Subchondral Bone Cyst Development in Osteoarthritis: From Pathophysiology to Bone Microarchitecture Changes and Clinical Implementations
Abstract
1. Introduction
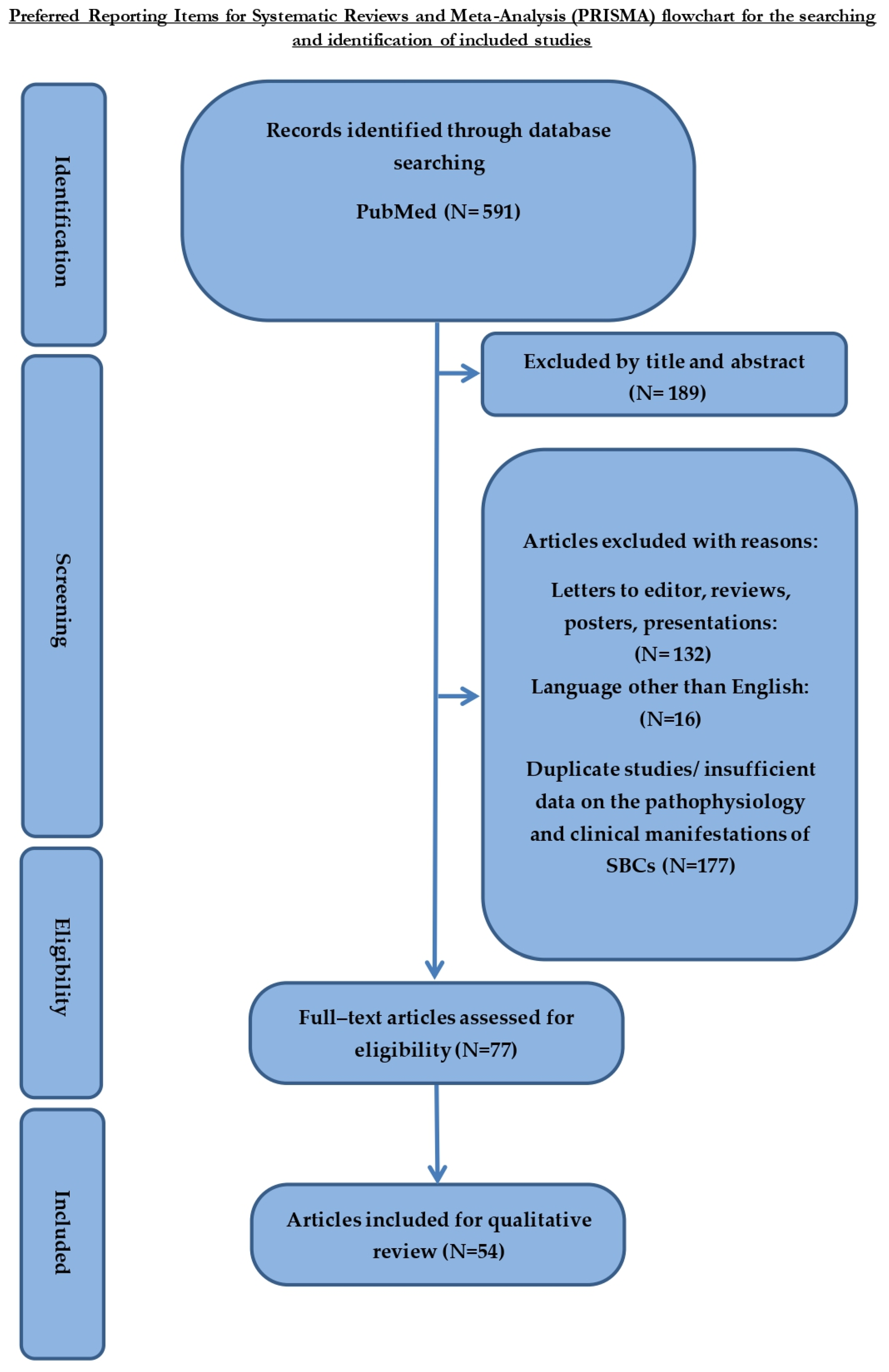
2. Materials and Methods
2.1. Research Strategy
2.2. Inclusion and Exclusion Criteria
3. Results
3.1. Pathophysiology
3.2. Subchondral Bone Cysts and Bone Turnover
3.3. Clinical Relevance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Goldring, M.B.; Goldring, S.R. Articular cartilage and subchondral bone in the pathogenesis of osteoarthritis. Ann. N. Y. Acad. Sci. 2010, 1192, 230–237. [Google Scholar] [CrossRef]
- Loeser, R.F.; Goldring, S.R.; Scanzello, C.R.; Goldring, M.B. Osteoarthritis: A disease of the joint as an organ. Arthritis Rheum. 2012, 64, 1697–1707. [Google Scholar] [CrossRef] [PubMed]
- Lories, R.J.; Luyten, F.P. The bone–cartilage unit in osteoarthritis. Nat. Rev. Rheumatol. 2010, 7, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.-Z.; Zheng, H.-A.; Jiang, Y.; Tu, Y.-H.; Jiang, P.-H.; Yang, A.-L. Mechanical and biological link between cartilage and subchondral bone in osteoarthritis. Arthritis Care Res. 2012, 64, 960–967. [Google Scholar] [CrossRef] [PubMed]
- McErlain, D.D.; Milner, J.S.; Ivanov, T.G.; Jencikova-Celerin, L.; Pollmann, S.I.; Holdsworth, D.W. Subchondral cysts create increased intra-osseous stress in early knee OA: A finite element analysis using simulated lesions. Bone 2011, 48, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Raynauld, J.-P.; Martel-Pelletier, J.; Berthiaume, M.-J.; Abram, F.; Choquette, D.; Haraoui, B.; Beary, J.F.; Cline, G.A.; Meyer, J.M. Correlation between bone lesion changes and cartilage volume loss in patients with osteoarthritis of the knee as assessed by quantitative magnetic resonance imaging over a 24-month period. Ann. Rheum. Dis. 2007, 67, 683–688. [Google Scholar] [CrossRef]
- Wu, H.; Webber, C.; Fuentes, C.O.; Bensen, R.; Beattie, K.; Adachi, J.D.; Xie, X.; Jabbari, F.; Levy, D.R. Prevalence of knee abnormalities in patients with osteoarthritis and anterior cruciate ligament injury identified with peripheral magnetic resonance imaging: A pilot study. Can. Assoc. Radiol. J. 2007, 58, 167–175. [Google Scholar]
- Crema, M.; Roemer, F.; Marra, M.; Niu, J.; Lynch, J.; Felson, D.; Guermazi, A. Contrast-enhanced MRI of subchondral cysts in patients with or at risk for knee osteoarthritis: The MOST study. Eur. J. Radiol. 2010, 75, e92–e96. [Google Scholar] [CrossRef]
- Javaid, M.; Lynch, J.; Tolstykh, I.; Guermazi, A.; Roemer, F.; Aliabadi, P.; McCulloch, C.; Curtis, J.; Felson, D.; Lane, N.; et al. Pre-radiographic MRI findings are associated with onset of knee symptoms: The most study. Osteoarthr. Cartil. 2010, 18, 323–328. [Google Scholar] [CrossRef]
