Impact of Insulin Resistance on Ovarian Sensitivity and Pregnancy Outcomes in Patients with Polycystic Ovary Syndrome Undergoing IVF
Abstract
:1. Introduction
2. Materials and Methods
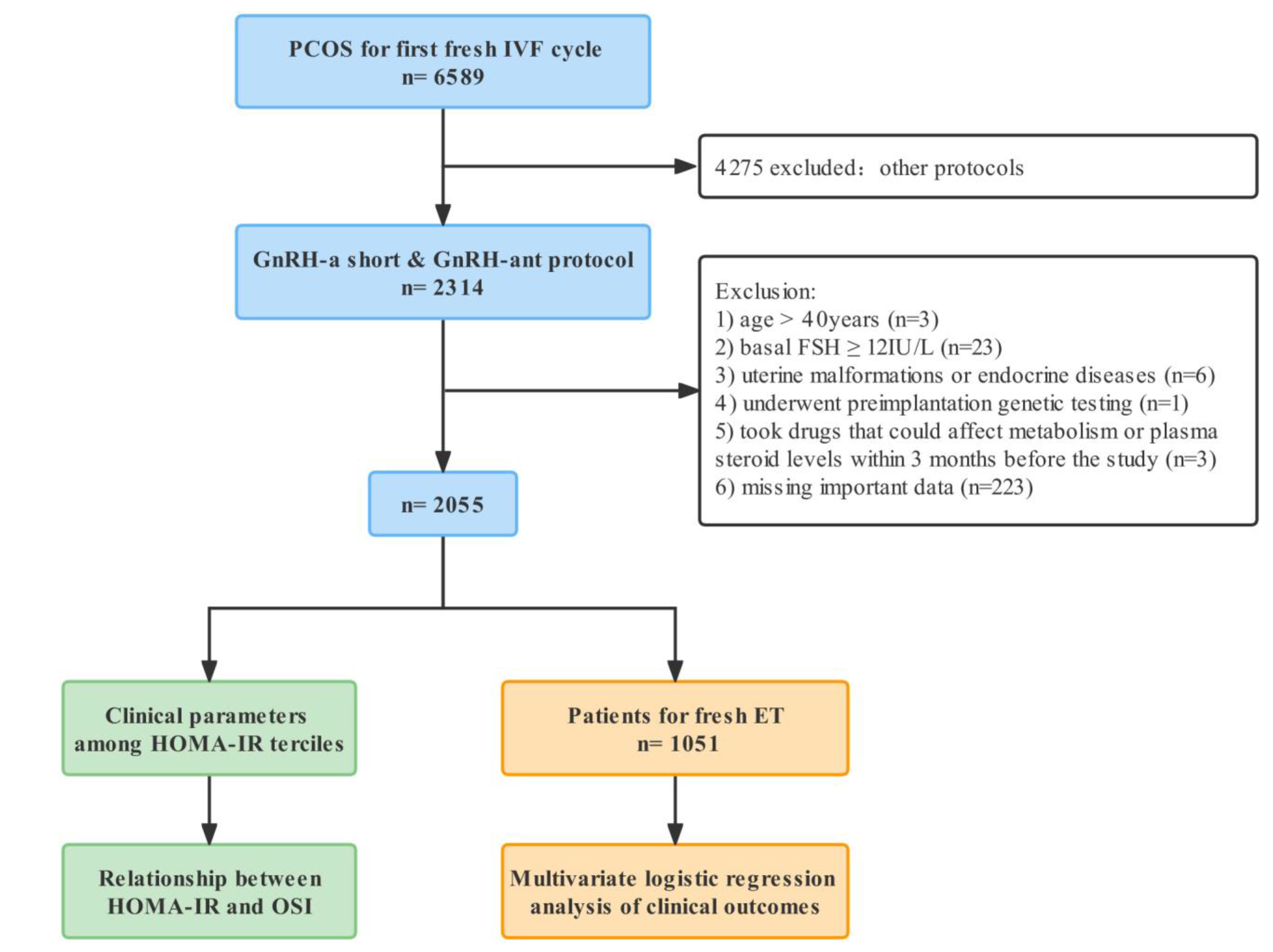
2.1. Study Design and Patients
2.2. Controlled Ovarian Stimulation Protocol
2.3. Hormone Measurements
2.4. Indicators
2.5. Statistical Analysis
3. Results
3.1. Demographic and Characteristics
3.2. Relationship between Clinical Characteristics and OSI
3.3. Effect of HOMA-IR on OSI in All Patients and BMI Subgroups
3.4. Effect of HOMA-IR on Pregnancy Outcomes
4. Discussion
4.1. The Cutoffs of HOMA-IR
4.2. Clinical Characteristics and IR
4.3. IR Affecting Ovarian Response
4.4. IR and Pregnancy Outcomes
4.5. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bozdag, G.; Mumusoglu, S.; Zengin, D.; Karabulut, E.; Yildiz, B.O. The prevalence and phenotypic features of polycystic ovary syndrome: A systematic review and meta-analysis. Hum. Reprod. 2016, 31, 2841–2855. [Google Scholar] [CrossRef] [PubMed]
- Escobar-Morreale, H. Polycystic ovary syndrome: Definition, aetiology, diagnosis and treatment. Nat. Rev. Endocrinol. 2018, 14, 270–284. [Google Scholar] [CrossRef] [PubMed]
- Glueck, C.J.; Goldenberg, N. Characteristics of obesity in polycystic ovary syndrome: Etiology, treatment, and genetics. Metabolism 2019, 92, 108–120. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, Y.; Fang, X.; Kwak-Kim, J.; Wu, L. Insulin Resistance Adversely Affect IVF Outcomes in Lean Women without PCOS. Front. Endocrinol. 2021, 12, 734638. [Google Scholar] [CrossRef] [PubMed]
- Bergman, R.N.; Finegood, D.T.; Ader, M. Assessment of Insulin Sensitivity in vivo. Endocr. Rev. 1985, 6, 45–86. [Google Scholar] [CrossRef]
- Greenwood, E.A.; Huddleston, H.G. Insulin resistance in polycystic ovary syndrome: Concept versus cutoff. Fertil. Steril. 2019, 112, 827–828. [Google Scholar] [CrossRef] [Green Version]
- Diamanti-Kandarakis, E.; Dunaif, A. Insulin Resistance and the Polycystic Ovary Syndrome Revisited: An Update on Mechanisms and Implications. Endocr. Rev. 2012, 33, 981–1030. [Google Scholar] [CrossRef]
- Kezele, P.R.; Nilsson, E.E.; Skinner, M.K. Insulin but not insulin-like growth factor-1 promotes the primordial to primary follicle transition. Mol. Cell. Endocrinol. 2002, 192, 37–43. [Google Scholar] [CrossRef]
