How to Solve the Conundrum of Heparin-Induced Thrombocytopenia during Cardiopulmonary Bypass
Abstract
1. Introduction
2. Pathophysiology of HIT
3. Standard Heparin Protocol
4. Alternative Protocols
4.1. Non-Heparin Anticoagulants
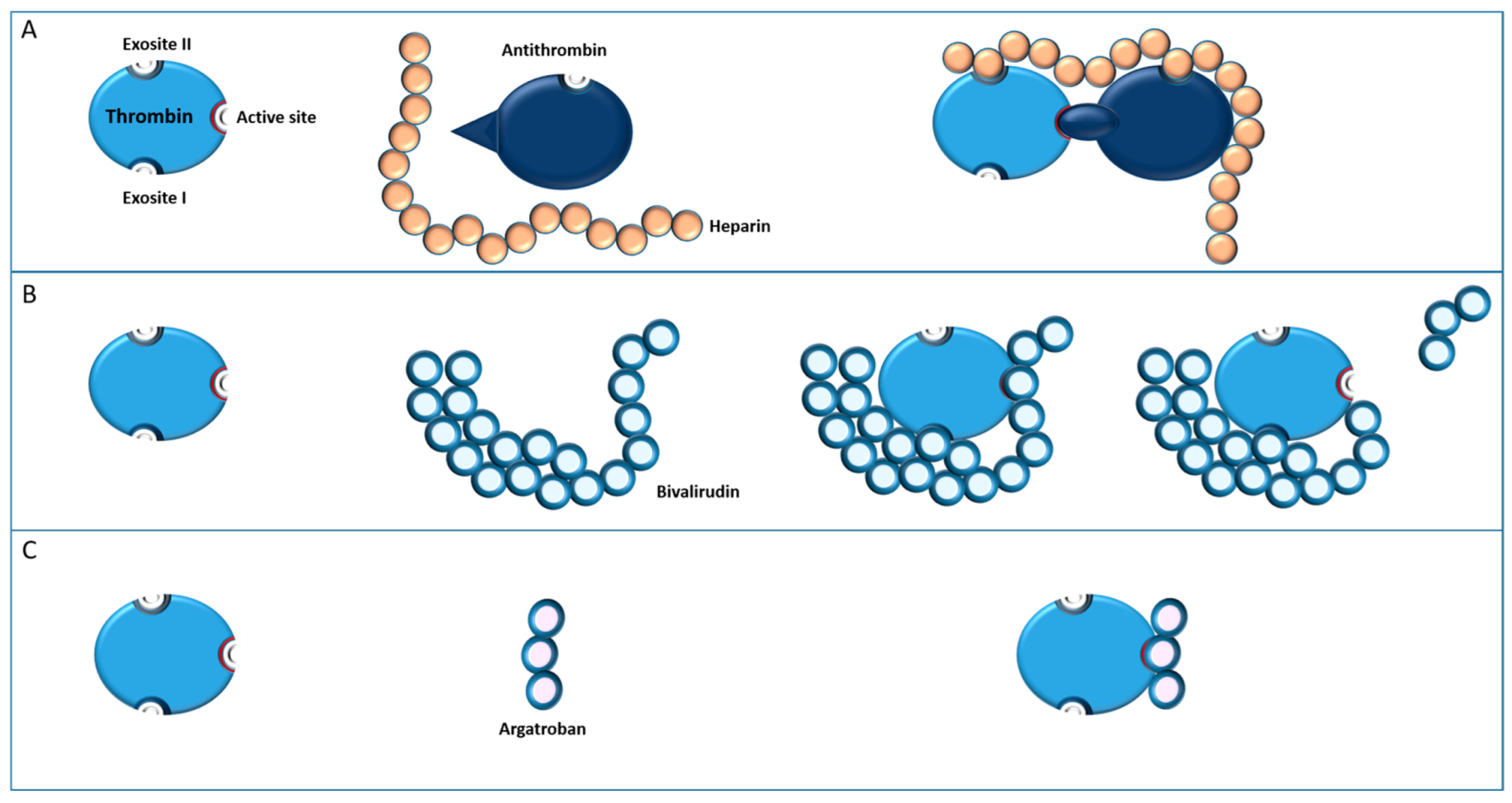
4.1.1. Bivalirudin
4.1.2. Argatroban
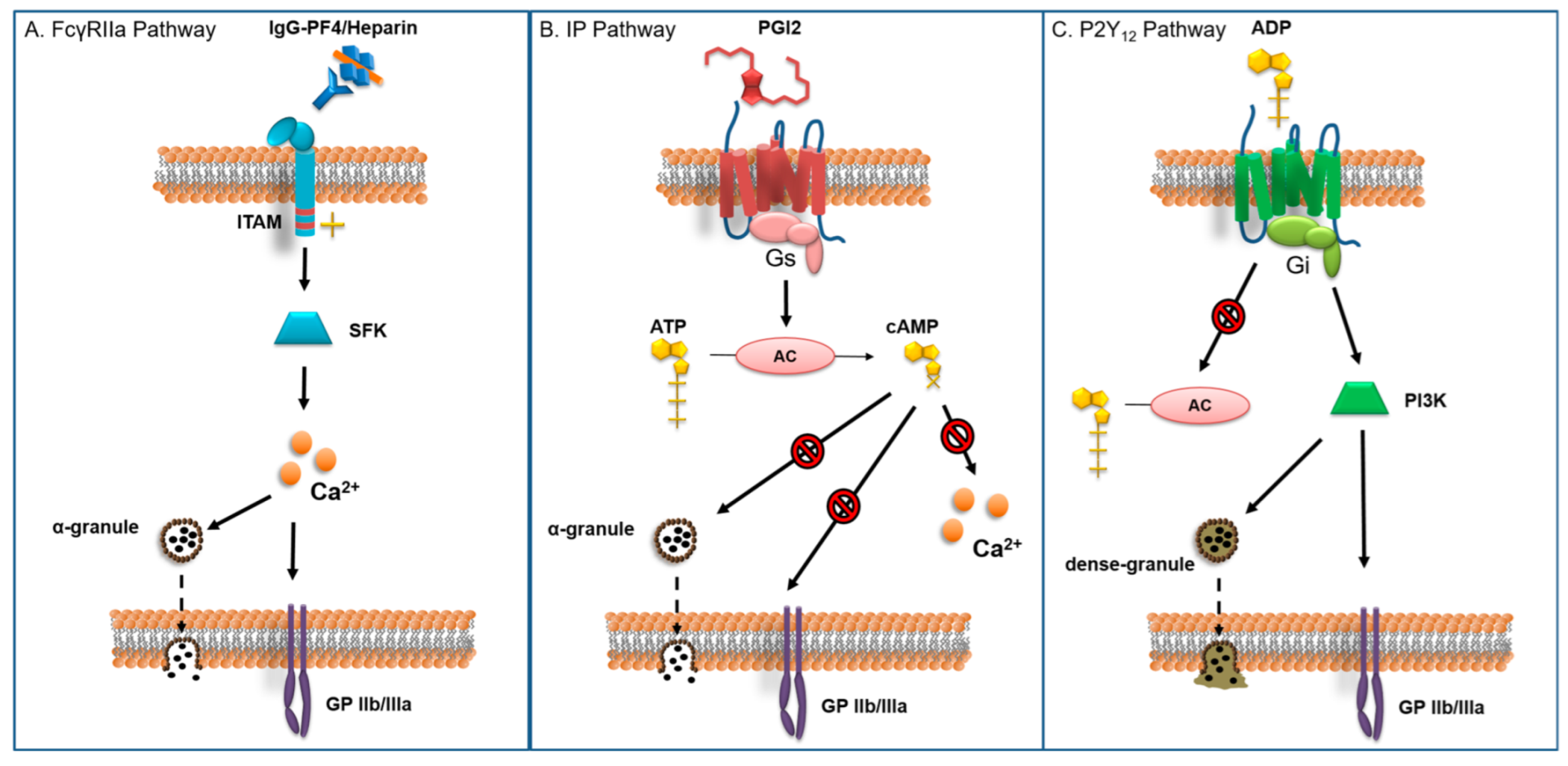
4.2. Heparin Associated with Potent Antiplatelet Agents
4.2.1. Prostacyclin Receptor Agonists
Epoprostenol
Iloprost
4.2.2. GP IIb/IIIa Antagonists
4.2.3. P2Y12 Receptor Antagonists
4.3. Intravenous Immunoglobulin (IVIG)
5. Conclusions
6. How We Do It in Lausanne
6.1. Rationale
6.2. HIT Phases and Timing
6.3. Dosing, and Practical Aspects (Table 2)
6.3.1. General Aspects
| Cangrelor | Tirofiban | Bivalirudin | ||
|---|---|---|---|---|
| Surgical flush solution | Citrate 3.8% | Citrate 3.8% | Citrate 3.8% | |
| Cell Saver solution | Citrate 4% | Citrate 4% | Citrate 4% | |
| Pump prime | 50 mg | |||
| Before CPB | Bolus | 30 µg/kg | 10 µg/kg | 1 mg/kg |
| perfusion | 4 µg/kg/min | 0.15 µg/kg/min | 2.5 mg/kg/h | |
| During CPB | perfusion | 4 µg/kg/min | 0.15 µg/kg/min | 2.5 mg/kg/h |
| bolus | 0.1–0.5 mg/kg | |||
| End of perfusion | 15 prior to end of CPB | 1 h prior to end of CPB | At start of protamine | |
| Bolus in circuit post CPB | 50 mg | |||
| Unplanned return on CPB | Bolus 30 µg/kg Perfusion 4 µg/kg/min | Bolus 5 µg/kg | Bolus 0.5 mg/kg Perfusion 2.5 mg/kg/h | |
6.3.2. Cangrelor-Heparin Protocol
- Preparation
- Drug administration before and during bypass
- End of bypass
- After bypass
6.3.3. Tirofiban-Heparin Protocol
- Preparation
- Drug administration before and during bypass:
- End of bypass
- After bypass
6.3.4. Bivalirudin Protocol
- Preparation
- Drug administration before and during bypass:
- Additional measures during bypass
- End of bypass
- After bypass
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Salter, B.S.; Weiner, M.M.; Trinh, M.A.; Heller, J.; Evans, A.S.; Adams, D.H.; Fischer, G.W. Heparin-induced thrombocytopenia: A comprehensive clinical review. J. Am. Coll. Cardiol. 2016, 67, 2519–2532. [Google Scholar] [CrossRef] [PubMed]
- Arepally, G.M.; Ortel, T.L. Clinical practice. Heparin-induced thrombocytopenia. N. Engl. J. Med. 2006, 355, 809–817. [Google Scholar] [CrossRef] [PubMed]
- Warkentin, T.E. The paradox of heparin-induced thrombocytopenia. J. Thromb. Haemost. 2009, 7, 1472–1473. [Google Scholar] [CrossRef]
- Greinacher, A.; Eichler, P.; Lubenow, N.; Kwasny, H.; Luz, M. Heparin-induced thrombocytopenia with thromboembolic complications: Meta-analysis of 2 prospective trials to assess the value of parenteral treatment with lepirudin and its therapeutic aPTT range. Blood 2000, 96, 846–851. [Google Scholar] [CrossRef] [PubMed]
- Warkentin, T.E.; Kelton, J.G. A 14-year study of heparin-induced thrombocytopenia. Am. J. Med. 1996, 101, 502–507. [Google Scholar] [CrossRef]
