Inhibition of the Activating Transcription Factor 6 Branch of Endoplasmic Reticulum Stress Ameliorates Brain Injury after Deep Hypothermic Circulatory Arrest
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Drug Intervention
2.2. Hypothermic OGD/R Experiments
2.3. Cell Counting Kit (CCK)-8 Cell Viability Assay and Apoptosis Level Analysis
2.4. Apoptosis Detection
2.5. Animals and Experimental Protocol
2.6. DHCA Surgery
2.7. Modified Neurological Severity Scores (mNSS)
2.8. Histological Study
2.9. Enzyme-Linked Immunoassay (ELISA)
2.10. Western Blotting
2.11. Statistical Analysis
3. Results
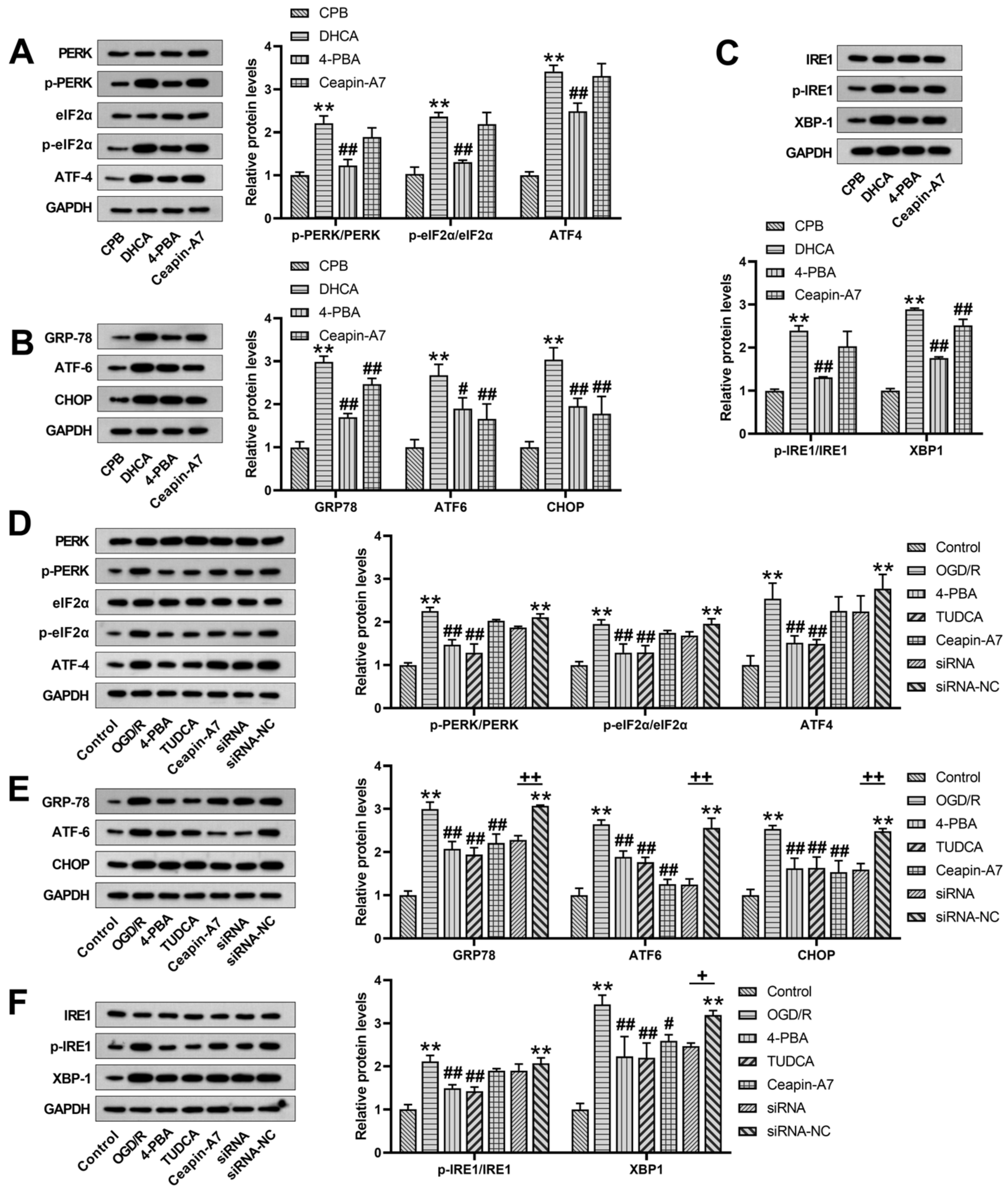
3.1. Changes in ER Stress Protein Expression after DHCA
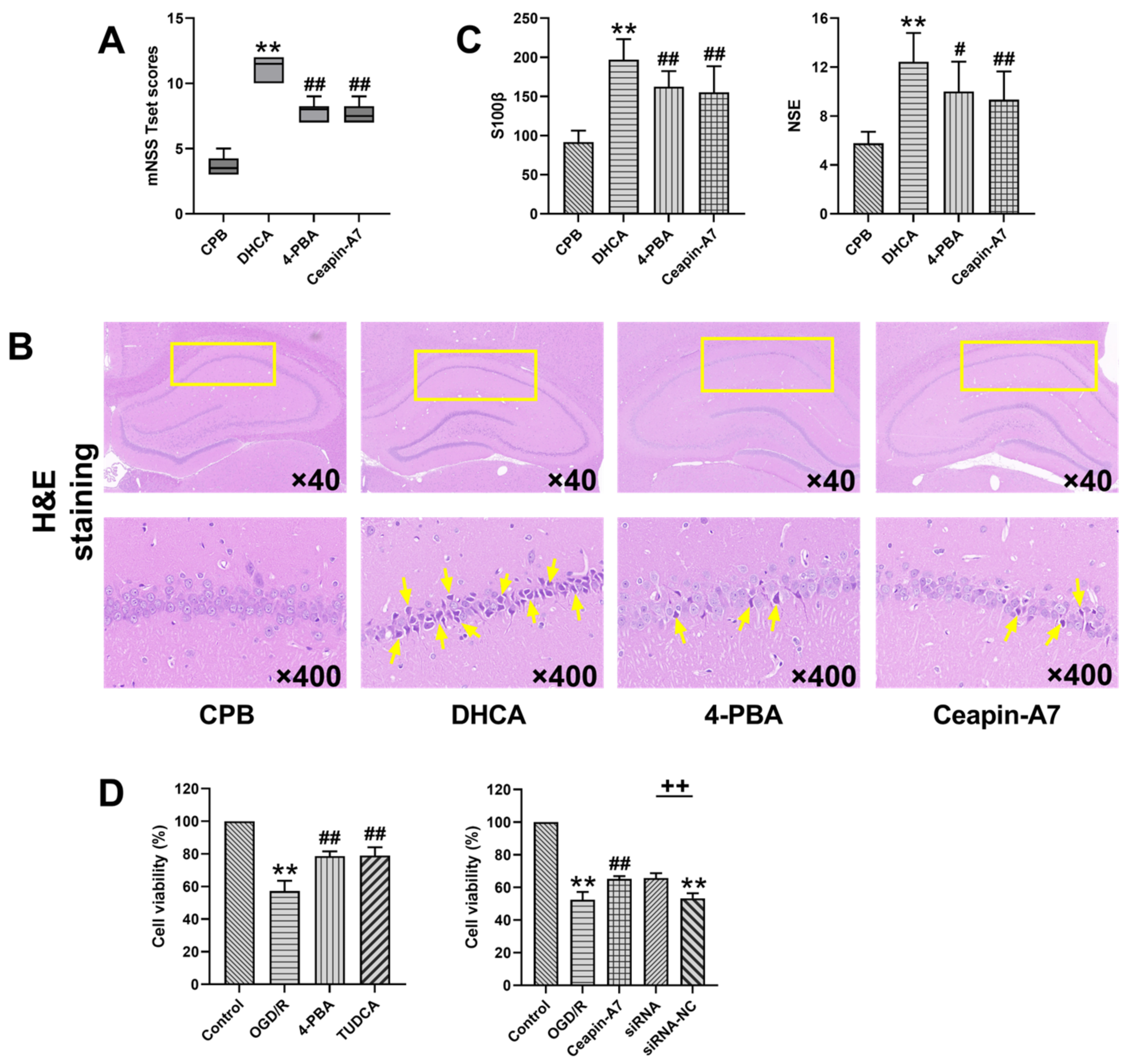
3.2. Inhibition of ER Stress and ATF6-Attenuated DHCA-Induced Brain Injury
3.3. Inhibition of ER Stress and ATF6-Inhibited Neuronal Apoptosis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Algra, S.O.; Jansen, N.J.; van der Tweel, I.; Schouten, A.N.; Groenendaal, F.; Toet, M.; van Oeveren, W.; van Haastert, I.C.; Schoof, P.H.; de Vries, L.S.; et al. Neurological injury after neonatal cardiac surgery: A randomized, controlled trial of 2 perfusion techniques. Circulation 2014, 129, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Griepp, R.B.; Stinson, E.B.; Hollingsworth, J.F.; Buehler, D. Prosthetic replacement of the aortic arch. J. Thorac. Cardiovasc. Surg. 1975, 70, 1051–1063. [Google Scholar] [CrossRef]
- Harrington, D.K.; Bonser, M.; Moss, A.; Heafield, M.T.; Riddoch, M.J.; Bonser, R.S. Neuropsychometric outcome following aortic arch surgery: A prospective randomized trial of retrograde cerebral perfusion. J. Thorac. Cardiovasc. Surg. 2003, 126, 638–644. [Google Scholar] [CrossRef]
- Liu, M.; Li, Y.; Liu, Y.; Yan, S.; Liu, G.; Zhang, Q.; Ji, B. Cold-inducible RNA-binding protein as a novel target to alleviate blood-brain barrier damage induced by cardiopulmonary bypass. J. Thorac. Cardiovasc. Surg. 2019, 157, 986–996.e985. [Google Scholar] [CrossRef]