- Phy, J.L.; Conover, C.A.; Abbott, D.H.; Zschunke, M.A.; Walker, D.L.; Session, D.R.; Tummon, I.S.; Thornhill, A.R.; Lesnick, T.G.; Dumesic, D.A. Insulin and Messenger Ribonucleic Acid Expression of Insulin Receptor Isoforms in Ovarian Follicles from Nonhirsute Ovulatory Women and Polycystic Ovary Syndrome Patients. J. Clin. Endocrinol. Metab. 2004, 89, 3561–3566. [Google Scholar] [CrossRef] [Green Version]
- Moghetti, P.; Tosi, F. Insulin resistance and PCOS: Chicken or egg? J. Endocrinol. Investig. 2021, 44, 233–244. [Google Scholar] [CrossRef]
- Hassani, F.; Oryan, S.; Eftekhari-Yazdi, P.; Bazrgar, M.; Moini, A.; Nasiri, N.; Ghaheri, A. Association between The Number of Retrieved Mature Oocytes and Insulin Resistance or Sensitivity in Infertile Women with Polycystic Ovary Syndrome. Int. J. Fertil. Steril. 2019, 12, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Fedorcsák, P.; Dale, P.O.; Storeng, R.; Åbyholm, T.; Tanbo, T. The effect of metformin on ovarian stimulation and in vitro fertilization in insulin-resistant women with polycystic ovary syndrome: An open-label randomized cross-over trial. Gynecol. Endocrinol. 2003, 17, 207–214. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Lu, Y.; Zhu, Q.; Wang, Y.; Lindheim, S.R.; Qi, J.; Li, X.; Ding, Y.; Shi, Y.; Wei, D.; et al. Influence of metabolic syndrome on female fertility and in vitro fertilization outcomes in PCOS women. Am. J. Obstet. Gynecol. 2019, 221, 138.e1–138.e12. [Google Scholar] [CrossRef] [PubMed]
- Fleming, R.; Deshpande, N.; Traynor, I.; Yates, R. Dynamics of FSH-induced follicular growth in subfertile women: Relationship with age, insulin resistance, oocyte yield and anti-Mullerian hormone. Hum. Reprod. 2006, 21, 1436–1441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fulghesu, A.M.; Villa, P.; Pavone, V.; Guido, M.; Apa, R.; Caruso, A.; Lanzone, A.; Rossodivita, A.; Mancuso, S. The Impact of Insulin Secretion on the Ovarian Response to Exogenous Gonadotropins in Polycystic Ovary Syndrome. J. Clin. Endocrinol. Metab. 1997, 82, 644–648. [Google Scholar] [CrossRef]
- Gallot, V.; da Silva, A.L.B.; Genro, V.; Grynberg, M.; Frydman, N.; Fanchin, R. Antral follicle responsiveness to follicle-stimulating hormone administration assessed by the Follicular Output RaTe (FORT) may predict in vitro fertilization-embryo transfer outcome. Hum. Reprod. 2012, 27, 1066–1072. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Fuentes, A.; Rouleau, J.P.; Vásquez, D.; Hernández, J.; Naftolin, F.; Palumbo, A. Volume-based follicular output rate improves prediction of the number of mature oocytes: A prospective comparative study. Fertil. Steril. 2022, 118, 885–892. [Google Scholar] [CrossRef]
