.; Waters, T.; Chi, L.; Chan, D.; Li, T.C. Lifestyle and demographic factors associated with human semen quality and sperm function. Syst. Biol. Reprod. Med. 2018, 64, 358–367. [Google Scholar] [CrossRef]
- Choy, J.T.; Amory, J.K. Nonsurgical Management of Oligozoospermia. J. Clin. Endocrinol. Metab. 2020, 105, e4194–e4207. [Google Scholar] [CrossRef]
- Chiu, Y.-L.; Jhou, M.-J.; Lee, T.-S.; Lu, C.-J.; Chen, M.-S. Health Data-Driven Machine Learning Algorithms Applied to Risk Indicators Assessment for Chronic Kidney Disease. Risk Manag. Healthc. Policy 2021, 14, 4401–4412. [Google Scholar] [CrossRef]
- Belladelli, F.; Boeri, L.; Pozzi, E.; Fallara, G.; Corsini, C.; Candela, L.; Cazzaniga, W.; Cignoli, D.; Pagliardini, L.; D’Arma, A.; et al. Triglycerides/Glucose Index Is Associated with Sperm Parameters and Sperm DNA Fragmentation in Primary Infertile Men: A Cross-Sectional Study. Metabolites 2022, 12, 143. [Google Scholar] [CrossRef]
- Arafa, M.; Agarwal, A.; Majzoub, A.; Panner Selvam, M.K.; Baskaran, S.; Henkel, R.; Elbardisi, H. Efficacy of Antioxidant Supplementation on Conventional and Advanced Sperm Function Tests in Patients with Idiopathic Male Infertility. Antioxidants 2020, 9, 219. [Google Scholar] [CrossRef]
- Akhter, M.S.; Hamali, H.A.; Iqbal, J.; Mobarki, A.A.; Rashid, H.; Dobie, G.; Madkhali, A.M.; Arishi, B.Y.H.; Ageeli, E.O.O.; Laghbi, O.S.H. Iron Deficiency Anemia as a Factor in Male Infertility: Awareness in Health College Students in the Jazan Region of Saudi Arabia. Int. J. Environ. Res. Public Health 2021, 18, 12866. [Google Scholar] [CrossRef]
- Marill, K.A. Advanced statistics: Linear regression, part II: Multiple linear regression. Acad Emerg Med. 2004, 11, 94–102. [Google Scholar] [CrossRef]
- Niazian, M.; Sadat-Noori, S.A.; Abdipour, M. Artificial neural network and multiple regression analysis models to predict essential oil content of ajowan (Carum copticum L.). J. Appl. Res. Med. Aromat. Plants 2018, 9, 124–131. [Google Scholar] [CrossRef]
- Tenekedjiev, K.; Abdussamie, N.; An, H.; Nikolova, N. Regression Diagnostics with Predicted Residuals of Linear Model with Improved Singular Value Classification Applied to Forecast the Hydrodynamic Efficiency of Wave Energy Converters. Appl. Sci. 2021, 11, 2990. [Google Scholar] [CrossRef]
- Miller, D.D.; Brown, E.W. Artificial Intelligence in Medical Practice: The Question to the Answer? Am. J. Med. 2018, 131, 129–133. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, P.-H.C.; Krause, J.; Peng, L. How to Read Articles That Use Machine Learning: Users’ Guides to the Medical Literature. JAMA 2019, 322, 1806–1816. [Google Scholar] [CrossRef] [PubMed]
- Triantafyllidis, A.K.; Tsanas, A. Applications of Machine Learning in Real-Life Digital Health Interventions: Review of the Literature. J. Med. Internet Res. 2019, 21, e12286. [Google Scholar] [CrossRef] [Green Version]
- Peiffer-Smadja, N.; Rawson, T.M.; Ahmad, R.; Buchard, A.; Georgiou, P.; Lescure, F.X.; Birgand, G.; Holmes, A.H. Machine learning for clinical decision support in infectious diseases: A narrative review of current applications. Clin. Microbiol. Infect. 2020, 26, 584–595. [Google Scholar] [CrossRef]