- Bancroft, L.W.; Peterson, J.J.; Kransdorf, M.J. Cysts, geodes, and erosions. Radiol. Clin. N. Am. 2004, 42, 73–87. [Google Scholar] [CrossRef]
- Li, G.Y.; Yin, J.M.; Gao, J.J.; Cheng, T.S.; Pavlos, N.J.; Zhang, C.Q.; Zheng, M.H. Subchondral bone in osteoarthritis: Insight into risk factors and microstructural changes. Arthritis Res. Ther. 2013, 15, 223. [Google Scholar] [CrossRef]
- Pouders, C.; De Maeseneer, M.; Van Roy, P.; Gielen, J.; Goossens, A.; Shahabpour, M. Prevalence and MRI-anatomic correlation of bone cysts in osteoarthritic knees. Am. J. Roentgenol. 2008, 190, 17–21. [Google Scholar] [CrossRef]
- Chiba, K.; Burghardt, A.J.; Osaki, M.; Majumdar, S. Three-dimensional analysis of subchondral cysts in hip osteoarthritis: An ex vivo HR-pQCT study. Bone 2014, 66, 140–145. [Google Scholar] [CrossRef]
- Gupta, K.B.; Duryea, J.; Weissman, B.N. Radiographic evaluation of osteoarthritis. Radiol. Clin. N. Am. 2004, 42, 11–41. [Google Scholar] [CrossRef]
- Parman, L.M.; Murphey, M.D. Alphabet soup: Cystic lesions of bone. Semin. Musculoskelet. Radiol. 2000, 4, 89–101. [Google Scholar] [CrossRef]
- Kaspiris, A.; Khaldi, L.; Grivas, T.; Vasiliadis, E.; Kouvaras, I.; Dagkas, S.; Chronopoulos, E.; Papadimitriou, E. Subchondral cyst development and MMP-1 expression during progression of osteoarthritis: An immunohistochemical study. Orthop. Traumatol. Surg. Res. 2013, 99, 523–529. [Google Scholar] [CrossRef]
- Carrino, J.; Blum, J.; Parellada, J.; Schweitzer, M.; Morrison, W. MRI of bone marrow edema-like signal in the pathogenesis of subchondral cysts. Osteoarthr. Cartil. 2006, 14, 1081–1085. [Google Scholar] [CrossRef]
- Bousson, V.; Lowitz, T.; Laouisset, L.; Engelke, K.; Laredo, J.-D. CT imaging for the investigation of subchondral bone in knee osteoarthritis. Osteoporos. Int. 2012, 23 (Suppl. S8), S861–S865. [Google Scholar] [CrossRef]
- Tanamas, S.K.; Wluka, A.E.; Pelletier, J.-P.; Martel-Pelletier, J.; Abram, F.; Wang, Y.; Cicuttini, F.M. The association between subchondral bone cysts and tibial cartilage volume and risk of joint replacement in people with knee osteoarthritis: A longitudinal study. Thromb. Haemost. 2010, 12, R58. [Google Scholar] [CrossRef]
- Ondrouch, A.S. Cyst formation in Osteoarthritis. J. Bone Jt. Surg. 1963, 45-B, 755–760. [Google Scholar] [CrossRef]
- Landells, J.W. The bone cysts of osteoarthritis. J. Bone Jt. Surg. 1953, 35-B, 643–649. [Google Scholar] [CrossRef] [PubMed]
- McErlain, D.D.; Ulici, V.; Darling, M.; Gati, J.S.; Pitelka, V.; Beier, F.; Holdsworth, D.W. An in vivo investigation of the initiation and progression of subchondral cysts in a rodent model of secondary osteoarthritis. Arthritis Res. Ther. 2012, 14, R26. [Google Scholar] [CrossRef] [PubMed]
