Cellular and Humoral Responses to Recombinant and Inactivated SARS-CoV-2 Vaccines in CKD Patients: An Observational Study
Abstract
1. Introduction
2. Methods
2.1. Participants
2.2. Data Collection
2.3. SARS-CoV-2 Antibody Test
2.4. Detection of SARS-CoV-2 Specific B Cells by Flow Cytometry
2.5. Statistical Analysis
3. Results
3.1. Characteristics of Participants
3.2. SARS-CoV-2 Vaccination in CKD Patients
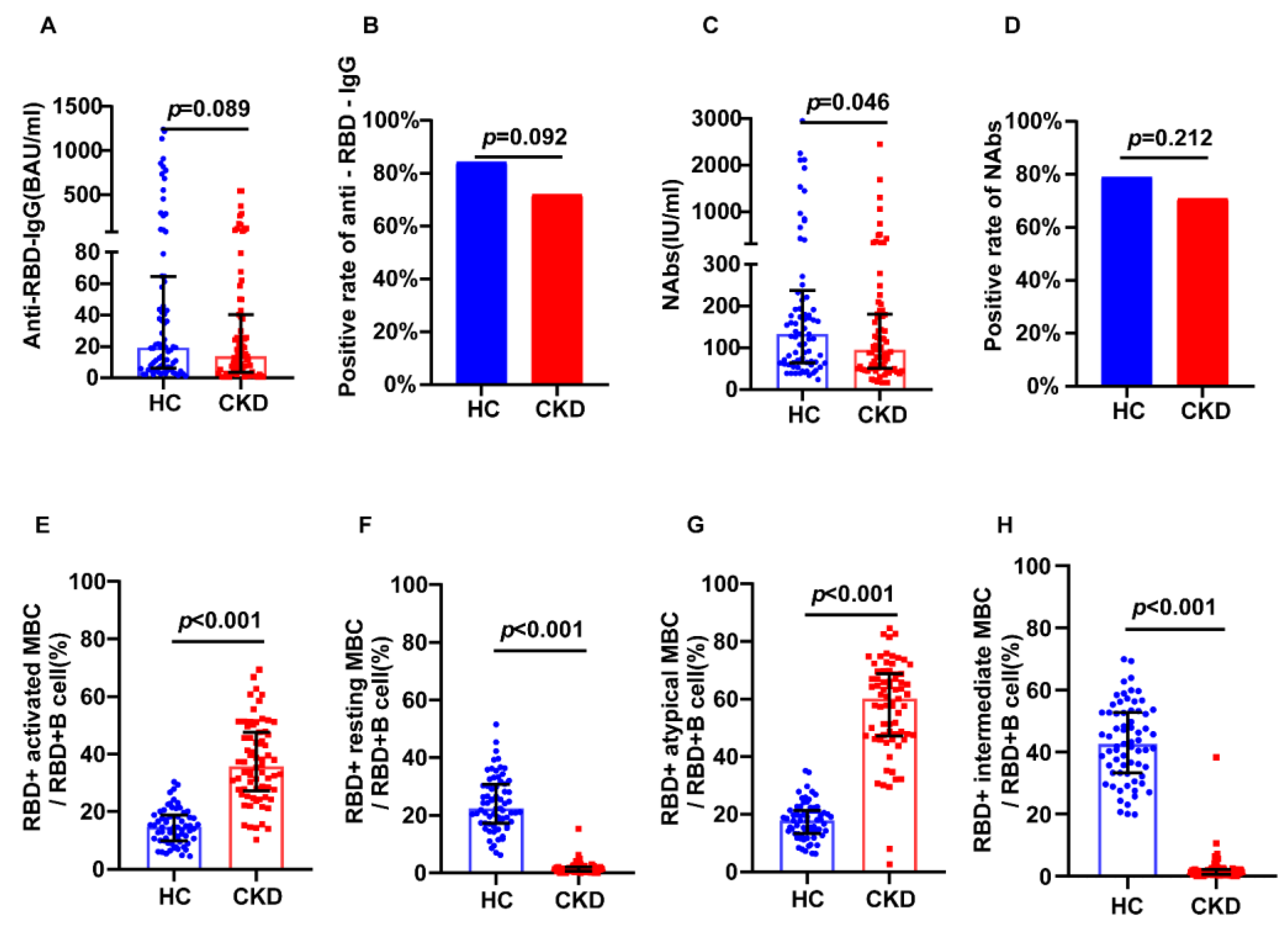
3.3. Inactivated SARS-CoV-2 Virus Vaccination in CKD
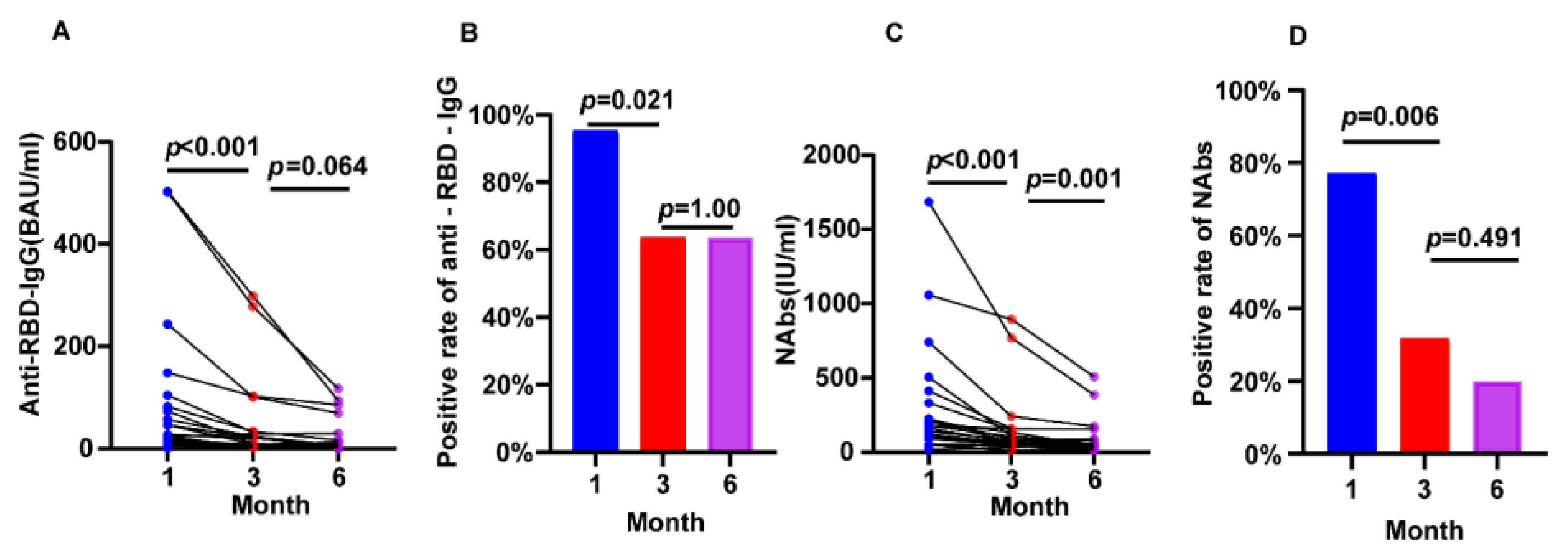
3.4. SARS-CoV-2 Vaccination in Hemodialysis Patients
3.5. Safety of SARS-CoV-2 Vaccination in CKD
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, X.; Wang, H.; Ai, J.; Shen, L.; Lin, K.; Yuan, G.; Sheng, X.; Jin, X.; Deng, Z.; Xu, J.; et al. Identification of CKD, Bedridden History and Cancer as Higher-Risk Comorbidities and Their Impact on Prognosis of Hospitalized Omicron Patients: A Multi-Centre Cohort Study. Emerg. Microbes Infect. 2022, 11, 2501–2509. [Google Scholar] [CrossRef]
- Loo, W.K.; Hasikin, K.; Suhaimi, A.; Yee, P.L.; Teo, K.; Xia, K.; Qian, P.; Jiang, Y.; Zhang, Y.; Dhanalakshmi, S.; et al. Systematic Review on COVID-19 Readmission and Risk Factors: Future of Machine Learning in COVID-19 Readmission Studies. Front. Public Health 2022, 10, 898254. [Google Scholar] [CrossRef]
- Chung, E.Y.M.; Palmer, S.C.; Natale, P.; Krishnan, A.; Cooper, T.E.; Saglimbene, V.M.; Ruospo, M.; Au, E.; Jayanti, S.; Liang, A.; et al. Incidence and Outcomes of COVID-19 in People With CKD: A Systematic Review and Meta-Analysis. Am. J. Kidney Dis. 2021, 78, 804–815. [Google Scholar] [CrossRef]
- Krueger, K.M.; Ison, M.G.; Ghossein, C. Practical Guide to Vaccination in All Stages of CKD, Including Patients Treated by Dialysis or Kidney Transplantation. Am. J. Kidney Dis. 2020, 75, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Danthu, C.; Hantz, S.; Dahlem, A.; Duval, M.; Ba, B.; Guibbert, M.; El Ouafi, Z.; Ponsard, S.; Berrahal, I.; Achard, J.M.; et al. Humoral Response after SARS-CoV-2 MRNA Vaccination in a Cohort of Hemodialysis Patients and Kidney Transplant Recipients. J. Am. Soc. Nephrol. 2021, 32, 2153–2158. [Google Scholar] [CrossRef]