- Song, Q.; Zheng, Y.-J.; Yang, J. Effects of Food Contamination on Gastrointestinal Morbidity: Comparison of Different Machine-Learning Methods. Int. J. Environ. Res. Public Health 2019, 16, 838. [Google Scholar] [CrossRef]
- Wu, T.-E.; Chen, H.-A.; Jhou, M.-J.; Chen, Y.-N.; Chang, T.-J.; Lu, C.-J. Evaluating the Effect of Topical Atropine Use for Myopia Control on Intraocular Pressure by Using Machine Learning. J. Clin. Med. 2021, 10, 111. [Google Scholar] [CrossRef]
- Huang, L.-Y.; Chen, F.-Y.; Jhou, M.-J.; Kuo, C.-H.; Wu, C.-Z.; Lu, C.-H.; Chen, Y.-L.; Pei, D.; Cheng, Y.-F.; Lu, C.-J. Comparing Multiple Linear Regression and Machine Learning in Predicting Diabetic Urine Albumin–Creatinine Ratio in a 4-Year Follow-Up Study. J. Clin. Med. 2022, 11, 3661. [Google Scholar] [CrossRef]
- Shah, S.H.; Angel, Y.; Houborg, R.; Ali, S.; McCabe, M.F. A Random Forest Machine Learning Approach for the Retrieval of Leaf Chlorophyll Content in Wheat. Remote Sens. 2019, 11, 920. [Google Scholar] [CrossRef]
- Wang, H.; Xu, Q.; Zhou, L. Seminal Quality Prediction Using Clustering-Based Decision Forests. Algorithms 2014, 7, 405–417. [Google Scholar] [CrossRef]
- Iqbal, I.; Mustafa, G.; Ma, J. Deep Learning-Based Morphological Classification of Human Sperm Heads. Diagnostics 2020, 10, 325. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Zhang, Y.; Martin, C.; Ma, X.; Shen, B. Translational Bioinformatics for Human Reproductive Biology Research: Examples, Opportunities and Challenges for a Future Reproductive Medicine. Int. J. Mol. Sci. 2023, 24, 4. [Google Scholar] [CrossRef]
- Tseng, C.-J.; Lu, C.-J.; Chang, C.-C.; Chen, G.-D.; Cheewakriangkrai, C. Integration of data mining classification techniques and ensemble learning to identify risk factors and diagnose ovarian cancer recurrence. Artif Intell Med. 2017, 78, 47–54. [Google Scholar] [CrossRef]
- Ting, W.-C.; Chang, H.-R.; Chang, C.-C.; Lu, C.-J. Developing a Novel Machine Learning-Based Classification Scheme for Predicting SPCs in Colorectal Cancer Survivors. Appl. Sci. 2020, 10, 1355. [Google Scholar] [CrossRef] [Green Version]
- Lee, T.-S.; Chen, I.-F.; Chang, T.-J.; Lu, C.-J. Forecasting Weekly Influenza Outpatient Visits Using a Two-Dimensional Hierarchical Decision Tree Scheme. Int. J. Environ. Res. Public Health 2020, 17, 4743. [Google Scholar] [CrossRef]
- Chang, C.-C.; Yeh, J.-H.; Chen, Y.-M.; Jhou, M.-J.; Lu, C.-J. Clinical Predictors of Prolonged Hospital Stay in Patients with Myasthenia Gravis: A Study Using Machine Learning Algorithms. J. Clin. Med. 2021, 10, 4393. [Google Scholar] [CrossRef]
- Chang, C.-C.; Huang, T.-H.; Shueng, P.-W.; Chen, S.-H.; Chen, C.-C.; Lu, C.-J.; Tseng, Y.-J. Developing a Stacked Ensemble-Based Classification Scheme to Predict Second Primary Cancers in Head and Neck Cancer Survivors. Int. J. Environ. Res. Public Health 2021, 18, 12499. [Google Scholar] [CrossRef]
- Wu, C.-W.; Shen, H.-L.; Lu, C.-J.; Chen, S.-H.; Chen, H.-Y. Comparison of Different Machine Learning Classifiers for Glaucoma Diagnosis Based on Spectralis OCT. Diagnostics 2021, 11, 1718. [Google Scholar] [CrossRef]