- Alviggi, C.; Conforti, A.; Esteves, S.C.; Vallone, R.; Venturella, R.; Staiano, S.; Castaldo, E.; Andersen, C.Y.; De Placido, G. Understanding Ovarian Hypo-Response to Exogenous Gonadotropin in Ovarian Stimulation and Its New Proposed Marker—The Follicle-To-Oocyte (FOI) Index. Front. Endocrinol. 2018, 9, 589. [Google Scholar] [CrossRef]
- Hassan, A.M.A.; Kotb, M.M.M.; Awadallah, A.M.A.; Shehata, N.; Wahba, A. Follicular sensitivity index (FSI): A novel tool to predict clinical pregnancy rate in IVF/ICSI cycles. J. Assist. Reprod. Genet. 2017, 34, 1317–1324. [Google Scholar] [CrossRef]
- Huber, M.; Hadziosmanovic, N.; Berglund, L.; Holte, J. Using the ovarian sensitivity index to define poor, normal, and high response after controlled ovarian hyperstimulation in the long gonadotropin-releasing hormone-agonist protocol: Suggestions for a new principle to solve an old problem. Fertil. Steril. 2013, 100, 1270–1276. [Google Scholar] [CrossRef]
- Vaegter, K.K.; Lakic, T.G.; Olovsson, M.; Berglund, L.; Brodin, T.; Holte, J. Which factors are most predictive for live birth after in vitro fertilization and intracytoplasmic sperm injection (IVF/ICSI) treatments? Analysis of 100 prospectively recorded variables in 8,400 IVF/ICSI single-embryo transfers. Fertil. Steril. 2017, 107, 641–648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Revelli, A.; Gennarelli, G.; Biasoni, V.; Chiadò, A.; Carosso, A.; Evangelista, F.; Paschero, C.; Filippini, C.; Benedetto, C. The Ovarian Sensitivity Index (OSI) Significantly Correlates with Ovarian Reserve Biomarkers, Is More Predictive of Clinical Pregnancy than the Total Number of Oocytes, and Is Consistent in Consecutive IVF Cycles. J. Clin. Med. 2020, 9, 1914. [Google Scholar] [CrossRef]
- Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil. Steril. 2004, 81, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Rotterdam ESHRE/ASRM-sponsored PCOS consensus workshop group Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum. Reprod. 2004, 19, 41–47. [CrossRef] [Green Version]
- Kim, J.J.; Hwang, K.R.; Oh, S.H.; Chae, S.J.; Yoon, S.H.; Choi, Y.M. Prevalence of insulin resistance in Korean women with polycystic ovary syndrome according to various homeostasis model assessment for insulin resistance cutoff values. Fertil. Steril. 2019, 112, 959–966. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-Y.; Freedman, N.D.; Haiman, S.C.A.; Le Marchand, L.; Wilkens, D.L.R.; Setiawan, V.W. Association of Coffee Consumption with Total and Cause-Specific Mortality Among Nonwhite Populations. Ann. Intern. Med. 2017, 167, 228–235. [Google Scholar] [CrossRef]
