Potential Involvement of Oxidative Stress in Ligamentum Flavum Hypertrophy
Abstract
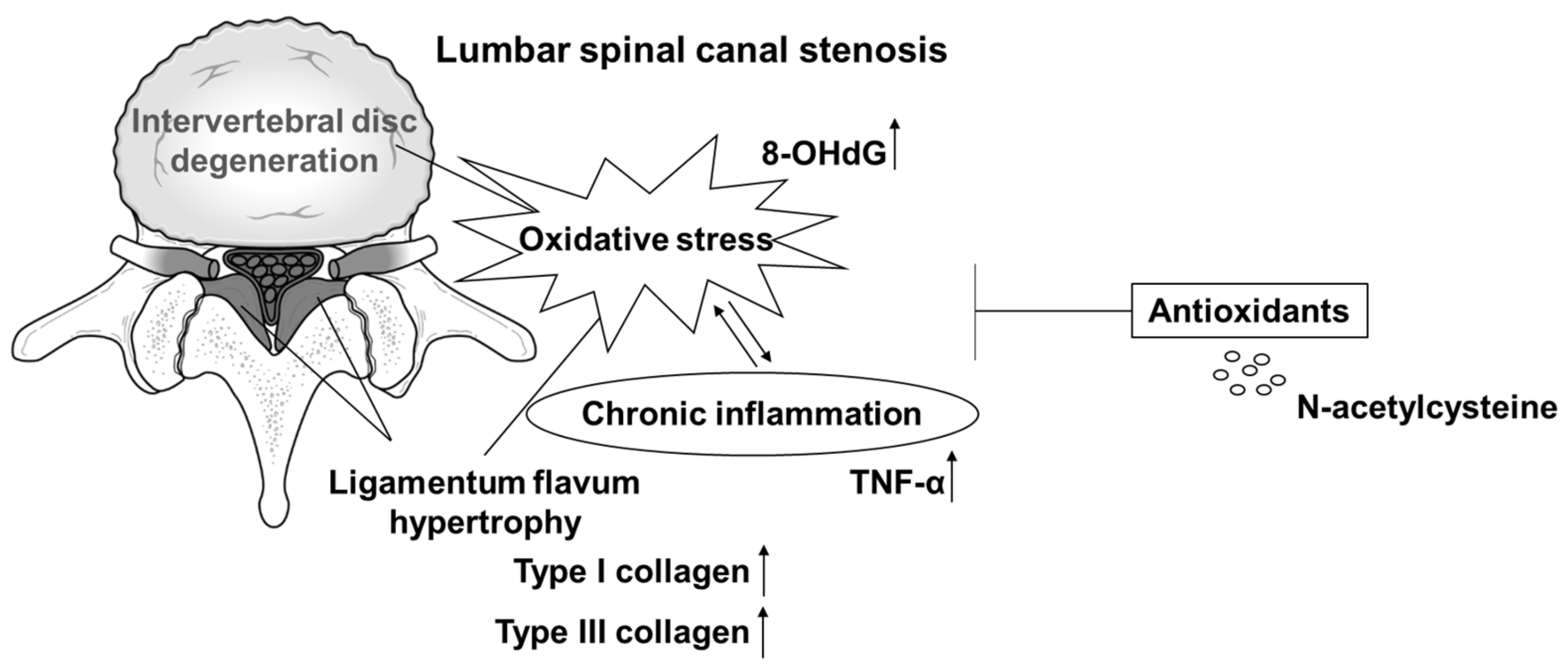
:1. Introduction
2. Materials and Methods
2.1. Human LF Samples
Magnetic Resonance Imaging
2.2. Immunostaining
2.3. Human LF Cells
2.4. Measurement of Intracellular ROS
2.5. Treatment
2.6. Real-Time RT-PCR
2.7. WB Analysis
2.8. Enzyme-Linked Immunosorbent Assay (ELISA)
2.9. Statistical Analysis
3. Results
3.1. Expression of TNF-α and 8-OHdG in LF Tissues
3.2. Intracellular ROS Level in LF Cells
3.3. Interaction between OS and Expression of LF Hypertrophy Markers in LF Cells
3.4. Intracellular Signaling of OS in LF Cells
3.5. Interaction between the OS and the Release of Type I Collagen in LF Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Szpalski, M.; Gunzburg, R. Lumbar spinal stenosis in the elderly: An overview. Eur. Spine J. 2003, 12, S170–S175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishimoto, Y.; Yoshimura, N.; Muraki, S.; Yamada, H.; Nagata, K.; Hashizume, H.; Takiguchi, N.; Minamide, A.; Oka, H.; Kawaguchi, H.; et al. Prevalence of symptomatic lumbar spinal stenosis and its association with physical performance in a population-based cohort in Japan: The Wakayama Spine Study. Osteoarthr. Cartil. 2012, 20, 1103–1108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yabuki, S.; Fukumori, N.; Takegami, M.; Onishi, Y.; Otani, K.; Sekiguchi, M.; Wakita, T.; Kikuchi, S.; Fukuhara, S.; Konno, S. Prevalence of lumbar spinal stenosis, using the diagnostic support tool, and correlated factors in Japan: A population-based study. J. Orthop. Sci. 2013, 18, 893–900. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buser, Z.; Ortega, B.; D’Oro, A.; Pannell, W.; Cohen, J.R.; Wang, J.; Golish, R.; Reed, M.; Wang, J.C. Spine degenerative conditions and their treatments: National trends in the United States of America. Glob. Spine J. 2018, 8, 57–67. [Google Scholar] [CrossRef] [Green Version]
- North American Spine Society. Evidence-Based Clinical Guidelines for Multidisciplinary Spine Care: Diagnosis and Treatment of Degenerative Lumbar Spinal Stenosis. 2011. Available online: http://www.spine.org/Documents/LumbarStenosis11.pdf (accessed on 27 January 2021).
- Marcolina, A.; Vu, K.; Annaswamy, T.M. Lumbar spinal stenosis and potential management with prostaglandin E1 analogs. Am. J. Phys. Med. Rehabil. 2021, 100, 297–302. [Google Scholar] [CrossRef]
- Turner, J.A.; Ersek, M.; Herron, L.; Deyo, R. Surgery for lumbar spinal stenosis. Attempted meta-analysis of the literature. Spine 1992, 17, 1–8. [Google Scholar] [CrossRef]
- Williams, M.G.; Wafai, A.M.; Podmore, M.D. Functional outcomes of laminectomy and laminotomy for the surgical management lumbar spine stenosis. J. Spine Surg. 2017, 3, 580–586. [Google Scholar] [CrossRef]