- Huang, Y.-C.; Cheng, Y.-C.; Jhou, M.-J.; Chen, M.; Lu, C.-J. Important Risk Factors in Patients with Nonvalvular Atrial Fibrillation Taking Dabigatran Using Integrated Machine Learning Scheme—A Post Hoc Analysis. J. Pers. Med. 2022, 12, 756. [Google Scholar] [CrossRef] [PubMed]
- Jhou, M.-J.; Chen, M.-S.; Lee, T.-S.; Yang, C.-T.; Chiu, Y.-L.; Lu, C.-J. A Hybrid Risk Factor Evaluation Scheme for Metabolic Syndrome and Stage 3 Chronic Kidney Disease Based on Multiple Machine Learning Techniques. Healthcare 2022, 10, 2496. [Google Scholar] [CrossRef]
- Sun, C.-K.; Tang, Y.-X.; Liu, T.-C.; Lu, C.-J. An Integrated Machine Learning Scheme for Predicting Mammographic Anomalies in High-Risk Individuals Using Questionnaire-Based Predictors. Int. J. Environ. Res. Public Health 2022, 19, 9756. [Google Scholar] [CrossRef]
- Liao, P.-C.; Chen, M.-S.; Jhou, M.-J.; Chen, T.-C.; Yang, C.-T.; Lu, C.-J. Integrating Health Data-Driven Machine Learning Algorithms to Evaluate Risk Factors of Early Stage Hypertension at Different Levels of HDL and LDL Cholesterol. Diagnostics 2022, 12, 1965. [Google Scholar] [CrossRef] [PubMed]
- Breiman, L. Random Forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Friedman, J. Greedy function approximation: A gradient boosting machine. Ann. Stat. 2001, 29, 1189–1232. Available online: http://www.jstor.org/stable/2699986 (accessed on 25 May 2022). [CrossRef]
- Guindo, M.L.; Kabir, M.H.; Chen, R.; Liu, F. Particle Swarm Optimization and Multiple Stacked Generalizations to Detect Nitrogen and Organic-Matter in Organic-Fertilizer Using Vis-NIR. Sensors 2021, 21, 4882. [Google Scholar] [CrossRef]
- Hastie, T.; Tibshirani, R.; Wainwright, M. Statistical Learning with Sparsity: The Lasso and Generalizations; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar] [CrossRef]
- Kwon, S.; Lee, S.; Na, O. Tuning parameter selection for the adaptive Lasso in the autoregressive model. J. Korean Stat. Soc. 2017, 46, 285–297. [Google Scholar] [CrossRef]
- Hoerl, A.E.; Kennard, R.W. Ridge regression: Biased estimation for non-orthogonal problems. Technometrics 1970, 12, 55–67. [Google Scholar] [CrossRef]
- Chen, T.; Guestrin, C. XGBoost: A Scalable Tree Boosting System. In Proceedings of the 22nd ACM SIGKDD International Conference on Knowledge Discovery and Data Mining, San Francisco, CA, USA, 13–17 August 2016; pp. 785–794. [Google Scholar]
- Breiman, L.; Cutler, A. RandomForest: Breiman and Cutler’s Random Forests for Classification and Regression. 2022. R Package Version, 4.7-1.1. Available online: https://CRAN.R-project.org/package=randomForest (accessed on 25 May 2022).
- Greenwell, B.; Boehmke, B.; Cunningham, J. Gbm: Generalized Boosted Regression Models. 2020. R Package Version, 2.1.8. Available online: https://CRAN.R-project.org/package=gbm (accessed on 25 May 2022).
- Friedman, J.; Hastie, T.; Tibshirani, R.; Narasimhan, B.; Tay, K.; Simon, N.; Qian, J.; Yang, J. Glmnet: Lasso and Elastic-Net Regularized Generalized Linear Models. 2022. R Package Version, 4.1-4. Available online: https://CRAN.R-project.org/package=glmnet (accessed on 25 May 2022).