- Wise, A. A Histological Analysis of Subchondral Bone Cysts in Osteoarthritic Hips. Ph.D. Thesis, Boston University, Boston, MA, USA, 2016; p. 61. [Google Scholar]
- Chan, P.M.B.; Wen, C.; Yang, W.C.; Yan, C.; Chiu, K.-Y. Is subchondral bone cyst formation in non-load-bearing region of osteoarthritic knee a vascular problem? Med. Hypotheses 2017, 109, 80–83. [Google Scholar] [CrossRef] [PubMed]
- Shabestari, M.; Kise, N.J.; Landin, M.A.; Sesseng, S.; Hellund, J.C.; Reseland, J.E.; Eriksen, E.F.; Haugen, I.K. Enhanced angiogenesis and increased bone turnover characterize bone marrow lesions in osteoarthritis at the base of the thumb. Bone Jt. Res. 2018, 7, 406–413. [Google Scholar] [CrossRef]
- Kaspiris, A.; Khaldi, L.; Chronopoulos, E.; Vasiliadis, E.; Grivas, T.B.; Kouvaras, I.; Dagkas, S.; Papadimitriou, E. Macrophage-specific metalloelastase (MMP-12) immunoexpression in the osteochondral unit in osteoarthritis correlates with BMI and disease severity. Pathophysiology 2015, 22, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Nakasone, A.; Guang, Y.; Wise, A.; Kim, L.; Babbin, J.; Rathod, S.; Mitchell, A.; Gerstenfeld, L.; Morgan, E. Structural features of subchondral bone cysts and adjacent tissues in hip osteoarthritis. Osteoarthr. Cartil. 2022, 30, 1130–1139. [Google Scholar] [CrossRef]
- Hick, A.-C.; Malaise, M.; Loeuille, D.; Conrozier, T.; Maugars, Y.; Pelousse, F.; Tits, C.; Henrotin, Y. Cartilage Biomarkers Coll2-1 and Coll2-1NO2 Are Associated with Knee OA MRI Features and Are Helpful in Identifying Patients at Risk of Disease Worsening. Cartilage 2021, 13, 1637S–1647S. [Google Scholar] [CrossRef]
- Wang, W.; Ding, R.; Zhang, N.; Hernigou, P. Subchondral bone cysts regress after correction of malalignment in knee osteoarthritis: Comply with Wolff’s law. Int. Orthop. 2020, 45, 445–451. [Google Scholar] [CrossRef]
- Lenz, C.G.; Zingg, P.O.; Dora, C. Do osteoarthritic subchondral bone cysts spontaneously consolidate after total hip replacement? HIP Int. 2018, 29, 398–404. [Google Scholar] [CrossRef]
- Takada, R.; Jinno, T.; Miyatake, K.; Yamauchi, Y.; Koga, D.; Yagishita, K.; Okawa, A. Longitudinal morphological change of acetabular subchondral bone cyst after total hip arthroplasty in developmental dysplasia of the hip. Eur. J. Orthop. Surg. Traumatol. 2018, 28, 621–625. [Google Scholar] [CrossRef]
- Mechlenburg, I.; Nyengaard, J.R.; Gelineck, J.; Søballe, K. Cartilage Thickness and Cyst Volume Are Unchanged 10 Years After Periacetabular Osteotomy in Patients without Hip Symptoms. Clin. Orthop. Relat. Res. 2015, 473, 2644–2649. [Google Scholar] [CrossRef]