- Okuda, T.; Baba, I.; Fujimoto, Y.; Tanaka, N.; Sumida, T.; Manabe, H.; Hayashi, Y.; Ochi, M. The pathology of ligamentum flavum in degenerative lumbar disease. Spine 2004, 29, 1689–1697. [Google Scholar] [CrossRef]
- Kosaka, H.; Sairyo, K.; Biyani, A.; Leaman, D.; Yeasting, R.; Higashino, K.; Sakai, T.; Katoh, S.; Sano, T.; Goel, V.K.; et al. Pathomechanism of loss of elasticity and hypertrophy of lumbar ligamentum flavum in elderly patients with lumbar spinal canal stenosis. Spine 2007, 32, 2805–2811. [Google Scholar] [CrossRef]
- Zhong, Z.M.; Zha, D.S.; Xiao, W.D.; Wu, S.H.; Wu, Q.; Zhang, Y.; Liu, F.Q.; Chen, J.T. Hypertrophy of ligamentum flavum in lumbar spine stenosis associated with the increased expression of connective tissue growth factor. J. Orthop. Res. 2011, 29, 1592–1597. [Google Scholar] [CrossRef]
- Habibi, H.; Suzuki, A.; Hayashi, K.; Salimi, H.; Terai, H.; Hori, Y.; Tamai, K.; Orita, K.; Ohyama, S.; Yabu, A.; et al. Expression and function of FGF9 in the hypertrophied ligamentum flavum of lumbar spinal stenosis patients. Spine J. 2021, 21, 1010–1020. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Yokota, K.; Kobayakawa, K.; Hara, M.; Kubota, K.; Harimaya, K.; Kawaguchi, K.; Hayashida, M.; Matsumoto, Y.; Doi, T.; et al. Experimental mouse model of lumbar ligamentum flavum hypertrophy. PLoS ONE 2017, 12, e0169717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saito, T.; Hara, M.; Kumamaru, H.; Kobayakawa, K.; Yokota, K.; Kijima, K.; Yoshizaki, S.; Harimaya, K.; Matsumoto, Y.; Kawaguchi, K.; et al. Macrophage infiltration is a causative factor for ligamentum flavum hypertrophy through the activation of collagen production in fibroblasts. Am. J. Pathol. 2017, 187, 2831–2840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sairyo, K.; Biyani, A.; Goel, V.K.; Leaman, D.W.; Booth, R., Jr.; Thomas, J.; Ebraheim, N.A.; Cowgill, I.A.; Mohan, S.E. Lumbar ligamentum flavum hypertrophy is due to accumulation of inflammation-related scar tissue. Spine 2007, 32, E340–E347. [Google Scholar] [CrossRef] [PubMed]
- Park, J.O.; Lee, B.H.; Kang, Y.M.; Kim, T.H.; Yoon, J.Y.; Kim, H.; Kwon, U.H.; Lee, K.I.; Lee, H.M.; Moon, S.H. Inflammatory cytokines induce fibrosis and ossification of human ligamentum flavum cells. J. Spinal Disord. Tech. 2013, 26, E6–E12. [Google Scholar] [CrossRef]
- Sun, C.; Zhang, H.; Wang, X.; Liu, X. Ligamentum flavum fibrosis and hypertrophy: Molecular pathways, cellular mechanisms, and future directions. FASEB J. 2020, 34, 9854–9868. [Google Scholar] [CrossRef]
- Yabu, A.; Suzuki, A.; Hayashi, K.; Hori, Y.; Terai, H.; Orita, K.; Habibi, H.; Salimi, H.; Kono, H.; Toyoda, H.; et al. Periostin increased by mechanical stress upregulates interleukin-6 expression in the ligamentum flavum. FASEB J. 2023, 37, e22726. [Google Scholar] [CrossRef]