- Greinacher, A. Clinical practice. Heparin-Induced Thrombocytopenia. N. Engl. J. Med. 2015, 373, 252–261. [Google Scholar] [CrossRef]
- Marchetti, M.; Zermatten, M.G.; Bertaggia Calderara, D.; Aliotta, A.; Alberio, L. Heparin-Induced Thrombocytopenia: A Review of New Concepts in Pathogenesis, Diagnosis, and Management. J. Clin. Med. 2021, 10, 683. [Google Scholar] [CrossRef]
- Mayo, K.H.; Ilyina, E.; Roongta, V.; Dundas, M.; Joseph, J.; Lai, C.K.; Maione, T.; Daly, T.J. Heparin binding to platelet factor-4. An NMR and site-directed mutagenesis study: Arginine residues are crucial for binding. Biochem. J. 1995, 312 Pt 2, 357–365. [Google Scholar] [CrossRef]
- Khandelwal, S.; Lee, G.M.; Hester, C.G.; Poncz, M.; McKenzie, S.E.; Sachais, B.S.; Rauova, L.; Kelsoe, G.; Cines, D.B.; Frank, M.; et al. The antigenic complex in HIT binds to B cells via complement and complement receptor 2 (CD21). Blood 2016, 128, 1789–1799. [Google Scholar] [CrossRef]
- Qiao, J.; Al-Tamimi, M.; Baker, R.I.; Andrews, R.K.; Gardiner, E.E. The platelet Fc receptor, FcγRIIa. Immunol. Rev. 2015, 268, 241–252. [Google Scholar] [CrossRef]
- Braune, S.; Küpper, J.H.; Jung, F. Effect of prostanoids on human platelet function: An overview. Int. J. Mol. Sci. 2020, 21, 9020. [Google Scholar] [CrossRef] [PubMed]
- Dorsam, R.T.; Kunapuli, S.P. Central role of the P2Y12 receptor in platelet activation. J. Clin. Investig. 2004, 113, 340–345. [Google Scholar] [CrossRef] [PubMed]
- Warkentin, T.E.; Hayward, C.P.; Boshkov, L.K.; Santos, A.V.; Sheppard, J.A.; Bode, A.P.; Kelton, J.G. Sera from patients with heparin-induced thrombocytopenia generate platelet-derived microparticles with procoagulant activity: An explanation for the thrombotic complications of heparin-induced thrombocytopenia. Blood 1994, 84, 3691–3699. [Google Scholar] [CrossRef] [PubMed]
- Greinacher, A.; Selleng, K.; Warkentin, T.E. Autoimmune heparin-induced thrombocytopenia. J. Thromb. Haemost. 2017, 15, 2099–2114. [Google Scholar] [CrossRef] [PubMed]
- Warkentin, T.E.; Kelton, J.G. Temporal aspects of heparin-induced thrombocytopenia. N. Engl. J. Med. 2001, 344, 1286–1292. [Google Scholar] [CrossRef] [PubMed]
- Cuker, A. Management of the multiple phases of heparin-induced thrombocytopenia. Thromb. Haemost. 2016, 116, 835–842. [Google Scholar] [CrossRef]
- Cuker, A.; Arepally, G.M.; Chong, B.H.; Cines, D.B.; Greinacher, A.; Gruel, Y.; Linkins, L.A.; Rodner, S.B.; Selleng, S.; Warkentin, T.E.; et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: Heparin-induced thrombocytopenia. Blood. Adv. 2018, 2, 3360–3392. [Google Scholar] [CrossRef] [PubMed]
- Chong, B.H.; Burgess, J.; Ismail, F. The clinical usefulness of the platelet aggregation test for the diagnosis of heparin-induced thrombocytopenia. Thromb. Haemost. 1993, 69, 344–350. [Google Scholar] [CrossRef]