- Kelly, M.P.; Kitamura, N.; Leung, S.B.; Engh, C.A., Sr. The natural history of osteoarthritic bone cysts after uncemented total hip arthroplasty. J. Arthroplast. 2007, 22, 1137–1142. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, T.; Guan, M.; Zhao, W.; Leung, F.-K.; Pan, H.; Cao, X.; Guo, X.; Lu, W. Bone turnover and articular cartilage differences localized to subchondral cysts in knees with advanced osteoarthritis. Osteoarthr. Cartil. 2015, 23, 2174–2183. [Google Scholar] [CrossRef]
- Chiba, K.; Nango, N.; Kubota, S.; Okazaki, N.; Taguchi, K.; Osaki, M.; Ito, M. Relationship between microstructure and degree of mineralization in subchondral bone of osteoarthritis: A synchrotron radiation µCT study. J. Bone Miner. Res. 2012, 27, 1511–1517. [Google Scholar] [CrossRef]
- Burnett, W.D.; Kontulainen, S.A.; McLennan, C.E.; Hazel, D.; Talmo, C.; Wilson, D.R.; Hunter, D.J.; Johnston, J.D. Knee osteoarthritis patients with more subchondral cysts have altered tibial subchondral bone mineral density. BMC Musculoskelet. Disord. 2019, 20, 14. [Google Scholar] [CrossRef]
- von Rechenberg, B.; Guenther, H.; McIlwraith, C.W.; Leutenegger, C.; Frisbie, D.D.; Akens, M.K.; Auer, J.A. Fibrous tissue of subchondral cystic lesions in horses produce local mediators and neutral metalloproteinases and cause bone resorption in vitro. Vet. Surg. 2000, 29, 420–429. [Google Scholar] [CrossRef]
- Sabokbar, A.; Crawford, R.; Murray, D.W.; Athanasou, N.A. Macrophage-osteoclast differentiation and bone resorption in osteoarthritic subchondral acetabular cysts. Acta Orthop. Scand. 2000, 71, 255–261. [Google Scholar] [CrossRef]
- Frazer, L.L.; Santschi, E.M.; Fischer, K.J. The impact of subchondral bone cysts on local bone stresses in the medial femoral condyle of the equine stifle joint. Med. Eng. Phys. 2017, 48, 158–167. [Google Scholar] [CrossRef]
- Ota, S.; Sasaki, E.; Sasaki, S.; Chiba, D.; Kimura, Y.; Yamamoto, Y.; Kumagai, M.; Ando, M.; Tsuda, E.; Ishibashi, Y. Relationship between abnormalities detected by magnetic resonance imaging and knee symptoms in early knee osteoarthritis. Sci. Rep. 2021, 11, 15179. [Google Scholar] [CrossRef]
- Kijima, H.; Yamada, S.; Konishi, N.; Kubota, H.; Tazawa, H.; Tani, T.; Suzuki, N.; Kamo, K.; Okudera, Y.; Fujii, M.; et al. The Differences in Imaging Findings Between Painless and Painful Osteoarthritis of the Hip. Clin. Med. Insights Arthritis Musculoskelet. Disord. 2020, 13, 1179544120946747. [Google Scholar] [CrossRef]
- Sayre, E.C.; Guermazi, A.; Esdaile, J.M.; Kopec, J.A.; Singer, J.; Thorne, A.; Nicolaou, S.; Cibere, J. Associations between MRI features versus knee pain severity and progression: Data from the Vancouver Longitudinal Study of Early Knee Osteoarthritis. PLoS ONE 2017, 12, e0176833. [Google Scholar] [CrossRef] [PubMed]
