Proposed Implementation of a Patient-Centered Self-Assessment Tool for Patients with Neuroendocrine Tumors among Academic and Community Practice Sites: The City of Hope Model
Abstract
1. Introduction
2. Patient-Centered Assessments
2.1. Disease-Agnostic Patient Assessments: Quality of Life
2.2. NET-Specific Patient Assessments
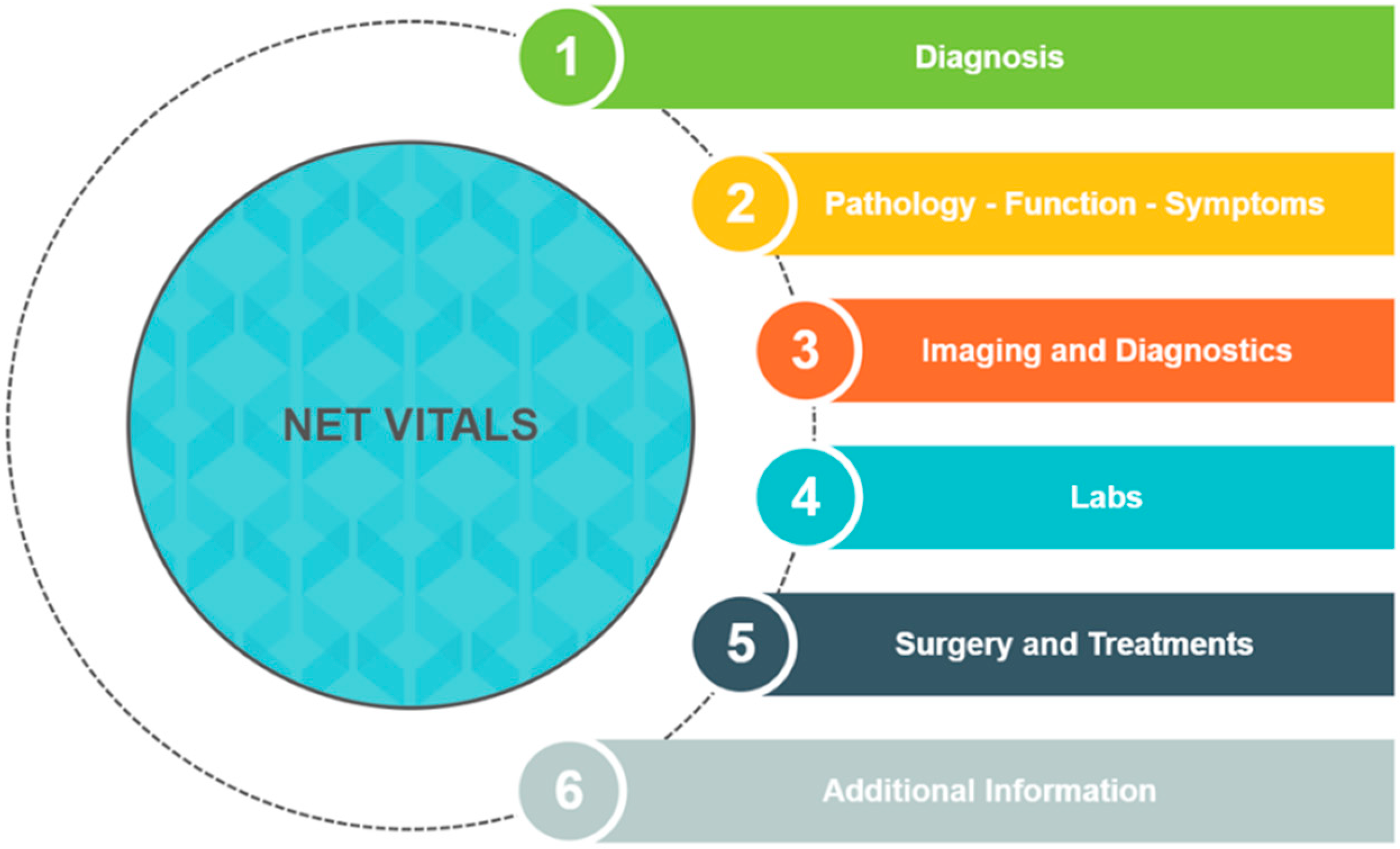
3. NET VITALS
4. Implementation of NET VITALS: The City of Hope Model
5. Opportunities and Challenges of NET VITALS Integration within the City of Hope Enterprise
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yao, J.C.; Hassan, M.; Phan, A.; Dagohoy, C.; Leary, C.; Mares, J.E.; Abdalla, E.K.; Fleming, J.B.; Vauthey, J.-N.; Rashid, A.; et al. One hundred years after “carcinoid”: Epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J. Clin. Oncol. 2008, 26, 3063–3072. [Google Scholar] [CrossRef] [PubMed]
- Dasari, A.; Shen, C.; Halperin, D.; Zhao, B.; Zhou, S.; Xu, Y.; Shih, T.; Yao, J.C. Trends in the Incidence, Prevalence, and Survival Outcomes in Patients with Neuroendocrine Tumors in the United States. JAMA Oncol. 2017, 3, 1335–1342. [Google Scholar] [CrossRef] [PubMed]
- Surveillance, Epidemiology, and End Results (SEER) Program SEER*Stat Database. Available online: https://seer.cancer.gov/ (accessed on 13 July 2021).