- Wang, Y.; Gu, T.; Shi, E.; Yu, L.; Wang, C.; Zhang, Y.; Fang, Q. Inhibition of microRNA-29c protects the brain in a rat model of prolonged hypothermic circulatory arrest. J. Thorac. Cardiovasc. Surg. 2015, 150, 675–684.e671. [Google Scholar] [CrossRef]
- Pinto, A.; Jahn, A.; Immohr, M.B.; Jenke, A.; Dohrn, L.; Kornfeld, M.; Lichtenberg, A.; Akhyari, P.; Boeken, U. Modulation of Immunologic Response by Preventive Everolimus Application in a Rat CPB Model. Inflammation 2016, 39, 1771–1782. [Google Scholar] [CrossRef] [PubMed]
- Senkal, C.E.; Ponnusamy, S.; Bielawski, J.; Hannun, Y.A.; Ogretmen, B. Antiapoptotic roles of ceramide-synthase-6-generated C16-ceramide via selective regulation of the ATF6/CHOP arm of ER-stress-response pathways. FASEB J. 2010, 24, 296–308. [Google Scholar] [CrossRef] [PubMed]
- Frand, A.R.; Cuozzo, J.W.; Kaiser, C.A. Pathways for protein disulphide bond formation. Trends Cell Biol. 2000, 10, 203–210. [Google Scholar] [CrossRef]
- Nakka, V.P.; Gusain, A.; Raghubir, R. Endoplasmic reticulum stress plays critical role in brain damage after cerebral ischemia/reperfusion in rats. Neurotox. Res. 2010, 17, 189–202. [Google Scholar] [CrossRef]
- Xiaohong, W.; Jun, Z.; Hongmei, G.; Fan, Q. CFLAR is a critical regulator of cerebral ischaemia-reperfusion injury through regulating inflammation and endoplasmic reticulum (ER) stress. Biomed. Pharmacother. 2019, 117, 109155. [Google Scholar] [CrossRef]
- Li, H.Q.; Xia, S.N.; Xu, S.Y.; Liu, P.Y.; Gu, Y.; Bao, X.Y.; Xu, Y.; Cao, X. γ-Glutamylcysteine Alleviates Ischemic Stroke-Induced Neuronal Apoptosis by Inhibiting ROS-Mediated Endoplasmic Reticulum Stress. Oxidative Med. Cell. Longev. 2021, 2021, 2961079. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.W.; Hu, Z.P. Neuroprotective effects of atorvastatin against cerebral ischemia/reperfusion injury through the inhibition of endoplasmic reticulum stress. Neural Regen. Res. 2015, 10, 1239–1244. [Google Scholar] [CrossRef] [PubMed]