- Magnusson, K.; Turkiewicz, A.; Kumm, J.; Zhang, F.; Englund, M. Relationship Between Magnetic Resonance Imaging Features and Knee Pain Over Six Years in Knees without Radiographic Osteoarthritis at Baseline. Arthritis Care Res. 2020, 73, 1659–1666. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, M.; Konishi, N. Subchondral cysts arise in the anterior acetabulum in dysplastic osteoarthritic hips. Clin. Orthop. Relat. Res. 2002, 404, 291–301. [Google Scholar] [CrossRef]
- Tanner, G.; Simon, P.; Sellers, T.; Christmas, K.N.; Otto, R.J.; Cuff, D.J.; Abdelfattah, A.; Mighell, M.A.; Frankle, M.A. Total shoulder arthroplasty with minimum 5-year follow-up: Does the presence of subchondral cysts in the glenoid increase risk of failure? J. Shoulder Elb. Surg. 2018, 27, 794–800. [Google Scholar] [CrossRef] [PubMed]
- DiDomenico, L.A.; Williams, K. Revisional total ankle arthroplasty because of a large tibial bone cyst. J. Foot Ankle Surg. 2008, 47, 453–456. [Google Scholar] [CrossRef]
- Krych, A.J.; King, A.; Berardelli, R.L.; Sousa, P.L.; Levy, B.A. Is Subchondral Acetabular Edema or Cystic Change on MRI a Contraindication for Hip Arthroscopy in Patients with Femoroacetabular Impingement? Am. J. Sports Med. 2015, 44, 454–459. [Google Scholar] [CrossRef]
- Besse, J.-L.; Lienhart, C.; Fessy, M.-H. Outcomes following cyst curettage and bone grafting for the management of periprosthetic cystic evolution after AES total ankle replacement. Clin. Podiatr. Med. Surg. 2013, 30, 157–170. [Google Scholar] [CrossRef]
- Zeng, G.; Foong, F.; Lie, D. Knee subchondroplasty for management of subchondral bone cysts: A novel treatment method. Singap. Med. J. 2021, 62, 492–496. [Google Scholar] [CrossRef]
- Wallis, T.W.; Goodrich, L.R.; McIlwraith, C.W.; Frisbie, D.D.; Hendrickson, D.A.; Trotter, G.W.; Baxter, G.M.; Kawcak, C.E. Arthroscopic injection of corticosteroids into the fibrous tissue of subchondral cystic lesions of the medial femoral condyle in horses: A retrospective study of 52 cases (2001–2006). Equine Vet. J. 2008, 40, 461–467. [Google Scholar] [CrossRef]
- Ortved, K.F.; Nixon, A.J.; Mohammed, H.O.; Fortier, L.A. Treatment of subchondral cystic lesions of the medial femoral condyle of mature horses with growth factor enhanced chondrocyte grafts: A retrospective study of 49 cases. Equine Vet. J. 2012, 44, 606–613. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, L.; Yang, W.; Cao, Y.; Shi, Y.; Li, X.; Zhang, Q. The effects of different doses of IGF-1 on cartilage and subchondral bone during the repair of full-thickness articular cartilage defects in rabbits. Osteoarthr. Cartil. 2017, 25, 309–320. [Google Scholar] [CrossRef]
- Tellegen, A.R.; Rudnik-Jansen, I.; Pouran, B.; De Visser, H.M.; Weinans, H.; Thomas, R.E.; Kik, M.J.L.; Grinwis, G.; Thies, J.C.; Woike, N.; et al. Controlled release of celecoxib inhibits inflammation, bone cysts and osteophyte formation in a preclinical model of osteoarthritis. Drug Deliv. 2018, 25, 1438–1447. [Google Scholar] [CrossRef]