- Harman, D. Aging: A theory based on free radical and radiation chemistry. J. Gerontol. 1956, 11, 298–300. [Google Scholar] [CrossRef] [Green Version]
- Basu, S.; Michaëlsson, K.; Olofsson, H.; Johansson, S.; Melhus, H. Association between oxidative stress and bone mineral density. Biochem. Biophys. Res. Commun. 2001, 288, 275–279. [Google Scholar] [CrossRef]
- Banfi, G.; Iorio, E.L.; Corsi, M.M. Oxidative stress, free radicals and bone remodeling. Clin. Chem. Lab. Med. 2008, 46, 1550–1555. [Google Scholar] [CrossRef]
- Loeser, R.F. Aging and osteoarthritis. Curr. Opin. Rheumatol. 2011, 23, 492–496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poveda, L.; Hottiger, M.; Boos, N.; Wuertz, K. Peroxynitrite induces gene expression in intervertebral disc cells. Spine 2009, 34, 1127–1133. [Google Scholar] [CrossRef] [PubMed]
- Dimozi, A.; Mavrogonatou, E.; Sklirou, A.; Kletsas, D. Oxidative stress inhibits the proliferation, induces premature senescence and promotes a catabolic phenotype in human nucleus pulposus intervertebral disc cells. Eur. Cell Mater. 2015, 30, 89–102. [Google Scholar] [CrossRef] [PubMed]
- Nasto, L.A.; Robinson, A.R.; Ngo, K.; Clauson, C.L.; Dong, Q.; St Croix, C.; Sowa, G.; Pola, E.; Robbins, P.D.; Kang, J.; et al. Mitochondrial-derived reactive oxygen species (ROS) play a causal role in aging-related intervertebral disc degeneration. J. Orthop. Res. 2013, 31, 1150–1157. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, S.; Fujita, N.; Hosogane, N.; Watanabe, K.; Ishii, K.; Toyama, Y.; Takubo, K.; Horiuchi, K.; Miyamoto, T.; Nakamura, M.; et al. Excessive reactive oxygen species are therapeutic targets for intervertebral disc degeneration. Arthritis Res. Ther. 2015, 17, 316. [Google Scholar] [CrossRef] [Green Version]
- Chuang, H.C.; Tsai, K.L.; Tsai, K.J.; Tu, T.Y.; Shyong, Y.J.; Jou, I.M.; Hsu, C.C.; Shih, S.S.; Liu, Y.F.; Lin, C.L. Oxidative stress mediates age-related hypertrophy of ligamentum flavum by inducing inflammation, fibrosis, and apoptosis through activating Akt and MAPK pathways. Aging 2020, 12, 24168–24183. [Google Scholar] [CrossRef]
- Hsu, Y.C.; Chuang, H.C.; Tsai, K.L.; Tu, T.Y.; Shyong, Y.J.; Kuo, C.H.; Liu, Y.F.; Shih, S.S.; Lin, C.L. Administration of N-acetylcysteine to regress the fibrogenic and proinflammatory effects of oxidative stress in hypertrophic ligamentum flavum cells. Oxid. Med. Cell Longev. 2022, 2022, 1380353. [Google Scholar] [CrossRef]
- Takeda, H.; Nagai, S.; Ikeda, D.; Kaneko, S.; Tsuji, T.; Fujita, N. Collagen profiling of ligamentum flavum in patients with lumbar spinal canal stenosis. J. Orthop. Sci. 2021, 26, 560–565. [Google Scholar] [CrossRef]
- Nagai, S.; Hachiya, K.; Takeda, H.; Ikeda, D.; Kawabata, S.; Watanabe, K.; Kaneko, S.; Fujita, N. Impact of oxidized LDL/LOX-1 system on ligamentum flavum hypertrophy. J. Orthop. Sci. 2022. [Google Scholar] [CrossRef]