- Chen, T.; He, T.; Benesty, M.; Khotilovich, V.; Tang, Y.; Cho, H.; Chen, K.; Mitchell, R.; Cano, I.; Zhou, T.; et al. Xgboost: Extreme Gradient Boosting. 2022. R Package Version, 1.5.0.2. Available online: https://CRAN.R-project.org/package=xgboost (accessed on 1 January 2022).
- Kuhn, M. Caret: Classification and Regression Training. 2022. R Package Version, 6.0-92. Available online: https://CRAN.R-project.org/package=caret (accessed on 25 May 2022).
- Chen, Q.; Yang, H.; Zhou, N.; Sun, L.; Bao, H.; Tan, L.; Chen, H.; Ling, X.; Zhang, G.; Huang, L.; et al. Inverse U-shaped Association between Sleep Duration and Semen Quality: Longitudinal Observational Study (MARHCS) in Chongqing, China. Sleep 2016, 39, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Jensen, T.K.; Andersson, A.M.; Skakkebæk, N.E.; Joensen, U.N.; Blomberg Jensen, M.; Lassen, T.H.; Nordkap, L.; Olesen, I.A.; Hansen, Å.M.; Rod, N.H.; et al. Association of sleep disturbances with reduced semen quality: A cross-sectional study among 953 healthy young Danish men. Am. J. Epidemiol. 2013, 177, 1027–1037. [Google Scholar] [CrossRef]
- Choi, J.H.; Lee, S.H.; Bae, J.H.; Shim, J.S.; Park, H.S.; Kim, Y.S.; Shin, C. Effect of sleep deprivation on the male reproductive system in rats. J. Korean Med. Sci. 2016, 31, 1624–1630. [Google Scholar] [CrossRef]
- Yazama, F.; Tai, A. Unexpected role of α-fetoprotein in spermatogenesis. PLoS ONE 2011, 6, e19387. [Google Scholar] [CrossRef]