- Warkentin, T.E.; Hayward, C.P.; Smith, C.A.; Kelly, P.M.; Kelton, J.G. Determinants of donor platelet variability when testing for heparin-induced thrombocytopenia. J. Lab. Clin. Med. 1992, 120, 371–379. [Google Scholar]
- Greinacher, A.; Michels, I.; Kiefel, V.; Mueller-Eckhardt, C. A rapid and sensitive test for diagnosing heparin-associated thrombocytopenia. Thromb. Haemost. 1991, 66, 734–736. [Google Scholar] [CrossRef]
- Koster, A.; Erdoes, G.; Nagler, M.; Birschmann, I.; Alberio, L. How would we treat our own heparin-induced thrombocytopenia during cardiac surgery? J. Cardiothorac. Vasc. Anesth. 2021, 35, 1585–1593. [Google Scholar] [CrossRef]
- Alberio, L.; Angelillo-Scherrer, A.; Asmis, L.; Casini, A.; Fontana, P.; Graf, L.; Hegemann, I.; Kremer Hovinga, J.A.; Korte, W.; Lecompte, T.; et al. Recommendations on the use of anticoagulants for the treatment of patients with heparin-induced thrombocytopenia in Switzerland. Swiss Med. Wkly. 2020, 150, w20210. [Google Scholar] [CrossRef] [PubMed]
- Wahba, A.; Milojevic, M.; Boer, C.; De Somer, F.; Gudbjartsson, T.; van den Goor, J.; Jones, T.J.; Lomivorotov, V.; Merkle, F.; Ranucci, M.; et al. 2019 EACTS/EACTA/EBCP guidelines on cardiopulmonary bypass in adult cardiac surgery. Eur. J. Cardiothorac. Surg. 2020, 57, 210–251. [Google Scholar] [CrossRef]
- Finley, A.; Greenberg, C. Review article: Heparin sensitivity and resistance: Management during cardiopulmonary bypass. Anesth. Analg. 2013, 116, 1210–1222. [Google Scholar] [CrossRef] [PubMed]
- Øvrum, E.; Tangen, G.; Tølløfsrud, S.; Skeie, B.; Ringdal, M.A.; Istad, R.; Øystese, R. Heparinized cardiopulmonary bypass circuits and low systemic anticoagulation: An analysis of nearly 6000 patients undergoing coronary artery bypass grafting. J. Thorac. Cardiovasc. Surg. 2011, 141, 1145–1149. [Google Scholar] [CrossRef]
- Boer, C.; Meesters, M.I.; Veerhoek, D.; Vonk, A.B.A. Anticoagulant and side-effects of protamine in cardiac surgery: A narrative review. Br. J. Anaesth. 2018, 120, 914–927. [Google Scholar] [CrossRef] [PubMed]
- Bauer, A.; Hausmann, H.; Schaarschmidt, J.; Szlapka, M.; Scharpenberg, M.; Eberle, T.; Hasenkam, J.M. Is 300 seconds ACT safe and efficient during MiECC procedures? Thorac. Cardiovasc. Surg. 2019, 67, 191–202. [Google Scholar] [CrossRef]
- Koster, A.; Faraoni, D.; Levy, J.H. Argatroban and bivalirudin for perioperative anticoagulation in cardiac surgery. Anesthesiology 2018, 128, 390–400. [Google Scholar] [CrossRef]
- Wanat-Hawthorne, A.; Tanaka, K.; Angona, R.; Feng, C.; Eaton, M. Survey of practice pattern in patients with heparin-induced thrombocytopenia requiring cardiopulmonary bypass. Anesth. Analg. 2021, 133, 1180–1186. [Google Scholar] [CrossRef]
- Shore-Lesserson, L.; Baker, R.A.; Ferraris, V.A.; Greilich, P.E.; Fitzgerald, D.; Roman, P.; Hammon, J.W. The society of thoracic surgeons, the society of cardiovascular anesthesiologists, and the American Society of ExtraCorporeal Technology: Clinical practice guidelines-anticoagulation during cardiopulmonary bypass. Anesth. Analg. 2018, 126, 413–424. [Google Scholar] [CrossRef]