- Sanderson, T.; Gallaway, M.; Kumar, R. Unfolding the Unfolded Protein Response: Unique Insights into Brain Ischemia. Int. J. Mol. Sci. 2015, 16, 7133–7142. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Sheng, H.; Shuai, L.; Zhao, S.; Wei, Y. Activation of the ATF6 branch of the unfolded protein response in neurons improves stroke outcome. J. Cereb. Blood Flow Metab. 2017, 37, 1069–1079. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Piao, M.; Guo, M.; Meng, L.; Yu, H. MicroRNA-211-5p attenuates spinal cord injury via targeting of activating transcription factor 6. Tissue Cell 2020, 68, 101459. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.W.; Chen, T.Y.; Hung, C.Y.; Tai, S.H.; Huang, S.Y.; Chang, C.C.; Hung, H.Y.; Lee, E.J. Melatonin protects brain against ischemia/reperfusion injury by attenuating endoplasmic reticulum stress. Int. J. Mol. Med. 2018, 42, 182–192. [Google Scholar] [CrossRef] [PubMed]
- Georgiadis, D.; Berger, A.; Kowatschev, E.; Lautenschläger, C.; Börner, A.; Lindner, A.; Schulte-Mattler, W.; Zerkowski, H.R.; Zierz, S.; Deufel, T. Predictive value of S-100beta and neuron-specific enolase serum levels for adverse neurologic outcome after cardiac surgery. J. Thorac. Cardiovasc. Surg. 2000, 119, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Hazelton, J.L.; Balan, I.; Elmer, G.I.; Kristian, T.; Rosenthal, R.E.; Krause, G.; Sanderson, T.H.; Fiskum, G. Hyperoxic reperfusion after global cerebral ischemia promotes inflammation and long-term hippocampal neuronal death. J. Neurotrauma 2010, 27, 753–762. [Google Scholar] [CrossRef]
- Li, Y.A.; Liu, Z.G.; Zhang, Y.P.; Hou, H.T.; He, G.W.; Xue, L.G.; Yang, Q.; Liu, X.C. Differential expression profiles of circular RNAs in the rat hippocampus after deep hypothermic circulatory arrest. Artif. Organs 2021, 45, 866–880. [Google Scholar] [CrossRef]
- Iurlaro, R.; Munoz-Pinedo, C. Cell death induced by endoplasmic reticulum stress. FEBS J. 2016, 283, 2640–2652. [Google Scholar] [CrossRef]