- Sanders, J.S.F.; Bemelman, F.J.; Messchendorp, A.L.; Baan, C.C.; Van Baarle, D.; Van Binnendijk, R.; Diavatopoulos, D.A.; Frölke, S.C.; Geers, D.; Geurtsvankessel, C.H.; et al. The RECOVAC Immune-Response Study: The Immunogenicity, Tolerability, and Safety of COVID-19 Vaccination in Patients with Chronic Kidney Disease, on Dialysis, or Living with a Kidney Transplant. Transplantation 2022, 106, 821–834. [Google Scholar] [CrossRef]
- Kim, W.; Zhou, J.Q.; Horvath, S.C.; Schmitz, A.J.; Sturtz, A.J.; Lei, T.; Liu, Z.; Kalaidina, E.; Thapa, M.; Alsoussi, W.B.; et al. Germinal Centre-Driven Maturation of B Cell Response to MRNA Vaccination. Nature 2022, 604, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Kardava, L.; Rachmaninoff, N.; Lau, W.W.; Buckner, C.M.; Trihemasava, K.; Blazkova, J.; Lopes De Assis, F.; Wang, W.; Zhang, X.; Wang, Y.; et al. Early Human B Cell Signatures of the Primary Antibody Response to MRNA Vaccination. Proc. Natl. Acad. Sci. USA 2022, 119, e2204607119. [Google Scholar] [CrossRef] [PubMed]
- Ao, L.; Lu, T.; Cao, Y.; Chen, Z.; Wang, Y.; Li, Z.; Ren, X.; Xu, P.; Peng, M.; Chen, M.; et al. Safety and Immunogenicity of Inactivated SARS-CoV-2 Vaccines in People Living with HIV. Emerg. Microbes Infect. 2022, 11, 1126–1134. [Google Scholar] [CrossRef]
- Benedetto, U.; Head, S.J.; Angelini, G.D.; Blackstone, E.H. Statistical Primer: Propensity Score Matching and Its Alternatives. Eur. J. Cardio-Thorac. Surg. 2018, 53, 1112–1117. [Google Scholar] [CrossRef]
- Liechti, T.; Günthard, H.F.; Trkola, A. OMIP-047: High-Dimensional Phenotypic Characterization of B Cells. Cytom. Part A 2018, 93, 592–596. [Google Scholar] [CrossRef] [PubMed]