- Kulke, M.H.; Hörsch, D.; Caplin, M.E.; Anthony, L.B.; Bergsland, E.; Öberg, K.; Welin, S.; Warner, R.R.P.; Lombard-Bohas, C.; Kunz, P.L.; et al. Telotristat Ethyl, a Tryptophan Hydroxylase Inhibitor for the Treatment of Carcinoid Syndrome. J. Clin. Oncol. 2017, 35, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Kulke, M.H.; Kennecke, H.F.; Murali, K.; Joish, V.N. Changes in Carcinoid Syndrome Symptoms Among Patients Receiving Telotristat Ethyl in US Clinical Practice: Findings from the TELEPRO-II Real-World Study. Cancer Manag. Res. 2021, 13, 7439–7446. [Google Scholar] [CrossRef] [PubMed]
- Vinik, A.I.; Woltering, E.A.; Warner, R.R.P.; Caplin, M.; O’Dorisio, T.M.; Wiseman, G.A.; Coppola, D.; Go, V.L.W. NANETS Consensus Guidelines for the Diagnosis of Neuroendocrine Tumor. Pancreas 2010, 39, 713–734. [Google Scholar] [CrossRef]
- Singh, S.; Granberg, D.; Wolin, E.; Warner, R.; Sissons, M.; Kolarova, T.; Goldstein, G.; Pavel, M.; Öberg, K.; Leyden, J. Patient-Reported Burden of a Neuroendocrine Tumor (NET) Diagnosis: Results from the First Global Survey of Patients with NETs. J. Glob. Oncol. 2017, 3, 43–53. [Google Scholar] [CrossRef]
- Wolin, E.M.; Leyden, J.; Goldstein, G.; Kolarova, T.; Hollander, R.; Warner, R.R.P. Patient-Reported Experience of Diagnosis, Management, and Burden of Neuroendocrine Tumors: Results from a Large Patient Survey in the United States. Pancreas 2017, 46, 639–647. [Google Scholar] [CrossRef]
- Feinberg, Y.; Law, C.; Singh, S.; Wright, F.C. Patient experiences of having a neuroendocrine tumour: A qualitative study. Eur. J. Oncol. Nurs. 2013, 17, 541–545. [Google Scholar] [CrossRef]
- Berger, O.; Grønberg, B.H.; Loge, J.H.; Kaasa, S.; Sand, K. Cancer patients’ knowledge about their disease and treatment before, during and after treatment: A prospective, longitudinal study. BMC Cancer 2018, 18, 381. [Google Scholar] [CrossRef]
- Yang, T.; Tan, T.; Yang, J.; Pan, J.; Hu, C.; Li, J.; Zou, Y. The impact of using three-dimensional printed liver models for patient education. J. Int. Med Res. 2018, 46, 1570–1578. [Google Scholar] [CrossRef]
- Aaronson, N.K.; Ahmedzai, S.; Bergman, B.; Bullinger, M.; Cull, A.; Duez, N.J.; Filiberti, A.; Flechtner, H.; Fleishman, S.B.; De Haes, J.C.J.M.; et al. The European Organization for Research and Treatment of Cancer QLQ-C30: A Quality-of-Life Instrument for Use in International Clinical Trials in Oncology. J. Natl. Cancer Inst. 1993, 85, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Galle, P.R.; Finn, R.S.; Qin, S.; Ikeda, M.; Zhu, A.X.; Kim, T.-Y.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.; et al. Patient-reported outcomes with atezolizumab plus bevacizumab versus sorafenib in patients with unresectable hepatocellular carcinoma (IMbrave150): An open-label, randomised, phase 3 trial. Lancet Oncol. 2021, 22, 991–1001. [Google Scholar] [CrossRef] [PubMed]
- Harbeck, N.; Iyer, S.; Turner, N.; Cristofanilli, M.; Ro, J.; André, F.; Loi, S.; Verma, S.; Iwata, H.; Bhattacharyya, H.; et al. Quality of life with palbociclib plus fulvestrant in previously treated hormone receptor-positive, HER2-negative metastatic breast cancer: Patient-reported outcomes from the PALOMA-3 trial. Ann. Oncol. 2016, 27, 1047–1054. [Google Scholar] [CrossRef] [PubMed]
- Hui, R.; Özgüroğlu, M.; Villegas, A.; Daniel, D.; Vicente, D.; Murakami, S.; Yokoi, T.; Chiappori, A.; Lee, K.H.; de Wit, M.; et al. Patient-reported outcomes with durvalumab after chemoradiotherapy in stage III, unresectable non-small-cell lung cancer (PACIFIC): A randomised, controlled, phase 3 study. Lancet Oncol. 2019, 20, 1670–1680. [Google Scholar] [CrossRef] [PubMed]