- Corsini, C.; Fallara, G.; Candela, L.; Raffo, M.; Pozzi, E.; Belladelli, F.; Capogrosso, P.; Boeri, L.; Costa, A.; Schifano, N.; et al. High serum alpha-fetoprotein levels in primary infertile men. Andrology 2023, 11, 86–92. [Google Scholar] [CrossRef]
- Jensen, T.K.; Andersson, A.M.; Jorgensen, N.; Andersen, A.G.; Carlsen, E.; Petersen, J.H.; Skakkebaek, N.E. Body mass index in relation to semen quality and reproductive hormones among 1,558 danish men. Fertil. Steril. 2004, 82, 863–870. [Google Scholar] [CrossRef] [PubMed]
- Ergün, A.; Köse, S.K.; Aydos, K.; Ata, A.; Avci, A. Correlation of seminal parameters with serum lipid profile and sex hormones. Arch. Androl. 2007, 53, 21–23. [Google Scholar] [CrossRef] [PubMed]
- Fogari, R.; Zoppi, A.; Preti, P.; Rinaldi, A.; Marasi, G.; Vanasia, A.; Mugellini, A. Sexual activity and plasma testosterone levels in hypertensive males. Am. J. Hypertens. 2002, 15, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, A.; Stewart, A.; Farquhar, C. Body mass index in relation to semen quality and reproductive hormones in New Zealand men: A cross-sectional study in fertility clinics. Hum. Reprod. 2013, 28, 3178–3187. [Google Scholar] [CrossRef] [PubMed]
- Edey, M.M. Male Sexual Dysfunction and Chronic Kidney Disease. Front. Med. 2017, 4, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
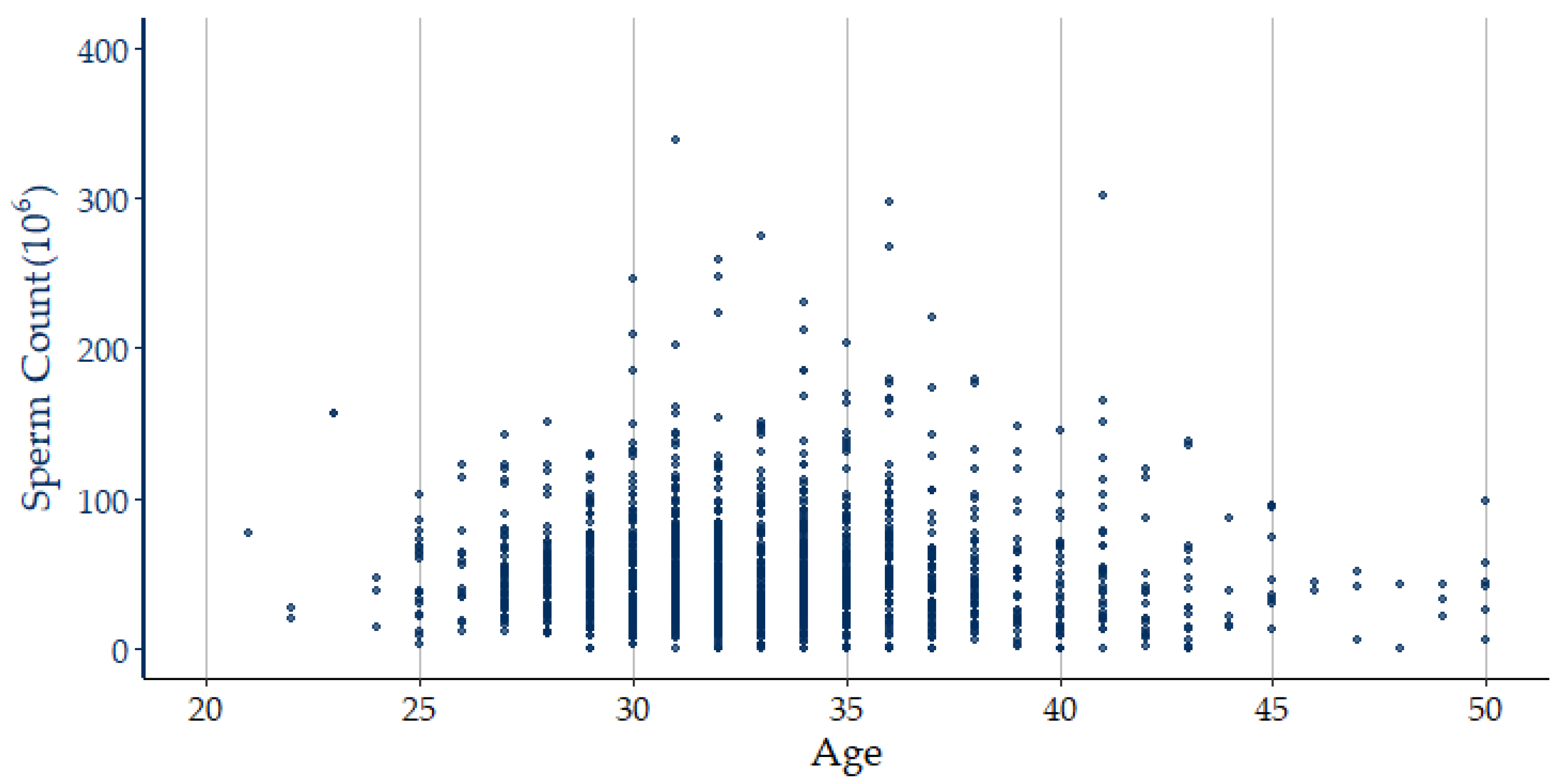

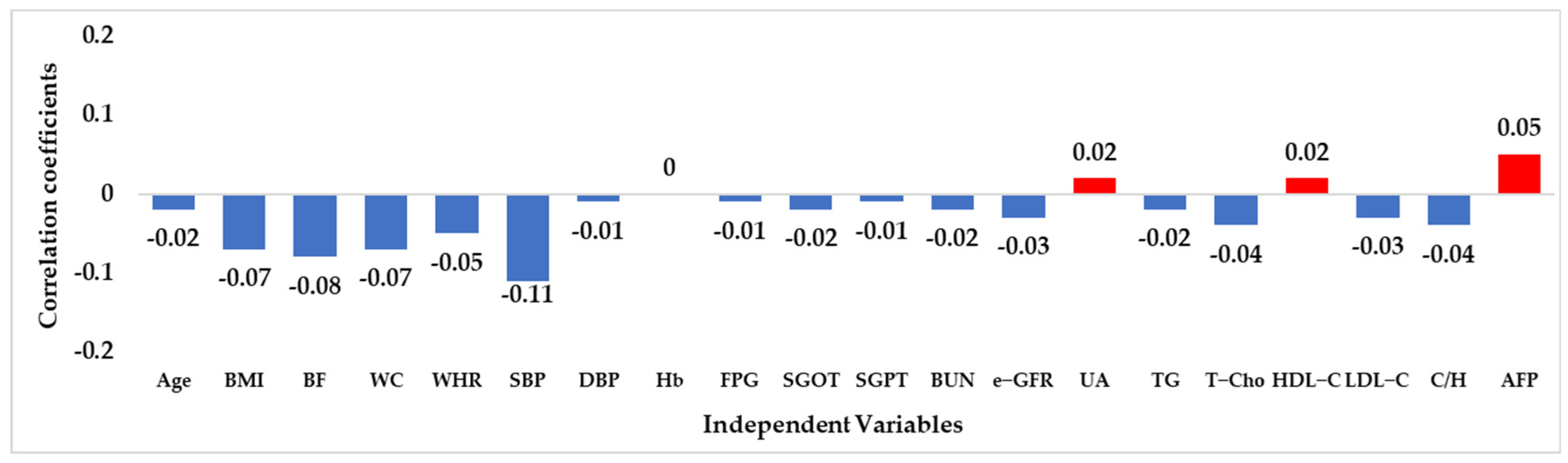
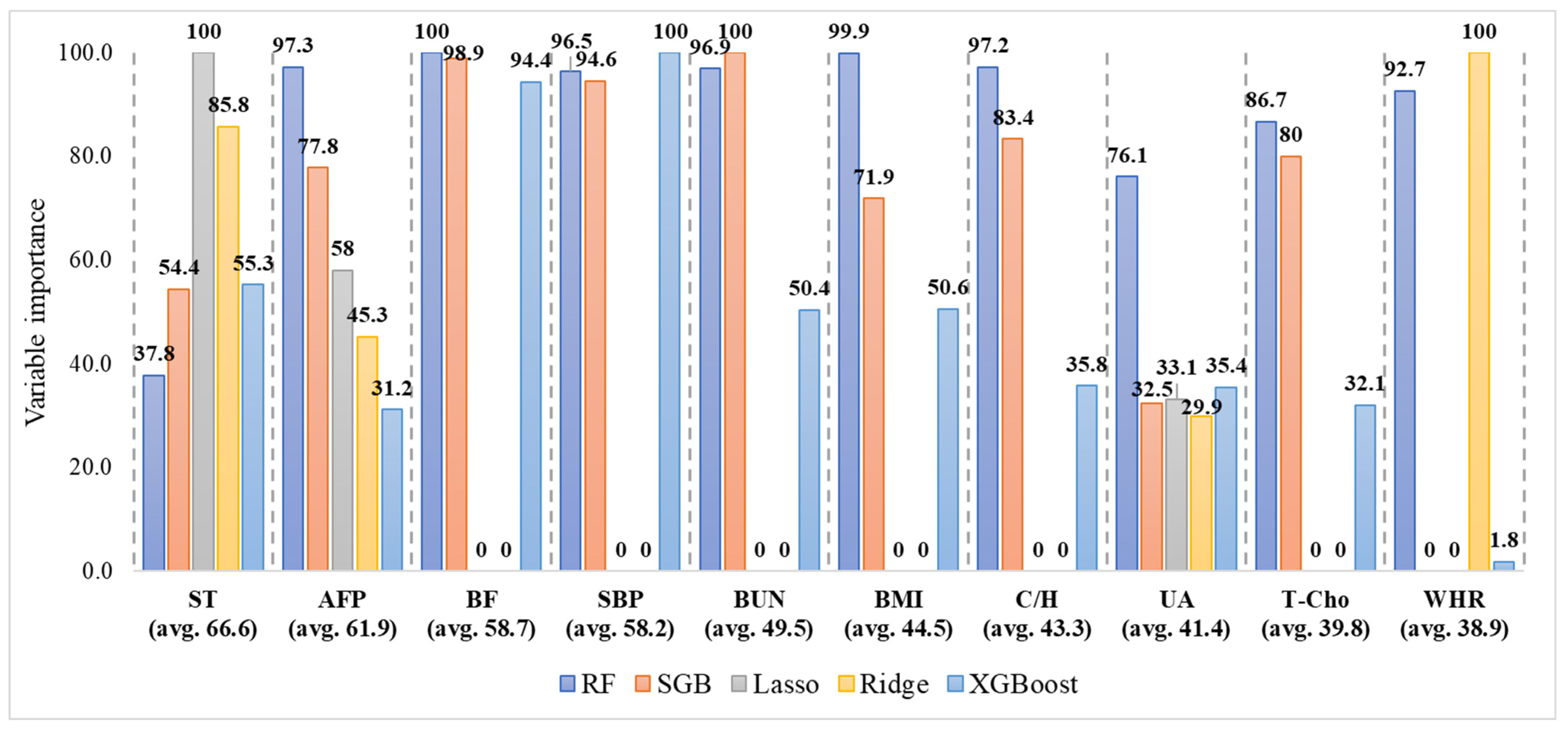
| Independent Variable | N = 1375; n (%) | Independent Variable | N = 1375; n (%) |