- Wang, A.Y.; Saini, H.; Tingen, J.N.; Sharma, V.; Flores, A.; Liu, D.; Olmos, M.; McPhail, E.D.; Safain, M.G.; Kryzanski, J.; et al. The relationship between wild-type transthyretin amyloid load and ligamentum flavum thickness in lumbar stenosis patients. World Neurosurg. 2022, 164, e113–e118. [Google Scholar] [CrossRef]
- Sakai, S.; Ito, T.; Hida, K.; Ito, A.; Harada, K.; Watanabe, K. Clinical outcome of lumbar spinal stenosis based on new classification according to hypertrophied ligamentum flavum. J. Orthop. Sci. 2017, 22, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.Y.; Ahmad, N.; Haqqi, T.M. Oxidative stress and inflammation in osteoarthritis pathogenesis: Role of polyphenols. Biomed. Pharmacother. 2020, 129, 110452. [Google Scholar] [CrossRef] [PubMed]
- Sadowska, A.M.; Manuel-Y-Keenoy, B.; De Backer, W.A. Antioxidant and anti-inflammatory efficacy of NAC in the treatment of COPD: Discordant in vitro and in vivo dose-effects: A review. Pulm. Pharmacol. Ther. 2007, 20, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Santus, P.; Corsico, A.; Solidoro, P.; Braido, F.; Di Marco, F.; Scichilone, N. Oxidative stress and respiratory system: Pharmacological and clinical reappraisal of N-acetylcysteine. COPD 2014, 11, 705–717. [Google Scholar] [CrossRef] [PubMed]





| Samples | n = 47 | |
|---|---|---|
| Patients | n = 39 | |
| Gender | Male | 18 |
| Female | 21 | |
| Age (years) | ≥80 | 3 |
| 60–80 | 20 | |
| 40–60 | 10 | |
| <40 | 6 | |
| Level | L2/3 | 2 |
| L3/4 | 9 | |
| L4/5 | 24 | |
| L5/S | 12 | |
| Diagnosis | LSS | 32 |
| LDH | 15 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ito, K.; Kise, H.; Suzuki, S.; Nagai, S.; Hachiya, K.; Takeda, H.; Kawabata, S.; Ikeda, D.; Takubo, K.; Kaneko, S.; et al. Potential Involvement of Oxidative Stress in Ligamentum Flavum Hypertrophy. J. Clin. Med. 2023, 12, 808. https://doi.org/10.3390/jcm12030808
Ito K, Kise H, Suzuki S, Nagai S, Hachiya K, Takeda H, Kawabata S, Ikeda D, Takubo K, Kaneko S, et al. Potential Involvement of Oxidative Stress in Ligamentum Flavum Hypertrophy. Journal of Clinical Medicine. 2023; 12(3):808. https://doi.org/10.3390/jcm12030808
Chicago/Turabian StyleIto, Kei, Hideki Kise, Satoshi Suzuki, Sota Nagai, Kurenai Hachiya, Hiroki Takeda, Soya Kawabata, Daiki Ikeda, Keiyo Takubo, Shinjiro Kaneko, and et al. 2023. "Potential Involvement of Oxidative Stress in Ligamentum Flavum Hypertrophy" Journal of Clinical Medicine 12, no. 3: 808. https://doi.org/10.3390/jcm12030808
APA StyleIto, K., Kise, H., Suzuki, S., Nagai, S., Hachiya, K., Takeda, H., Kawabata, S., Ikeda, D., Takubo, K., Kaneko, S., & Fujita, N. (2023). Potential Involvement of Oxidative Stress in Ligamentum Flavum Hypertrophy. Journal of Clinical Medicine, 12(3), 808. https://doi.org/10.3390/jcm12030808