- Bouraghda, A.; Gillois, P.; Albaladejo, P. Alternatives to heparin and protamine anticoagulation for cardiopulmonary bypass in cardiac surgery. Can. J. Anaesth. 2015, 62, 518–528. [Google Scholar] [CrossRef]
- Lee, C.J.; Ansell, J.E. Direct thrombin inhibitors. Br. J. Clin. Pharmacol. 2011, 72, 581–592. [Google Scholar] [CrossRef] [PubMed]
- Shammas, N.W. Bivalirudin: Pharmacology and clinical applications. Cardiovasc. Drug Rev. 2005, 23, 345–360. [Google Scholar] [CrossRef] [PubMed]
- Warkentin, T.E.; Greinacher, A.; Koster, A. Bivalirudin. Thromb. Haemost. 2008, 99, 830–839. [Google Scholar] [PubMed]
- Merry, A.F.; Raudkivi, P.J.; Middleton, N.G.; McDougall, J.M.; Nand, P.; Mills, B.P.; Webber, B.J.; Frampton, C.M.; White, H.D. Bivalirudin versus heparin and protamine in off-pump coronary artery bypass surgery. Ann. Thorac. Surg. 2004, 77, 925–931, discussion 931. [Google Scholar] [CrossRef]
- Smedira, N.G.; Dyke, C.M.; Koster, A.; Jurmann, M.; Bhatia, D.S.; Hu, T.; McCarthy, H.L., 2nd; Lincoff, A.M.; Spiess, B.D.; Aronson, S. Anticoagulation with bivalirudin for off-pump coronary artery bypass grafting: The results of the EVOLUTION-OFF study. J. Thorac. Cardiovasc. Surg. 2006, 131, 686–692. [Google Scholar] [CrossRef]
- Dyke, C.M.; Smedira, N.G.; Koster, A.; Aronson, S.; McCarthy, H.L., 2nd; Kirshner, R.; Lincoff, A.M.; Spiess, B.D. A comparison of bivalirudin to heparin with protamine reversal in patients undergoing cardiac surgery with cardiopulmonary bypass: The EVOLUTION-ON study. J. Thorac. Cardiovasc. Surg. 2006, 131, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Dyke, C.M.; Aldea, G.; Koster, A.; Smedira, N.; Avery, E.; Aronson, S.; Spiess, B.D.; Lincoff, A.M. Off-pump coronary artery bypass with bivalirudin for patients with heparin-induced thrombocytopenia or antiplatelet factor four/heparin antibodies. Ann. Thorac. Surg. 2007, 84, 836–839. [Google Scholar] [CrossRef]
- Koster, A.; Dyke, C.M.; Aldea, G.; Smedira, N.G.; McCarthy, H.L., 2nd; Aronson, S.; Hetzer, R.; Avery, E.; Spiess, B.; Lincoff, A.M. Bivalirudin during cardiopulmonary bypass in patients with previous or acute heparin-induced thrombocytopenia and heparin antibodies: Results of the CHOOSE-ON trial. Ann. Thorac. Surg. 2007, 83, 572–577. [Google Scholar] [CrossRef]
- Veale, J.J.; McCarthy, H.M.; Palmer, G.; Dyke, C.M. Use of bivalirudin as an anticoagulant during cardiopulmonary bypass. J. Extra Corpor. Technol. 2005, 37, 296–302. [Google Scholar]
- Sharma, G.H.; SKapoor, P. Perfusion Strategies for Bivalirudin Anticoagulation: AIIMS Protocol. J. Card Crit. Care 2022, 6, 54–58. [Google Scholar] [CrossRef]
- Morshuis, M.; Boergermann, J.; Gummert, J.; Koster, A. A modified technique for implantation of the HeartWare™ left ventricular assist device when using bivalirudin anticoagulation in patients with acute heparin-induced thrombocytopenia. Interact Cardiovasc. Thorac. Surg. 2013, 17, 225–226. [Google Scholar] [CrossRef]