|---|---|---|---|
| CS: Current smokers | ST: Sleep time (hours) | ||
| (1) Never | 911 (66.25%) | (1) <4 | 7 (0.51%) |
| (2) Passive smoking | 56 (4.07%) | (2) 4–6 | 265 (19.27%) |
| (3) Quit | 114 (8.29%) | (3) 6–7 | 811 (58.98%) |
| (4) Occasional | 58 (4.22%) | (4) 7–8 | 248 (18.04%) |
| (5) Addicted | 236 (17.16%) | (5) 8–9 | 44 (3.20%) |
| AD: Alcohol drinker | (6) >9 | NA | |
| (1) Never | 1143 (83.13%) | MetS | |
| (2) Quit | 17 (1.24%) | (1) No | 1241 (90.25%) |
| (3) 1–2 times a week | 169 (12.29%) | (2) Yes | 134 (9.75%) |
| (4) 3–4 times a week | 39 (2.84%) | Independent Variable | Mean ± SD |
| (5) 5–6 times a week | NA | Age | 33.22 ± 4.36 |
| (6) Addicted | 7 (0.51%) | BMI (body mass index, kg/m2) | 24.27 ± 3.37 |
| Vitamin C supplementation | BF (body fat, %) | 24.36 ± 5.57 | |
| (1) No | 1156 (84.07%) | WC (waist circumference, cm) | 82.26 ± 8.34 |
| (2) Yes | 219 (15.93%) | WHR (waist–hip ratio, %) | 0.84 ± 0.05 |
| Vitamin E supplementation | SBP (systolic blood pressure, mmHg) | 118.22 ± 12.60 | |
| (1) No | 1289 (93.75%) | DBP (diastolic blood pressure, mmHg) | 72.99 ± 9.62 |
| (2) Yes | 86 (6.25%) | Hb (hemoglobin, g/dL) | 15.22 ± 0.99 |
| Consumption of Omega-3 rich food | FPG (fasting plasma glucose, mg/dL) | 98.61 ± 10.60 | |
| (1) No | 1283 (93.31%) | SGOT (serum glutamic oxaloacetic transaminase, U/L) | 25.78 ± 20.02 |
| (2) Yes | 92 (6.69%) | SGPT (serum glutamic pyruvic transaminase, U/L) | 36.97 ± 36.02 |