- Case, S.M.; Fried, T.R.; O’Leary, J. How to ask: Older adults’ preferred tools in health outcome prioritization. Patient Educ. Couns. 2013, 91, 29–36. [Google Scholar] [CrossRef]
- Fried, T.R.; Tinetti, M.; Agostini, J.; Iannone, L.; Towle, V. Health outcome prioritization to elicit preferences of older persons with multiple health conditions. Patient Educ. Couns. 2011, 83, 278–282. [Google Scholar] [CrossRef] [PubMed]
- Case, S.M.; Towle, V.R.; Fried, T.R. Considering the Balance: Development of a Scale to Assess Patient Views on Trade-Offs in Competing Health Outcomes. J. Am. Geriatr. Soc. 2013, 61, 1331–1336. [Google Scholar] [CrossRef]
- El-Jawahri, A.; Traeger, L.; Park, E.R.; Greer, J.A.; Pirl, W.; Lennes, I.T.; Jackson, V.A.; Gallagher, E.R.; Temel, J.S. Associations among prognostic understanding, quality of life, and mood in patients with advanced cancer. Cancer 2014, 120, 278–285. [Google Scholar] [CrossRef]
- Nipp, R.D.; Greer, J.A.; El-Jawahri, A.; Moran, S.M.; Traeger, L.; Jacobs, J.M.; Jacobsen, J.C.; Gallagher, E.R.; Park, E.R.; Ryan, D.P.; et al. Coping and Prognostic Awareness in Patients with Advanced Cancer. J. Clin. Oncol. 2017, 35, 2551–2557. [Google Scholar] [CrossRef]
- Brazier, J.E.; Harper, R.; Jones, N.M.; O’Cathain, A.; Thomas, K.J.; Usherwood, T.; Westlake, L. Validating the SF-36 health survey questionnaire: New outcome measure for primary care. BMJ 1992, 305, 160–164. [Google Scholar] [CrossRef]
- Vinik, A.; Bottomley, A.; Korytowsky, B.; Bang, Y.-J.; Raoul, J.-L.; Valle, J.W.; Metrakos, P.; Hörsch, D.; Mundayat, R.; Reisman, A.; et al. Patient-Reported Outcomes and Quality of Life with Sunitinib Versus Placebo for Pancreatic Neuroendocrine Tumors: Results from an International Phase III Trial. Target. Oncol. 2016, 11, 815–824. [Google Scholar] [CrossRef] [PubMed]
- Strosberg, J.; Wolin, E.; Chasen, B.; Kulke, M.; Bushnell, D.; Caplin, M.; Baum, R.P.; Kunz, P.; Hobday, T.; Hendifar, A.; et al. Health-Related Quality of Life in Patients with Progressive Midgut Neuroendocrine Tumors Treated With 177Lu-Dotatate in the Phase III NETTER-1 Trial. J. Clin. Oncol. 2018, 36, 2578–2584. [Google Scholar] [CrossRef] [PubMed]
- Martini, C.; Buxbaum, S.; Rodrigues, M.; Nilica, B.; Scarpa, L.; Holzner, B.; Virgolini, I.; Gamper, E.-M. Quality of Life in Patients with Metastatic Gastroenteropancreatic Neuroendocrine Tumors Receiving Peptide Receptor Radionuclide Therapy: Information from a Monitoring Program in Clinical Routine. J. Nucl. Med. 2018, 59, 1566–1573. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Navalkissoor, S.; Quigley, A.-M.; Gnanasegaran, G.; Mandair, D.; Toumpanakis, C.; Caplin, M.E.; Hayes, A.R. 177Lu-DOTATATE in older patients with metastatic neuroendocrine tumours: Safety, efficacy and health-related quality of life. Eur. J. Nucl. Med. 2021, 48, 3582–3594. [Google Scholar] [CrossRef]
- Sorbye, H.; Meyer, L.S.; Mordal, K.E.; Myhre, S.; Thiis-Evensen, E. Patient reported symptoms, coping and quality of life during somatostatin analogue treatment for metastatic small- intestinal neuroendocrine tumours. Health Qual. Life Outcomes 2020, 18, 188. [Google Scholar] [CrossRef]
- Scandurra, C.; Modica, R.; Maldonato, N.M.; Dolce, P.; Dipietrangelo, G.G.; Centello, R.; Di Vito, V.; Bottiglieri, F.; de Cicco, F.; Giannetta, E.; et al. Quality of Life in Patients with Neuroendocrine Neoplasms: The Role of Severity, Clinical Heterogeneity, and Resilience. J. Clin. Endocrinol. Metab. 2020, 106, e316–e327. [Google Scholar] [CrossRef]