- Carr, E.J.; Kronbichler, A.; Graham-Brown, M.; Abra, G.; Argyropoulos, C.; Harper, L.; Lerma, E.V.; Suri, R.S.; Topf, J.; Willicombe, M.; et al. Review of Early Immune Response to SARS-CoV-2 Vaccination Among Patients With CKD. Kidney Int. Rep. 2021, 6, 2292–2304. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.Y.; Fang, T.C.; Liao, H.W.; Chen, T.H.; Chang, J.H.; Lin, Y.C.; Kao, C.C.; Liu, M.C.; Chang, H.W.; Hung, C.S.; et al. The Humoral Immune Response of the ChAdOx1 NCoV-19 Vaccine in Maintenance Dialysis Patients without Prior COVID-19 Infection. Vaccines 2022, 10, 338. [Google Scholar] [CrossRef]
- Quiroga, B.; Soler, M.J.; Ortiz, A.; Vaquera, S.M.; Jesús, C.; Mantecón, J.; Useche, G.; Gabriela Sánchez Márquez, M.; Carnerero, M.; Teresa, M.; et al. Safety and Immediate Humoral Response of COVID-19 Vaccines in Chronic Kidney Disease Patients: The SENCOVAC Study. Nephrol. Dial. Transplant. 2021, 37, 1868–1878. [Google Scholar] [CrossRef]
- Zhang, Z.; Mateus, J.; Coelho, C.H.; Dan, J.M.; Moderbacher, C.R.; Gálvez, R.I.; Cortes, F.H.; Grifoni, A.; Tarke, A.; Chang, J.; et al. Humoral and Cellular Immune Memory to Four COVID-19 Vaccines. Cell 2022, 185, 2434–2451.e17. [Google Scholar] [CrossRef] [PubMed]
- Sutton, H.J.; Aye, R.; Idris, A.H.; Vistein, R.; Nduati, E.; Kai, O.; Mwacharo, J.; Li, X.; Gao, X.; Andrews, T.D.; et al. Atypical B Cells Are Part of an Alternative Lineage of B Cells That Participates in Responses to Vaccination and Infection in Humans. Cell Rep. 2021, 34, 108684. [Google Scholar] [CrossRef]
- Ogega, C.O.; Skinner, N.E.; Blair, P.W.; Park, H.S.; Littlefield, K.; Ganesan, A.; Dhakal, S.; Ladiwala, P.; Antar, A.A.R.; Ray, S.C.; et al. Durable SARS-CoV-2 B Cell Immunity after Mild or Severe Disease. J. Clin. Investig. 2021, 131, e145516. [Google Scholar] [CrossRef]
- Portugal, S.; Obeng-Adjei, N.; Moir, S.; Crompton, P.D.; Pierce, S.K. Atypical Memory B Cells in Human Chronic Infectious Diseases: An Interim Report. Cell Immunol. 2017, 321, 18–25. [Google Scholar] [CrossRef]