- Zhao, Y.; Fang, Y.; Zhao, H.; Li, J.; Duan, Y.; Shi, W.; Huang, Y.; Gao, L.; Luo, Y. Chrysophanol inhibits endoplasmic reticulum stress in cerebral ischemia and reperfusion mice. Eur. J. Pharmacol. 2018, 818, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, J.H.; Sheng, J.; Shao, A. Immunoreactive Cells After Cerebral Ischemia. Front. Immunol. 2019, 10, 2781. [Google Scholar] [CrossRef] [PubMed]
- Haupt, M.; Zechmeister, B.; Bosche, B.; Lieschke, S.; Zheng, X.; Zhang, L.; Venkataramani, V.; Jin, F.; Hein, K.; Weber, M.S.; et al. Lithium enhances post-stroke blood-brain barrier integrity, activates the MAPK/ERK1/2 pathway and alters immune cell migration in mice. Neuropharmacology 2020, 181, 108357. [Google Scholar] [CrossRef] [PubMed]
- Feng, D.; Wang, B.; Wang, L.; Abraham, N.; Tao, K.; Huang, L.; Shi, W.; Dong, Y.; Qu, Y. Pre-ischemia melatonin treatment alleviated acute neuronal injury after ischemic stroke by inhibiting endoplasmic reticulum stress-dependent autophagy via PERK and IRE1 signalings. J. Pineal Res. 2017, 62, e12395. [Google Scholar] [CrossRef]
- Gharibani, P.M.; Modi, J.; Pan, C.; Menzie, J.; Ma, Z.; Chen, P.C.; Tao, R.; Prentice, H.; Wu, J.Y. The mechanism of taurine protection against endoplasmic reticulum stress in an animal stroke model of cerebral artery occlusion and stroke-related conditions in primary neuronal cell culture. Adv. Exp. Med. Biol. 2013, 776, 241–258. [Google Scholar] [CrossRef]
- Oyadomari, S.; Mori, M. Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ. 2004, 11, 381–389. [Google Scholar] [CrossRef]
- McCullough, K.D.; Martindale, J.L.; Klotz, L.O.; Aw, T.Y.; Holbrook, N.J. Gadd153 sensitizes cells to endoplasmic reticulum stress by down-regulating Bcl2 and perturbing the cellular redox state. Mol. Cell. Biol. 2001, 21, 1249–1259. [Google Scholar] [CrossRef]
- Kumar, R.; Azam, S.; Sullivan, J.M.; Owen, C.; Cavener, D.R.; Zhang, P.; Ron, D.; Harding, H.P.; Chen, J.J.; Han, A.; et al. Brain ischemia and reperfusion activates the eukaryotic initiation factor 2alpha kinase, PERK. J. Neurochem. 2001, 77, 1418–1421. [Google Scholar] [CrossRef]
- Xu, W.; Lu, X.; Zheng, J.; Li, T.; Gao, L.; Lenahan, C.; Shao, A.; Zhang, J.; Yu, J. Melatonin Protects Against Neuronal Apoptosis via Suppression of the ATF6/CHOP Pathway in a Rat Model of Intracerebral Hemorrhage. Front. Neurosci. 2018, 12, 638. [Google Scholar] [CrossRef]