- Kong, X.; Huang, X.; Zhao, M.; Xu, B.; Xu, R.; Song, Y.; Yu, Y.; Yang, W.; Zhang, J.; Liu, L.; et al. Platelet Count Affects Efficacy of Folic Acid in Preventing First Stroke. J. Am. Coll. Cardiol. 2018, 71, 2136–2146. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, J.; Li, A.; Yang, N.; Cui, N.; Hao, G.; Gao, B.-L. Impact of Elevated Progesterone in Late Follicular Phase on Early Pregnancy Outcomes and Live Birth Rate After Fresh Embryo Transfers. Front. Cell Dev. Biol. 2022, 10, 855455. [Google Scholar] [CrossRef]
- Greenwood, E.A.; Pasch, L.A.; Cedars, M.I.; Legro, R.S.; Eisenberg, E.; Huddleston, H.G. Insulin resistance is associated with depression risk in polycystic ovary syndrome. Fertil. Steril. 2018, 110, 27–34. [Google Scholar] [CrossRef]
- Li, Y.; Tan, J.; Wang, Q.; Duan, C.; Hu, Y.; Huang, W. Comparing the individual effects of metformin and rosiglitazone and their combination in obese women with polycystic ovary syndrome: A randomized controlled trial. Fertil. Steril. 2020, 113, 197–204. [Google Scholar] [CrossRef]
- Chen, Y.; Zheng, X.; Ma, D.; Zheng, S.; Han, Y.; Su, W.; Liu, W.; Xiao, F.; Lin, M.; Yan, X.; et al. Neck circumference is a good predictor for insulin resistance in women with polycystic ovary syndrome. Fertil. Steril. 2021, 115, 753–760. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Qiao, J. Association of Insulin Resistance and Elevated Androgen Levels with Polycystic Ovarian Syndrome (PCOS): A Review of Literature. J. Healthc. Eng. 2022, 2022, 9240569. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.S.; Kakoly, N.S.; Tan, J.W.J.; Fitzgerald, G.; Khomami, M.B.; Joham, A.E.; Cooray, S.; Misso, M.L.; Norman, R.; Harrison, C.L.; et al. Metabolic syndrome in polycystic ovary syndrome: A systematic review, meta-analysis and meta-regression. Obes. Rev. 2019, 20, 339–352. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Yang, X.; Li, J.; Yu, J.; Wu, X. Effect of hyperinsulinaemia and insulin resistance on endocrine, metabolic and fertility outcomes in women with polycystic ovary syndrome undergoing ovulation induction. Clin. Endocrinol. 2019, 91, 440–448. [Google Scholar] [CrossRef]
- Homburg, R.; Pariente, C.; Lunenfeld, B.; Jacobs, H. The role of insulin-like growth factor-1 (IGF-1) and IGF binding protein-1 (IGFBP-1) in the pathogenesis of polycystic ovary syndrome. Hum. Reprod. 1992, 7, 1379–1383. [Google Scholar] [CrossRef]