- Holla, P.; Ambegaonkar, A.; Sohn, H.; Pierce, S.K. Exhaustion May Not Be in the Human B Cell Vocabulary, at Least Not in Malaria. Immunol. Rev. 2019, 292, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Frosch, A.E.; Odumade, O.A.; Taylor, J.J.; Ireland, K.; Ayodo, G.; Ondigo, B.; Narum, D.L.; Vulule, J.; John, C.C. Decrease in Numbers of Naive and Resting B Cells in HIV-Infected Kenyan Adults Leads to a Proportional Increase in Total and Plasmodium Falciparum– Specific Atypical Memory B Cells. J. Immunol. 2017, 198, 4629–4638. [Google Scholar] [CrossRef]
- Wu, C.; Fu, Q.; Guo, Q.; Chen, S.; Goswami, S.; Sun, S.; Li, T.; Cao, X.; Chu, F.; Chen, Z.; et al. Lupus-Associated Atypical Memory B Cells Are MTORC1-Hyperactivated and Functionally Dysregulated. Ann. Rheum. Dis. 2019, 78, 1090–1100. [Google Scholar] [CrossRef] [PubMed]
- Comarmond, C.; Lorin, V.; Marques, C.; Maciejewski-Duval, A.; Joher, N.; Planchais, C.; Touzot, M.; Biard, L.; Hieu, T.; Quiniou, V.; et al. TLR9 Signalling in HCV-Associated Atypical Memory B Cells Triggers Th1 and Rheumatoid Factor Autoantibody Responses. J. Hepatol. 2019, 71, 908–919. [Google Scholar] [CrossRef] [PubMed]
- Hopp, C.S.; Skinner, J.; Anzick, S.L.; Tipton, C.M.; Peterson, M.E.; Li, S.; Doumbo, S.; Kayentao, K.; Ongoiba, A.; Martens, C.; et al. Atypical B Cells Up-Regulate Costimulatory Molecules during Malaria and Secrete Antibodies with T Follicular Helper Cell Support. Sci. Immunol. 2022, 7, eabn1250. [Google Scholar] [CrossRef] [PubMed]
- Pape, K.A.; Dileepan, T.; Kabage, A.J.; Kozysa, D.; Batres, R.; Evert, C.; Matson, M.; Lopez, S.; Krueger, P.D.; Graiziger, C.; et al. High-Affinity Memory B Cells Induced by SARS-CoV-2 Infection Produce More Plasmablasts and Atypical Memory B Cells than Those Primed by MRNA Vaccines. Cell Rep. 2021, 37, 109823. [Google Scholar] [CrossRef] [PubMed]


| Variables | CKD Group | Control Group | p Value | Control Group * | p Value * |
|---|---|---|---|---|---|
| Age (years) | 47.25 ± 14.31 | 47 (34–58) | 0.682 | 42 (25–53) | 0.051 |
| ≤60 | 64/79 | 334/420 | 0.763 | 67/79 | 0.526 |
| >60 | 15/79 | 86/420 | 12/79 | ||
| Gender (male, (n%)) | 53/79 (67%) | 218/420 (52%) | 0.013 | 47/79 (59%) | 0.409 |
| BMI (kg/m2) | 24.70 ± 4.25 | 25.04 (20.55–27.33) | 0.381 | 23.85 ± 3.79 | 0.102 |