| Consumption of sugar-containing beverages | BUN (blood urea nitrogen, mg/dL) | ± 3.01 | |
| (1) No or less than 1 cup per week | 356 (25.89%) | e-GFR (estimated glomerular filtration rate, ml/min/1.73m2) | ± 11.23 |
| (2) 1 to 3 cups per week | 460 (33.45%) | UA (uric acid, mg/dL) | 6.68 ± 1.27 |
| (3) 4 to 6 cups per week | 266 (19.35%) | TG (triglyceride, mg/dL) | 118.3 ± 68.94 |
| (4) 1 cup per day | 198 (14.40%) | T-Cho (total cholesterol, mg/dl) | 193.42 ± 32.54 |
| (5) 2 or more than 2 cups per day | 95 (6.91%) | HDL-C (high-density lipoprotein cholesterol, mg/dL) | 52.36 ± 11.67 |
| Daily physical activity | LDL-C (low-density lipoprotein cholesterol, mg/dL) | 119.55 ± 30.63 | |
| (1) Sedentary most of the time | 928 (67.49%) | C/H (T-Cho/HDL-C) | 3.85 ± 0.96 |
| (2) Frequent repeated sitting and ambulation | 311 (22.62%) | AFP (alpha–fetoprotein, ng/mL) | 2.74 ± 1.33 |
| (3) Standing or ambulation most of the time | 111 (8.07%) | Dependent Variable | Mean ± SD |
| (4) Requires whole body muscle usage most of the time | 25 (1.82%) | S-C (sperm count) | 53.3 ± 42.24 |
| Metric | Description | Calculation |
|---|---|---|
| SMAPE | Symmetric mean absolute percentage error | |
| RAE | Relative absolute error | |
| RRSE | Root relative squared error | |
| RMSE | Root mean squared error |
| Methods | SMAPE | RAE | RRSE | RMSE |
|---|---|---|---|---|
| RF | 0.537 | 0.984 | 1.014 | 53.060 |
| SGB | 0.536 | 0.977 | 1.017 | 53.218 |
| Lasso | 0.534 | 0.972 | 1.005 | 52.608 |
| Ridge | 0.530 | 0.964 | 1.006 | 52.674 |