- Bergh, C.; Carlsson, B.; Olsson, J.-H.; Selleskog, U.; Hillensjö, T. Regulation of androgen production in cultured human thecal cells by insulin-like growth factor I and insulin. Fertil. Steril. 1993, 59, 323–331. [Google Scholar] [CrossRef]
- Armanini, D.; Boscaro, M.; Bordin, L.; Sabbadin, C. Controversies in the Pathogenesis, Diagnosis and Treatment of PCOS: Focus on Insulin Resistance, Inflammation, and Hyperandrogenism. Int. J. Mol. Sci. 2022, 23, 4110. [Google Scholar] [CrossRef]
- Nestler, J.E.; Jakubowicz, D.J.; de Vargas, A.F.; Brik, C.; Quintero, N.; Medina, F. Insulin Stimulates Testosterone Biosynthesis by Human Thecal Cells from Women with Polycystic Ovary Syndrome by Activating Its Own Receptor and Using Inositolglycan Mediators as the Signal Transduction System1. J. Clin. Endocrinol. Metab. 1998, 83, 2001–2005. [Google Scholar] [CrossRef] [Green Version]
- Xu, P.; Huang, B.-Y.; Zhan, J.-H.; Liu, M.-T.; Fu, Y.; Su, Y.-Q.; Sun, Q.-Y.; Wang, W.-H.; Chen, D.-J.; Liu, J.-Q. Insulin Reduces Reaction of Follicular Granulosa Cells to FSH Stimulation in Women with Obesity-Related Infertility During IVF. J. Clin. Endocrinol. Metab. 2019, 104, 2547–2560. [Google Scholar] [CrossRef]
- Anjali, G.; Kaur, S.; Lakra, R.; Taneja, J.; Kalsey, G.S.; Nagendra, A.; Shrivastav, T.; Devi, M.G.; Malhotra, N.; Kriplani, A.; et al. FSH stimulates IRS-2 expression in human granulosa cells through cAMP/SP1, an inoperative FSH action in PCOS patients. Cell. Signal. 2015, 27, 2452–2466. [Google Scholar] [CrossRef]
- Chahal, N.; Geethadevi, A.; Kaur, S.; Lakra, R.; Nagendra, A.; Shrivastav, T.; De Pascali, F.; Reiter, E.; Crépieux, P.; Devi, M.G.; et al. Direct impact of gonadotropins on glucose uptake and storage in preovulatory granulosa cells: Implications in the pathogenesis of polycystic ovary syndrome. Metabolism 2021, 115, 154458. [Google Scholar] [CrossRef] [PubMed]
- Eng, G.S.; Sheridan, R.A.; Wyman, A.; Chi, M.M.-Y.; Bibee, K.P.; Jungheim, E.S.; Moley, K.H. AMP Kinase Activation Increases Glucose Uptake, Decreases Apoptosis, and Improves Pregnancy Outcome in Embryos Exposed to High IGF-I Concentrations. Diabetes 2007, 56, 2228–2234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, Y.; Pan, Y.; Li, P.; Wang, Z.; Wang, Z.; Shi, Y. Ovarian Sensitivity Decreased Significantly in Patients with Insulin Resistance Undergoing in vitro Fertilization and Embryo Transfer. Front. Physiol. 2022, 12, 809419. [Google Scholar] [CrossRef]