- Haugland, T.; Veenstra, M.; Vatn, M.H.; Wahl, A.K. Improvement in Stress, General Self-Efficacy, and Health Related Quality of Life following Patient Education for Patients with Neuroendocrine Tumors: A Pilot Study. Nurs. Res. Pract. 2013, 2013, 695820. [Google Scholar] [CrossRef]
- Li, D.; Sun, C.-L.; Kim, H.; Crook, C.; Zhang, Y.-H.; Allen, R.; Ballena, R.; Hyder, S.; Koczywas, M.; Chung, V.; et al. Patient-Defined Goals and Preferences Among Adults with Advanced Neuroendocrine Tumors. J. Natl. Compr. Canc. Netw. 2022, 20, 1330–1337.e1333. [Google Scholar] [CrossRef]
- Coughlin, C.C.; Pérez, M.; Kumar, M.G.; Jeffe, D.B.; Bayliss, S.J.; Sternhell-Blackwell, K. Skin cancer risk education in pediatric solid organ transplant patients: An evaluation of knowledge, behavior, and perceptions over time. Pediatr. Transplant. 2017, 21, e12817. [Google Scholar] [CrossRef]
- Leyden, J.; Pavlakis, N.; Chan, D.; Michael, M.; Clarke, S.; Khasraw, M.; Price, T. Patient-reported experience of the impact and burden of neuroendocrine tumors: Oceania patient results from a large global survey. Asia-Pacific J. Clin. Oncol. 2018, 14, 256–263. [Google Scholar] [CrossRef]
- Leyden, S.; Kolarova, T.; Bouvier, C.; Caplin, M.; Conroy, S.; Davies, P.; Dureja, S.; Falconi, M.; Ferolla, P.; Fisher, G.; et al. Unmet needs in the international neuroendocrine tumor (NET) community: Assessment of major gaps from the perspective of patients, patient advocates and NET health care professionals. Int. J. Cancer 2020, 146, 1316–1323. [Google Scholar] [CrossRef] [PubMed]
- Davies, A.H.G.; Larsson, G.; Ardill, J.; Friend, E.; Jones, L.; Falconi, M.; Bettini, R.; Koller, M.; Sezer, O.; Fleissner, C.; et al. Development of a disease-specific quality of life questionnaire module for patients with gastrointestinal neuroendocrine tumours. Eur. J. Cancer 2006, 42, 477–484. [Google Scholar] [CrossRef]
- Yadegarfar, G.; Friend, L.; Jones, L.; Plum, L.M.; Ardill, J.; Taal, B.; Larsson, G.; Jeziorski, K.; Kwekkeboom, D.; Ramage, J.K.; et al. Validation of the EORTC QLQ-GINET21 questionnaire for assessing quality of life of patients with gastrointestinal neuroendocrine tumours. Br. J. Cancer 2013, 108, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Milanetto, A.C.; Nordenström, E.; Sundlöv, A.; Almquist, M. Health-Related Quality of Life After Surgery for Small Intestinal Neuroendocrine Tumours. World J. Surg. 2018, 42, 3231–3239. [Google Scholar] [CrossRef] [PubMed]
- Spolverato, G.; Bagante, F.; Wagner, D.; Buettner, S.; Gupta, R.; Kim, Y.; Maqsood, H.; Pawlik, T.M. Quality of life after treatment of neuroendocrine liver metastasis. J. Surg. Res. 2015, 198, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Vinik, E.; Carlton, C.A.; Silva, M.P.; Vinik, A.I. Development of the Norfolk Quality of Life Tool for Assessing Patients with Neuroendocrine Tumors. Pancreas 2009, 38, e87–e95. [Google Scholar] [CrossRef]
- Bouma, G.; de Hosson, L.D.; van Woerkom, C.E.; van Essen, H.; de Bock, G.H.; Admiraal, J.M.; Reyners, A.K.L.; Walenkamp, A.M.E. Web-based information and support for patients with a newly diagnosed neuroendocrine tumor: A feasibility study. Support. Care Cancer 2017, 25, 2075–2083. [Google Scholar] [CrossRef]
- De Hosson, L.D.; Bouma, G.; Stelwagen, J.; Van Essen, H.; De Bock, G.H.; De Groot, D.J.A.; De Vries, E.G.E.; Walenkamp, A.M.E. Web-based personalised information and support for patients with a neuroendocrine tumour: Randomised controlled trial. Orphanet J. Rare Dis. 2019, 14, 60. [Google Scholar] [CrossRef]
- NET Cancer Health Storylines: All of Your Tools for Managing NET Cancer in One Place. Available online: https://www.healthstorylines.com/net-cancer-healthstorylines (accessed on 21 October 2022).