| Author and Year | Objective | Type of Study and Materials | Methods and Measurements | Follow-Up | Baseline | Results | Conclusions | |
|---|---|---|---|---|---|---|---|---|
| 1 | Nakasone et al., 2022, [27] | The aetiology and origin of SBCs in hip OA | Observational study on femoral heads which were collected from 34 patients during THA | MCT, histological and immune-cytological analysis | N/A | N/A | SBCs in 91% of femoral heads, location: mostly close surface of the femoral head Few correlations BMI, age and sex Composition: fibrous (most common) or fatty Containing: vasculature, nerve fibres, cartilage islands, and bony spicules High-density bone, adjacent to SBCs Lower cartilage thickness on regions overlying SBCs mCT: stiffness of the primary compressive group was mildly affected by SBCs | Structural changes of SBCs: extensive perturbations in cellular activity, culminating in a multitude of osseous tissue types with heterogeneous changes in bone and cartilage morphology, which favour OA progression. |
| 2 | Hick et al., 2021, [28] | Correlation between serum levels of Coll2-1 and Coll2-1NO2 with MRI findings and clinical outcomes in knee OA. | Prospective study 121 pts with knee OA | Pts followed up for 1 year with pain, function, and MRI assessment (PRODIGE study). Type II collagen-specific biomarkers Coll2-1 and Coll2-1NO2 were directly measured in serum using immunoassays at baseline and after 3-, 6-, and 12-month follow-ups. | One-year follow-up for pain, function, and MRI assessment (PRODIGE study) Serum biomarkers: serum using immunoassays at baseline and after 3-, 6-, and 12-month follow-ups | N/A | Coll2-1 significantly correlated with periarticular cysts/bursitis, subarticular bone attrition, SBCs and articular cartilage integrity Coll2-1NO2 correlated with worse symptoms from patellofemoral, and medial femorotibial compartments, loosening bodies and osteophytes | Serum cartilage biomarkers Coll2-1 and Coll2-1NO2 are associated with several knee OA features The baseline value of Coll2-1NO2 is positively correlated with pain worsening |
| 3 | Wang et al., 2020, [29] | To discuss the mechanical function of SBCs and their relationship with Wolff’s law | Retrospective 140 symptomatic OA knees (120 pts) scheduled for HTO | Baseline MRI before HTO SBCs and BMLs count, Hip–knee–ankle axis pre-op and evolution after HTO | 5 years | SBCs were detected in 72 knees, with 70 (97%) showing a BML surrounding the SBC | Average vol 9.6 ± 4.1 mm3 with adjacent cartilage present with no full-thickness defects Mean HKA axis was 7.3 ± 3 degrees of varus After 5 y regression of SBCs in 50 knees → revision for TKA 2/50 (4%), no regression 22 knees → revision for TKA 18/22 (82%) Mean SBC vol for regression group after 5 y → 6.3 ± 2.8 mm3 | Regression of SBCs may be related to the restoration of an appropriate load |