- Ketel, I.J.G.; Stehouwer, C.D.A.; Serné, E.H.; Korsen, T.J.M.; Hompes, P.G.A.; Smulders, Y.M.; de Jongh, R.T.; Homburg, R.; Lambalk, C.B. Obese but Not Normal-Weight Women with Polycystic Ovary Syndrome Are Characterized by Metabolic and Microvascular Insulin Resistance. J. Clin. Endocrinol. Metab. 2008, 93, 3365–3372. [Google Scholar] [CrossRef] [Green Version]
- Zhu, S.; Zhang, B.; Jiang, X.; Li, Z.; Zhao, S.; Cui, L.; Chen, Z.-J. Metabolic disturbances in non-obese women with polycystic ovary syndrome: A systematic review and meta-analysis. Fertil. Steril. 2019, 111, 168–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sunkara, S.K.; Rittenberg, V.; Raine-Fenning, N.; Bhattacharya, S.; Zamora, J.; Coomarasamy, A. Association between the number of eggs and live birth in IVF treatment: An analysis of 400 135 treatment cycles. Hum. Reprod. 2011, 26, 1768–1774. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.-F.; Zhang, J.; Xu, Y.-M.; Cao, Z.-Y.; Wang, Y.-Z.; Hao, G.-M.; Gao, B.-L. High BMI and Insulin Resistance Are Risk Factors for Spontaneous Abortion in Patients with Polycystic Ovary Syndrome Undergoing Assisted Reproductive Treatment: A Systematic Review and Meta-Analysis. Front. Endocrinol. 2020, 11, 592495. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Guo, J.; Zhang, Q.; Zhang, C. Insulin Resistance is a Risk Factor for Early Miscarriage and Macrosomia in Patients with Polycystic Ovary Syndrome from the First Embryo Transfer Cycle: A Retrospective Cohort Study. Front. Endocrinol. 2022, 13, 853473. [Google Scholar] [CrossRef]
- Tian, L.; Shen, H.; Lu, Q.; Norman, R.; Wang, J. Insulin Resistance Increases the Risk of Spontaneous Abortion after Assisted Reproduction Technology Treatment. J. Clin. Endocrinol. Metab. 2007, 92, 1430–1433. [Google Scholar] [CrossRef] [Green Version]
- He, F.-F.; Li, Y.-M. Role of gut microbiota in the development of insulin resistance and the mechanism underlying polycystic ovary syndrome: A review. J. Ovarian Res. 2020, 13, 73. [Google Scholar] [CrossRef]
- Katz, A.; Nambi, S.S.; Mather, K.; Baron, A.D.; Follmann, D.A.; Sullivan, G.; Quon, M.J. Quantitative Insulin Sensitivity Check Index: A Simple, Accurate Method for Assessing Insulin Sensitivity in Humans. J. Clin. Endocrinol. Metab. 2000, 85, 2402–2410. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | HOMA-IR ≤ 1.97 (n = 685) | 1.97 < HOMA-IR ≤ 3.71 (n = 685) | HOMA-IR > 3.71 (n = 685) | p-Value |
|---|---|---|---|---|
| Age (years) | 29.12 ± 3.61 | 29.25 ± 3.64 | 29.28 ± 3.89 | 0.698 |