- Awad, H.; Bryant, R.; Malik, O.; Dimitrova, G.; Sai-Sudhakar, C.B. Thrombosis during off pump LVAD placement in a patient with heparin induced thrombocytopenia using bivalirudin. J. Cardiothorac. Surg. 2013, 8, 115. [Google Scholar] [CrossRef] [PubMed]
- Hillebrand, J.; Sindermann, J.; Schmidt, C.; Mesters, R.; Martens, S.; Scherer, M. Implantation of left ventricular assist devices under extracorporeal life support in patients with heparin-induced thrombocytopenia. J. Artif. Organs. 2015, 18, 291–299. [Google Scholar] [CrossRef]
- Martin, M.E.; Kloecker, G.H.; Laber, D.A. Argatroban for anticoagulation during cardiac surgery. Eur. J. Haematol. 2007, 78, 161–166. [Google Scholar] [CrossRef]
- Jeske, W.P.; Fareed, J.; Hoppensteadt, D.A.; Lewis, B.; Walenga, J.M. Pharmacology of argatroban. Expert Rev. Hematol. 2010, 3, 527–539. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, M.; Barelli, S.; Gleich, T.; Gomez, F.J.; Goodyer, M.; Grandoni, F.; Alberio, L. Managing argatroban in heparin-induced thrombocytopenia: A retrospective analysis of 729 treatment days in 32 patients with confirmed heparin-induced thrombocytopenia. Br. J. Haematol. 2022, 197, 766–790. [Google Scholar] [CrossRef] [PubMed]
- Swan, S.K.; Hursting, M.J. The pharmacokinetics and pharmacodynamics of argatroban: Effects of age, gender, and hepatic or renal dysfunction. Pharmacotherapy 2000, 20, 318–329. [Google Scholar] [CrossRef]
- Tanigawa, Y.; Yamada, T.; Matsumoto, K.; Nakagawachi, A.; Torikai, A.; Sakaguchi, Y. Non-recovery of ACT in a patient with heparin-induced thrombocytopenia type II during mitral valve replacement using argatroban anticoagulation. J. Anesth. 2013, 27, 951–955. [Google Scholar] [CrossRef]
- König, G.; Obser, T.; Marggraf, O.; Schneppenheim, S.; Budde, U.; Schneppenheim, R.; Brehm, M.A. Alteration in GPIIb/IIIa Binding of VWD-Associated von Willebrand Factor Variants with C-Terminal Missense Mutations. Thromb. Haemost. 2019, 119, 1102–1111. [Google Scholar] [CrossRef]
- Scala, E.; Gerschheimer, C.; Gomez, F.J.; Alberio, L.; Marcucci, C. Potential and Limitations of the New P2Y12 Inhibitor, Cangrelor, in Preventing Heparin-Induced Platelet Aggregation During Cardiac Surgery: An In Vitro Study. Anesth. Analg. 2020, 131, 622–630. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, S.P.; Mazzeffi, M.A.; Strauss, E.; Hollis, A.; Tanaka, K.A. Prostacyclins in Cardiac Surgery: Coming of Age. Semin. Cardiothorac. Vasc. Anesth. 2018, 22, 306–323. [Google Scholar] [CrossRef] [PubMed]
- Aouifi, A.; Blanc, P.; Piriou, V.; Bastien, O.H.; Ffrench, P.; Hanss, M.; Lehot, J.J. Cardiac surgery with cardiopulmonary bypass in patients with type II heparin-induced thrombocytopenia. Ann. Thorac. Surg. 2001, 71, 678–683. [Google Scholar] [CrossRef] [PubMed]