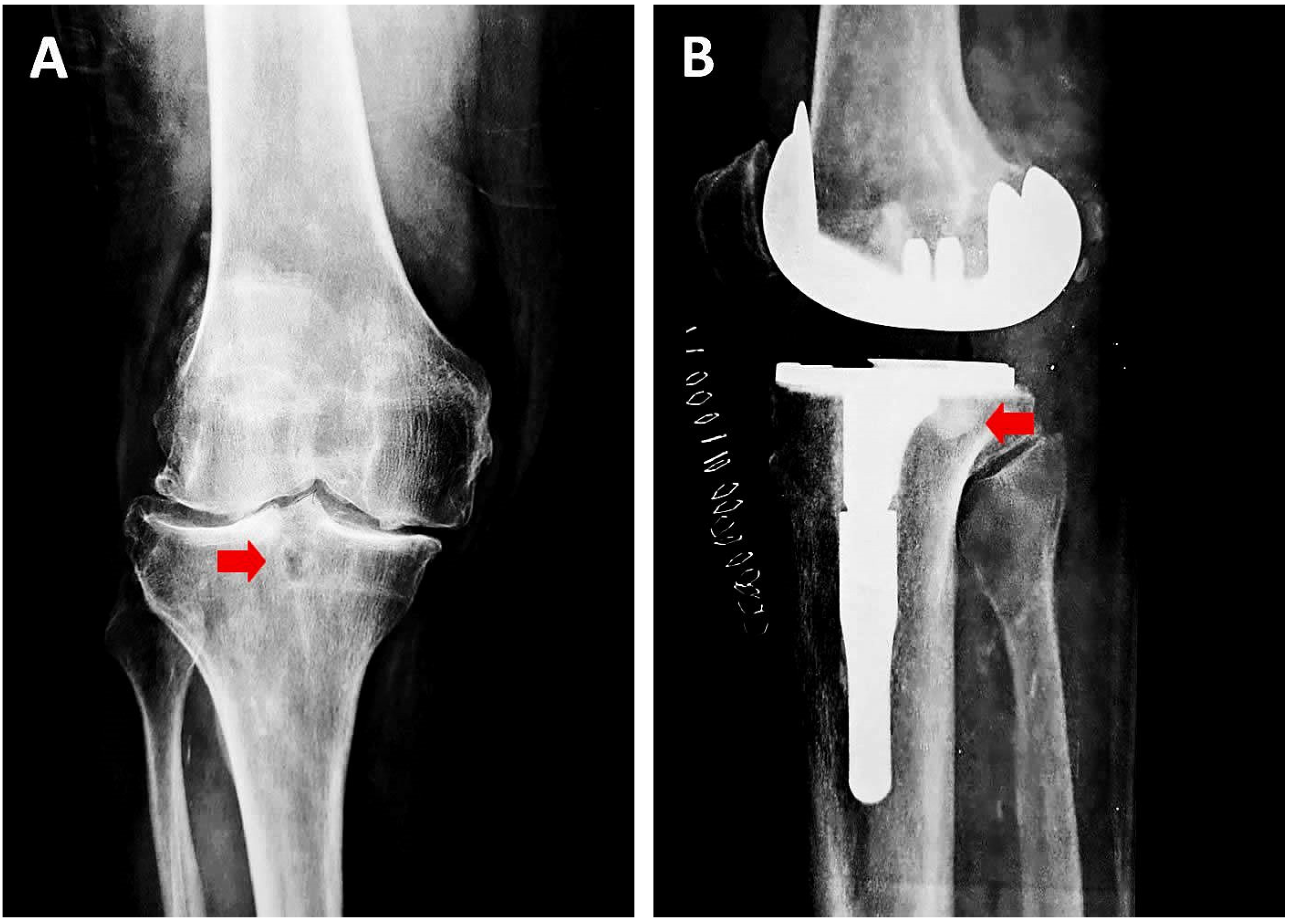
| 4 | Lenz et al., 2019, [30] | To investigate if acetabular SBCs consolidate spontaneously after THA | Retrospective Primary THA for 54 hip joints in 52 pts | AP hip radiograph preop, postop and at the latest follow-up Number of SBCs, size and progression | 6.3 years (5–9) | 88 cysts with a mean size of 9.3 ± 10 mm2 (range 0.9–57 mm2) | 71 SBCs (80.7%) in 38 hips disappeared in follow-up 17 SBCs (19.3%) in 16 hips are still visible (mostly in Charnley zone I) 15/17 SBCs decreased from 19.8 ± 18 mm2 preop to 13.3 ± 13.3 mm2 postop 2/17 SBCs progressed from 8.4 ± 2 preop to 15.8 ± 10.9 mm2 postop | Post THR, most neglected SBCs decrease in size. Larger cysts may persist without impact on surgical outcomes. No radiological signs of loosening were observed when acetabular SBCs are neglected during primary THR. |
| 5 | Takada et al., 2017, [31] | To clarify morphological changes of acetabular SBCs after THA for OA secondary to dysplasia | Retrospective 261 primary uncemented THA in 208 pts | Baseline CT preop Number of SBCs and evolution | 7–10 years (mean 8.4) | Preop CT → SBCs detected in 128 cases (49%) | Mean cross-sectional area of SBCs 3 m postop was 159.87 ± 130.05 mm2 No new SBC formation in 7–10-year follow-up Mean cross-sectional area of SBCs 7–10 y postop was 110.18 ± 130.05 mm2 (mean regression of 70.0 ± 51.6 mm2) | Acetabular SBCs do not expand after THA. The longitudinal morphological change of acetabular SBCs does not influence long-term implant fixation in THA. |
| 6 | Mechlenburg et al., 2012, [32] | To investigate whether the volume of acetabular and femoral head SBCs would change after periacetabular osteotomy | Prospective 26 pts scheduled for PAO | MRI preop Number of SBCs and total cyst volume. Pts also filled HOOS 4 years after PAO | 1 and 2.5 as well as 10 years | 22 SBCs in 12 pts (21 acetabular) | Mean total acetabular SBC volume per pt = 3.44 cm3 SD = 6.71 23 SBCs in 15 pts 1-year postop and 18 SBCs in 15 pts 2,5 years postop Mean total acetabular SBC volume per pt decreased → to 1.96 cm3 SD 3.97 1 y postop → to 0.96 cm3 SD 1.70 2, 5 y postop | No. of pts having SBCs did not change notably but the mean total cyst volume /pt decreased significantly |
| 7 | Tanamas et al., 2010, [19] | To determine the correlation between cartilage loss and risk of joint replacement with SBCs and BMLs | Retrospective Symptomatic knee in 132 pts | Baseline MRI Tibial cartilage volume, SBCs, BMLs, Risk for TKR over 4 years | 2 years | BSCs in 47.7% of subjects 98.1 of which had also BMLs | Over 2 y, 23.9% progress, 13% new SBC, 11.4% regress Baseline presence of BSC associated with lower tibial cartilage vol compared to those having only BML or neither | Annual cartilage loss is greatest in those with BSC compared to BML or neither As severity increased from no BMLs or SBCs → only BMLs → SBCs the risk of TKR increased |