| XGBoost | 0.532 | 0.968 | 1.011 | 52.913 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, H.-H.; Hsieh, S.-J.; Chen, M.-S.; Jhou, M.-J.; Liu, T.-C.; Shen, H.-L.; Yang, C.-T.; Hung, C.-C.; Yu, Y.-Y.; Lu, C.-J. Machine Learning Predictive Models for Evaluating Risk Factors Affecting Sperm Count: Predictions Based on Health Screening Indicators. J. Clin. Med. 2023, 12, 1220. https://doi.org/10.3390/jcm12031220
Huang H-H, Hsieh S-J, Chen M-S, Jhou M-J, Liu T-C, Shen H-L, Yang C-T, Hung C-C, Yu Y-Y, Lu C-J. Machine Learning Predictive Models for Evaluating Risk Factors Affecting Sperm Count: Predictions Based on Health Screening Indicators. Journal of Clinical Medicine. 2023; 12(3):1220. https://doi.org/10.3390/jcm12031220
Chicago/Turabian StyleHuang, Hung-Hsiang, Shang-Ju Hsieh, Ming-Shu Chen, Mao-Jhen Jhou, Tzu-Chi Liu, Hsiang-Li Shen, Chih-Te Yang, Chung-Chih Hung, Ya-Yen Yu, and Chi-Jie Lu. 2023. "Machine Learning Predictive Models for Evaluating Risk Factors Affecting Sperm Count: Predictions Based on Health Screening Indicators" Journal of Clinical Medicine 12, no. 3: 1220. https://doi.org/10.3390/jcm12031220
APA StyleHuang, H.-H., Hsieh, S.-J., Chen, M.-S., Jhou, M.-J., Liu, T.-C., Shen, H.-L., Yang, C.-T., Hung, C.-C., Yu, Y.-Y., & Lu, C.-J. (2023). Machine Learning Predictive Models for Evaluating Risk Factors Affecting Sperm Count: Predictions Based on Health Screening Indicators. Journal of Clinical Medicine, 12(3), 1220. https://doi.org/10.3390/jcm12031220