| <24 | 36/79 | 151/420 | 0.105 | 45/79 | 0.152 |
| ≥24 | 43/79 | 269/420 | 34/79 | ||
| Acquisition time (months) | |||||
| <3 | 61/79 | 330/420 | 0.788 | 59/79 | 0.71 |
| ≥3 | 18/79 | 90/420 | 20/79 | ||
| Vaccines | |||||
| Recombinant vaccine (n%) | 16/79 | 177/420 | <0.001 | 18/79 | 0.15 |
| Inactivated vaccine (n%) | 63/79 | 243/420 | 61/79 | ||
| Comorbidities | |||||
| Diabetes | 11/79 | 15/420 | <0.001 | 0/79 | <0.001 |
| Hypertension | 35/79 | 36/220 | <0.001 | 4/79 | <0.001 |
| Cardiovascular diseases | 14/79 | 3/420 | <0.001 | 0/79 | <0.001 |
| Variables | CKD Group | Control Group | p Value | Control Group * | p Value * |
|---|---|---|---|---|---|
| Age (years) | 48.60 ± 14.28 | 49 (34–60) | 0.642 | 48 (36–64) | 0.918 |
| ≤60 | 51/63 | 184/243 | 0.287 | 43/63 | 0.102 |
| >60 | 12/63 | 59/243 | 20/63 | ||
| Gender (male, (n%)) | 41/63 | 127/243 | 0.068 | 42/63 | 0.814 |
| BMI (kg/m2) | 24.56 (21.22–27.23) | 24.96 ± 3.55 | 0.293 | 25.65 ± 3.02 | 0.064 |
| <24 | 29/63 | 152/243 | 0.017 | 17/63 | 0.026 |
| ≥24 | 34/63 | 91/243 | 46/63 | ||
| Acquisition time (months) | |||||
| <3 | 48/63 | 166/243 | 0.224 | 53/63 | 0.264 |
| ≥3 | 15/63 | 77/243 | 10/63 | ||
| Diabetes | 11/63 | 8/243 | <0.001 | 0/63 | <0.001 |
| Hypertension | 35/63 | 24/243 | <0.001 | 3/63 | <0.001 |
| Cardiovascular diseases | 14/63 | 2/243 | <0.001 | 0/63 | <0.001 |
| Variables | CKD Group | Control Group | p Value |
|---|---|---|---|
| Age (years) | 48.00 ± 14.16 | 47 (34–58) | 0.604 |
| ≤60 | 19/23 | 334/420 | 1 |
| >60 | 4/23 | 86/420 | |
| Gender (male, (n%)) | 14/23 (61%) | 218/420 (52%) | 0.402 |
| BMI (kg/m2) | 22.16 ± 4.38 | 25.04 (20.55–27.33) | 0.007 |
| <24 | 0.008 | ||
| ≥24 | |||
| Acquisition time (months) | |||
| <3 | 21/23 | 330/420 | 0.189 |
| ≥3 | 2/23 | 90/420 | |
| Vaccines | |||
| Recombinant vaccine (n%) | 3/23 | 177/420 | 0.006 |
| Inactivated vaccine (n%) | 20/23 | 243/420 | |
| Comorbidities | |||
| Diabetes | 1/23 | 15/420 | 0.58 |
| Hypertension | 0/23 | 36/220 | 0.243 |
| Cardiovascular diseases | 0/23 | 3/420 | 1 |