- Krause, W.; Krais, T. Pharmacokinetics and pharmacodynamics of the prostacyclin analogue iloprost in man. Eur. J. Clin. Pharmacol. 1986, 30, 61–68. [Google Scholar] [CrossRef]
- Whittle, B.J.; Silverstein, A.M.; Mottola, D.M.; Clapp, L.H. Binding and activity of the prostacyclin receptor (IP) agonists, treprostinil and iloprost, at human prostanoid receptors: Treprostinil is a potent DP1 and EP2 agonist. Biochem. Pharmacol. 2012, 84, 68–75. [Google Scholar] [CrossRef]
- Gomberg-Maitland, M.; Olschewski, H. Prostacyclin therapies for the treatment of pulmonary arterial hypertension. Eur. Respir. J. 2008, 31, 891–901. [Google Scholar] [CrossRef]
- Knudsen, L.; Schurawlew, A.; Nickel, N.; Tiede, H.; Ghofrani, H.A.; Wilkens, H.; Ewert, R.; Halank, M.; Klose, H.; Bäzner, C.; et al. Long-term effects of intravenous iloprost in patients with idiopathic pulmonary arterial hypertension deteriorating on non-parenteral therapy. BMC Pulm. Med. 2011, 11, 56. [Google Scholar] [CrossRef]
- Palatianos, G.; Michalis, A.; Alivizatos, P.; Lacoumenda, S.; Geroulanos, S.; Karabinis, A.; Iliopoulou, E.; Soufla, G.; Kanthou, C.; Khoury, M.; et al. Perioperative use of iloprost in cardiac surgery patients diagnosed with heparin-induced thrombocytopenia-reactive antibodies or with true HIT (HIT-reactive antibodies plus thrombocytopenia): An 11-year experience. Am. J. Hematol. 2015, 90, 608–617. [Google Scholar] [CrossRef]
- Dannenberg, L.; Wolff, G.; Naguib, D.; Pöhl, M.; Zako, S.; Helten, C.; Mourikis, P.; Levkau, B.; Hohlfeld, T.; Zeus, T.; et al. Safety and efficacy of Tirofiban in STEMI-patients. Int. J. Cardiol. 2019, 274, 35–39. [Google Scholar] [CrossRef]
- Batchelor, W.B.; Tolleson, T.R.; Huang, Y.; Larsen, R.L.; Mantell, R.M.; Dillard, P.; Davidian, M.; Zhang, D.; Cantor, W.J.; Sketch, M.H., Jr.; et al. Randomized COMparison of platelet inhibition with abciximab, tiRofiban and eptifibatide during percutaneous coronary intervention in acute coronary syndromes: The COMPARE trial. Comparison Of Measurements of Platelet aggregation with Aggrastat, Reopro, and Eptifibatide. Circulation 2002, 106, 1470–1476. [Google Scholar]
- McClellan, K.J.; Goa, K.L. Tirofiban. A review of its use in acute coronary syndromes. Drugs 1998, 56, 1067–1080. [Google Scholar] [CrossRef] [PubMed]
- Commin, P.L.; Rozec, B.; Trossaërt, M.; Le Teurnier, Y.; Fournet, X.; Blanloeil, Y. Use of heparin and platelet GPIIbIIIa inhibitor tirofiban for cardiac surgery in patients for suspicion of heparin-induced thrombocytopenia. Ann. Fr. Anesth. Reanim. 2006, 25, 1153–1157. [Google Scholar] [CrossRef] [PubMed]
- Durand, M.; Lecompte, T.; Hacquard, M.; Carteaux, J.P. Heparin-induced thrombocytopenia and cardiopulmonary bypass: Anticoagulation with unfractionated heparin and the GPIIb/IIIa inhibitor tirofiban and successful use of rFVIIa for post-protamine bleeding due to persistent platelet blockade. Eur. J. Cardiothorac. Surg. 2008, 34, 687–689. [Google Scholar] [CrossRef]