- Xu, W.; Gao, L.; Li, T.; Zheng, J.; Shao, A.; Zhang, J. Apelin-13 Alleviates Early Brain Injury after Subarachnoid Hemorrhage via Suppression of Endoplasmic Reticulum Stress-mediated Apoptosis and Blood-Brain Barrier Disruption: Possible Involvement of ATF6/CHOP Pathway. Neuroscience 2018, 388, 284–296. [Google Scholar] [CrossRef]
- Zhang, S.; Zhao, X.; Hao, J.; Zhu, Y.; Wang, Y.; Wang, L.; Guo, S.; Yi, H.; Liu, Y.; Liu, J. The role of ATF6 in Cr(VI)-induced apoptosis in DF-1 cells. J. Hazard. Mater. 2021, 410, 124607. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.-P.; Yang, Q.; Li, Y.-A.; Yu, M.-H.; He, G.-W.; Zhu, Y.-X.; Liu, Z.-G.; Liu, X.-C. Inhibition of the Activating Transcription Factor 6 Branch of Endoplasmic Reticulum Stress Ameliorates Brain Injury after Deep Hypothermic Circulatory Arrest. J. Clin. Med. 2023, 12, 814. https://doi.org/10.3390/jcm12030814
Zhang Y-P, Yang Q, Li Y-A, Yu M-H, He G-W, Zhu Y-X, Liu Z-G, Liu X-C. Inhibition of the Activating Transcription Factor 6 Branch of Endoplasmic Reticulum Stress Ameliorates Brain Injury after Deep Hypothermic Circulatory Arrest. Journal of Clinical Medicine. 2023; 12(3):814. https://doi.org/10.3390/jcm12030814
Chicago/Turabian StyleZhang, You-Peng, Qin Yang, Yi-Ai Li, Ming-Huan Yu, Guo-Wei He, Yu-Xiang Zhu, Zhi-Gang Liu, and Xiao-Cheng Liu. 2023. "Inhibition of the Activating Transcription Factor 6 Branch of Endoplasmic Reticulum Stress Ameliorates Brain Injury after Deep Hypothermic Circulatory Arrest" Journal of Clinical Medicine 12, no. 3: 814. https://doi.org/10.3390/jcm12030814
APA StyleZhang, Y.-P., Yang, Q., Li, Y.-A., Yu, M.-H., He, G.-W., Zhu, Y.-X., Liu, Z.-G., & Liu, X.-C. (2023). Inhibition of the Activating Transcription Factor 6 Branch of Endoplasmic Reticulum Stress Ameliorates Brain Injury after Deep Hypothermic Circulatory Arrest. Journal of Clinical Medicine, 12(3), 814. https://doi.org/10.3390/jcm12030814