| CKD Patients (n = 79) | Controls (n = 420) | p Value | |
|---|---|---|---|
| Overall adverse events | 5 | 55 | 0.09 |
| Local adverse events | |||
| Pain | / | 27 | 0.021 |
| Redness | / | 4 | 0.384 |
| Rash | / | 7 | 0.248 |
| Systemic adverse events | |||
| Fatigue | 1 | 6 | 0.9102 |
| Dizziness | / | 3 | 0.451 |
| Diarrhea | / | 1 | 0.664 |
| Laryngeal pain | / | / | / |
| Cough | / | 1 | 0.664 |
| Chest distress | / | / | / |
| Chest pain | / | / | / |
| Chill | / | / | / |
| Proteinuria | 2 | / | 0.001 |
| Elevated blood pressure | / | / | / |
| Fever | 1 | 1 | 0.185 |
| Inappetence | / | / | / |
| Muscle pain | / | 2 | 0.541 |
| Nausea | 1 | 4 | 0.806 |
| Palpitation | / | / | / |
| Pruitus | / | / | / |
| Grade 3 and 4 adverse events | 1 | / | 0.022 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.; He, J.; Tang, B.; Zhou, Q.; Hu, Y.; Yu, Y.; Chen, J.; Liu, Y.; Li, C.; Ren, H.; et al. Cellular and Humoral Responses to Recombinant and Inactivated SARS-CoV-2 Vaccines in CKD Patients: An Observational Study. J. Clin. Med. 2023, 12, 1225. https://doi.org/10.3390/jcm12031225
Zhang S, He J, Tang B, Zhou Q, Hu Y, Yu Y, Chen J, Liu Y, Li C, Ren H, et al. Cellular and Humoral Responses to Recombinant and Inactivated SARS-CoV-2 Vaccines in CKD Patients: An Observational Study. Journal of Clinical Medicine. 2023; 12(3):1225. https://doi.org/10.3390/jcm12031225
Chicago/Turabian StyleZhang, Siliang, Jiaoxia He, Bin Tang, Qin Zhou, Yudong Hu, Yuan Yu, Jianwei Chen, Yi Liu, Chunmeng Li, Hong Ren, and et al. 2023. "Cellular and Humoral Responses to Recombinant and Inactivated SARS-CoV-2 Vaccines in CKD Patients: An Observational Study" Journal of Clinical Medicine 12, no. 3: 1225. https://doi.org/10.3390/jcm12031225
APA StyleZhang, S., He, J., Tang, B., Zhou, Q., Hu, Y., Yu, Y., Chen, J., Liu, Y., Li, C., Ren, H., & Liao, X. (2023). Cellular and Humoral Responses to Recombinant and Inactivated SARS-CoV-2 Vaccines in CKD Patients: An Observational Study. Journal of Clinical Medicine, 12(3), 1225. https://doi.org/10.3390/jcm12031225