- Koster, A.; Meyer, O.; Fischer, T.; Kukucka, M.; Krabatsch, T.; Bauer, M.; Kuppe, H.; Hetzer, R. One-year experience with the platelet glycoprotein IIb/IIIa antagonist tirofiban and heparin during cardiopulmonary bypass in patients with heparin-induced thrombocytopenia type II. J. Thorac. Cardiovasc. Surg. 2001, 122, 1254–1255. [Google Scholar] [CrossRef]
- Koster, A.; Kukucka, M.; Bach, F.; Meyer, O.; Fischer, T.; Mertzlufft, F.; Loebe, M.; Hetzer, R.; Kuppe, H. Anticoagulation during cardiopulmonary bypass in patients with heparin-induced thrombocytopenia type II and renal impairment using heparin and the platelet glycoprotein IIb-IIIa antagonist tirofiban. Anesthesiology 2001, 94, 245–251. [Google Scholar] [CrossRef]
- Sible, A.M.; Nawarskas, J.J. Cangrelor: A New Route for P2Y12 Inhibition. Cardiol. Rev. 2017, 25, 133–139. [Google Scholar] [CrossRef]
- Baker, D.E.; Ingram, K.T. Cangrelor. Hosp. Pharm. 2015, 50, 922–929. [Google Scholar] [PubMed]
- Ueno, M.; Ferreiro, J.L.; Angiolillo, D.J. Update on the clinical development of cangrelor. Expert Rev. Cardiovasc. Ther. 2010, 8, 1069–1077. [Google Scholar] [CrossRef]
- Gernhofer, Y.K.; Ross, M.; Khoche, S.; Pretorius, V. The use of cangrelor with heparin for left ventricular assist device implantation in a patient with acute heparin-induced thrombocytopenia. J. Cardiothorac. Surg. 2018, 13, 30. [Google Scholar] [CrossRef]
- Girgis, A.M.; Golts, E.; Humber, D.; Banks, D.A. Successful Use of Cangrelor and Heparin for Cardiopulmonary Bypass in a Patient With Heparin-Induced Thrombocytopenia and End-Stage Renal Disease: A Case Report. A A Pract. 2019, 13, 10–12. [Google Scholar] [CrossRef]
- Scala, E.; Pitta-Gros, B.; Pantet, O.; Iafrate, M.; Kirsch, M.; Marcucci, C.; Alberio, L. Cardiac Surgery Successfully Managed with Cangrelor in a Patient with Persistent Anti-PF4/Heparin Antibodies 8 Years after Heparin-Induced Thrombocytopenia. J. Cardiothorac. Vasc. Anesth. 2019, 33, 3073–3077. [Google Scholar] [CrossRef]
- Seider, S.; Ross, M.; Pretorius, V.; Maus, T. The Use of Cangrelor and Heparin for Anticoagulation in a Patient Requiring Pulmonary Thromboendarterectomy Surgery with Suspected Heparin-Induced Thrombocytopenia. J. Cardiothorac. Vasc. Anesth. 2019, 33, 1050–1053. [Google Scholar] [CrossRef] [PubMed]
- Gernhofer, Y.K.; Banks, D.A.; Golts, E.; Pretorius, V. Novel Use of Cangrelor with Heparin during Cardiopulmonary Bypass in Patients with Heparin-Induced Thrombocytopenia Who Require Cardiovascular Surgery: A Case Series. Semin. Thorac. Cardiovasc. Surg. 2020, 32, 763–769. [Google Scholar] [CrossRef] [PubMed]
- Dougherty, J.A.; Yarsley, R.L. Intravenous Immune Globulin (IVIG) for Treatment of Autoimmune Heparin-Induced Thrombocytopenia: A Systematic Review. Ann. Pharmacother. 2021, 55, 198–215. [Google Scholar] [CrossRef]