| 8 | Kelly et al., 2007, [33] | To quantify the radiographic changes of OA acetabular SBCs after uncemented THA | Retrospective 130 primary uncemented THA | Baseline postop radiographs SBCs count and 2-dimensional size | 10 years | 41 SBCs identified postop with a mean size of 1 ± 0.9 cm2 (range 0.2–4.5) | 4 SBCs (10%) expanded after 10 y with an average increase in the size of 5.1 ± 8.6 cm2 (713%) 27 SBCs (66%) regressed. 23 (56%) disappeared at a mean of 5.2 ± 2.7 years (2/23 had been grafted) 10 SBCs (24%) quasi-static | No difference among the 3 SBCs groups in initial size, pt age, pt weight, different cup types, pt sex, and bone graft The only variable correlated was SBC location → zone II more likely to progress (75% of SBCs that progressed were in zone II) Recommendation to place the cup to seal SBC + grafting at the time of primary THA to prevent progression and osteolysis |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaspiris, A.; Hadjimichael, A.C.; Lianou, I.; Iliopoulos, I.D.; Ntourantonis, D.; Melissaridou, D.; Savvidou, O.D.; Papadimitriou, E.; Chronopoulos, E. Subchondral Bone Cyst Development in Osteoarthritis: From Pathophysiology to Bone Microarchitecture Changes and Clinical Implementations. J. Clin. Med. 2023, 12, 815. https://doi.org/10.3390/jcm12030815
Kaspiris A, Hadjimichael AC, Lianou I, Iliopoulos ID, Ntourantonis D, Melissaridou D, Savvidou OD, Papadimitriou E, Chronopoulos E. Subchondral Bone Cyst Development in Osteoarthritis: From Pathophysiology to Bone Microarchitecture Changes and Clinical Implementations. Journal of Clinical Medicine. 2023; 12(3):815. https://doi.org/10.3390/jcm12030815
Chicago/Turabian StyleKaspiris, Angelos, Argyris C. Hadjimichael, Ioanna Lianou, Ilias D. Iliopoulos, Dimitrios Ntourantonis, Dimitra Melissaridou, Olga D. Savvidou, Evangelia Papadimitriou, and Efstathios Chronopoulos. 2023. "Subchondral Bone Cyst Development in Osteoarthritis: From Pathophysiology to Bone Microarchitecture Changes and Clinical Implementations" Journal of Clinical Medicine 12, no. 3: 815. https://doi.org/10.3390/jcm12030815
APA StyleKaspiris, A., Hadjimichael, A. C., Lianou, I., Iliopoulos, I. D., Ntourantonis, D., Melissaridou, D., Savvidou, O. D., Papadimitriou, E., & Chronopoulos, E. (2023). Subchondral Bone Cyst Development in Osteoarthritis: From Pathophysiology to Bone Microarchitecture Changes and Clinical Implementations. Journal of Clinical Medicine, 12(3), 815. https://doi.org/10.3390/jcm12030815