- Adams, J.R.; Ray, D.; Willmon, R.; Pulgar, S.; Dasari, A. Living with Neuroendocrine Tumors: Assessment of Quality of Life Through a Mobile Application. JCO Clin. Cancer Informatics 2019, 3, 1–10. [Google Scholar] [CrossRef]
- Wagner, M.; Samaha, D.; Khoury, H.; O’Neil, W.M.; Lavoie, L.; Bennetts, L.; Badgley, D.; Gabriel, S.; Berthon, A.; Dolan, J.; et al. Development of a Framework Based on Reflective MCDA to Support Patient–Clinician Shared Decision-Making: The Case of the Management of Gastroenteropancreatic Neuroendocrine Tumors (GEP-NET) in the United States. Adv. Ther. 2017, 35, 81–99. [Google Scholar] [CrossRef]
- Li, D.; Imbesi, G.J.; Yen, L.; Kim, H.; Sun, C.-L.; Crook, C.J.; Ballena, R.; Zhang, Y.-H.; Allen, R.; Sedrak, M.; et al. Feasibility and Satisfaction of Using NET VITALS Self-assessment Tool Among Patients with Neuroendocrine Tumors. Pancreas 2022, 51, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Learn Advocate Connect Neuroendocrine Tumor Society. NET VITALS-Your NET Communication Tool. Available online: https://www.lacnets.org/netvitals (accessed on 13 July 2021).
- Salgia, R.; Kulkarni, P. Integrating Clinical and Translational Research Networks—Building Team Medicine. J. Clin. Med. 2020, 9, 2975. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crook, C.J.; Yen, L.; Ta, K.; Karimi, M.; Nguyen, D.; Lee, R.T.; Li, D. Proposed Implementation of a Patient-Centered Self-Assessment Tool for Patients with Neuroendocrine Tumors among Academic and Community Practice Sites: The City of Hope Model. J. Clin. Med. 2023, 12, 1229. https://doi.org/10.3390/jcm12031229
Crook CJ, Yen L, Ta K, Karimi M, Nguyen D, Lee RT, Li D. Proposed Implementation of a Patient-Centered Self-Assessment Tool for Patients with Neuroendocrine Tumors among Academic and Community Practice Sites: The City of Hope Model. Journal of Clinical Medicine. 2023; 12(3):1229. https://doi.org/10.3390/jcm12031229
Chicago/Turabian StyleCrook, Christiana Joy, Lisa Yen, Kathleen Ta, Misagh Karimi, Danny Nguyen, Richard T. Lee, and Daneng Li. 2023. "Proposed Implementation of a Patient-Centered Self-Assessment Tool for Patients with Neuroendocrine Tumors among Academic and Community Practice Sites: The City of Hope Model" Journal of Clinical Medicine 12, no. 3: 1229. https://doi.org/10.3390/jcm12031229
APA StyleCrook, C. J., Yen, L., Ta, K., Karimi, M., Nguyen, D., Lee, R. T., & Li, D. (2023). Proposed Implementation of a Patient-Centered Self-Assessment Tool for Patients with Neuroendocrine Tumors among Academic and Community Practice Sites: The City of Hope Model. Journal of Clinical Medicine, 12(3), 1229. https://doi.org/10.3390/jcm12031229