- Warkentin, T.E. High-dose intravenous immunoglobulin for the treatment and prevention of heparin-induced thrombocytopenia: A review. Expert Rev. Hematol. 2019, 12, 685–698. [Google Scholar] [CrossRef] [PubMed]
- Shock, A.; Humphreys, D.; Nimmerjahn, F. Dissecting the mechanism of action of intravenous immunoglobulin in human autoimmune disease: Lessons from therapeutic modalities targeting Fcγ receptors. J. Allergy Clin. Immunol. 2020, 146, 492–500. [Google Scholar] [CrossRef] [PubMed]
- Koster, A.; Nazy, I.; Birschmann, I.E.; Smith, J.W.; Sheppard, J.I.; Warkentin, T.E. High-dose IVIG plus cangrelor platelet “anesthesia” during urgent heparin-CPB in a patient with recent SRA-negative HIT-thrombosis with persisting platelet-activating antibodies. Res. Pract. Thromb. Haemost. 2020, 4, 1060–1064. [Google Scholar] [CrossRef]
- Warkentin, T.E.; Sheppard, J.A. Serological investigation of patients with a previous history of heparin-induced thrombocytopenia who are reexposed to heparin. Blood 2014, 123, 2485–2493. [Google Scholar] [CrossRef]
- Ranucci, M.; Baryshnikova, E. Sensitivity of Viscoelastic Tests to Platelet Function. J. Clin. Med. 2020, 9, 189. [Google Scholar] [CrossRef]

| HIT Phase | Postpone Surgery, If Possible | Intraoperative Anticoagulation Strategy | Pre-/Post-Operative Anticoagulation Strategy |
|---|---|---|---|
| Acute | Yes | Alternative protocol | Non-heparin molecule |
| Subacute A | Yes | Alternative protocol | Non-heparin molecule |
| Subacute B | No | Alternative protocol | Non-heparin molecule |
| Remote | No | Standard heparin | Non-heparin molecule |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Revelly, E.; Scala, E.; Rosner, L.; Rancati, V.; Gunga, Z.; Kirsch, M.; Ltaief, Z.; Rusca, M.; Bechtold, X.; Alberio, L.; et al. How to Solve the Conundrum of Heparin-Induced Thrombocytopenia during Cardiopulmonary Bypass. J. Clin. Med. 2023, 12, 786. https://doi.org/10.3390/jcm12030786
Revelly E, Scala E, Rosner L, Rancati V, Gunga Z, Kirsch M, Ltaief Z, Rusca M, Bechtold X, Alberio L, et al. How to Solve the Conundrum of Heparin-Induced Thrombocytopenia during Cardiopulmonary Bypass. Journal of Clinical Medicine. 2023; 12(3):786. https://doi.org/10.3390/jcm12030786
Chicago/Turabian StyleRevelly, Etienne, Emmanuelle Scala, Lorenzo Rosner, Valentina Rancati, Ziyad Gunga, Matthias Kirsch, Zied Ltaief, Marco Rusca, Xavier Bechtold, Lorenzo Alberio, and et al. 2023. "How to Solve the Conundrum of Heparin-Induced Thrombocytopenia during Cardiopulmonary Bypass" Journal of Clinical Medicine 12, no. 3: 786. https://doi.org/10.3390/jcm12030786
APA StyleRevelly, E., Scala, E., Rosner, L., Rancati, V., Gunga, Z., Kirsch, M., Ltaief, Z., Rusca, M., Bechtold, X., Alberio, L., & Marcucci, C. (2023). How to Solve the Conundrum of Heparin-Induced Thrombocytopenia during Cardiopulmonary Bypass. Journal of Clinical Medicine, 12(3), 786. https://doi.org/10.3390/jcm12030786