| BMI (kg/m2) | 22.48 ± 3.24 | 24.39 ± 3.44 | 26.20 ± 3.53 | <0.001 |
| Infertility duration (years) | 3.49 ± 2.54 | 3.67 ± 2.28 | 3.77 ± 2.74 | 0.114 |
| Infertility type | 0.298 | |||
| Primary infertility | 475 (69.34%) | 461 (67.30%) | 448 (65.40%) | |
| Secondary infertility | 210 (30.66%) | 224 (32.70%) | 237 (34.60%) | |
| AMH (ng/mL) | 7.59 ± 3.39 | 7.13 ± 3.26 | 6.80 ± 2.98 | <0.001 |
| Basal FSH (IU/L) | 7.00 ± 1.81 | 6.86 ± 1.75 | 6.75 ± 1.77 | 0.038 |
| Basal LH (IU/L) | 6.94 ± 4.95 | 7.24 ± 5.23 | 7.43 ± 4.85 | 0.016 |
| Basal T (ng/mL) | 0.61 ± 0.34 | 0.65 ± 0.36 | 0.66 ± 0.36 | 0.011 * |
| AFC | 19.58 ± 7.02 | 19.54 ± 6.89 | 19.91 ± 6.93 | 0.556 |
| PCOS phenotypes | 0.105 | |||
| HA + OA + PCO | 130 (18.98%) | 124 (18.10%) | 118 (17.23%) | |
| HA + OA | 93 (13.58%) | 97 (14.16%) | 89 (12.99%) | |
| HA + PCO | 205 (29.93%) | 206 (30.07%) | 172 (25.11%) | |
| OA + PCO | 257 (37.52%) | 258 (37.66%) | 306 (44.67%) | |
| Blood pressure | ||||
| Systolic (mmHg) | 112.14 ± 8.04 | 113.07 ± 8.20 | 114.28 ± 10.23 | <0.001 |
| Diastolic (mmHg) | 72.72 ± 6.68 | 73.49 ± 7.12 | 74.17 ± 7.94 | 0.001 |
| Triglyceride (mmol/L) | 1.11 ± 0.57 | 1.32 ± 0.60 | 1.48 ± 0.79 | <0.001 * |
| Cholesterol (mmol/L) | 4.37 ± 0.68 | 4.40 ± 0.66 | 4.49 ± 0.69 | 0.002 |
| Fasting glucose (mmol/L) | 5.16 ± 0.36 | 5.28 ± 0.36 | 5.46 ± 0.38 | <0.001 |
| Fasting insulin (mIU/L) | 5.48 ± 2.03 | 11.54 ± 2.07 | 24.58 ± 7.22 | <0.001 |
| HOMA-IR | 1.25 ± 0.46 | 2.70 ± 0.48 | 5.98 ± 1.89 | <0.001 |
| Protocol | <0.001 | |||
| GnRH-antagonist | 211 (30.80%) | 256 (37.37%) | 277 (40.44%) | |
| GnRH-antagonist | 474 (69.20%) | 429 (62.63%) | 408 (59.56%) | |
| FSH initial dosage (IU) | 189.21 ± 48.83 | 197.52 ± 49.94 | 205.55 ± 52.07 | <0.001 |
| FSH total dosage (IU) | 2049.24 ± 747.39 | 2206.55 ± 849.45 | 2379.65 ± 926.39 | <0.001 |
| Duration of COS (days) | 10.28 ± 2.69 | 10.36 ± 2.45 | 10.68 ± 2.58 | 0.012 |
| E2 on trigger day (pg/mL) | 4285.59 ± 1158.08 | 3982.59 ± 1379.20 | 3825.08 ± 1385.84 | <0.001 |
| No. of oocyte retrieved | 17.28 ± 10.06 | 16.38 ± 9.11 | 15.13 ± 8.10 | 0.005 * |
| OSI | 9.90 ± 7.57 | 8.88 ± 6.73 | 7.38 ± 4.83 | <0.001 * |
| Variable | Value | Crude β (95% CI) | p-Value |
|---|---|---|---|
| Age (years) | 29.22 ± 3.72 | −0.26 (−0.33, −0.18) | <0.0001 |
| Age subgroups (years) | |||
| [20, 30) | 1184 (57.62%) | 0 | |
| [30, 35) | 686 (33.38%) | −0.53 (−0.94, 0.29) | 0.297 |
| [35, 40] | 185 (9.00%) | −2.94 (−3.96, −1.93) | <0.0001 |
| BMI (kg/m2) | 24.35 ± 3.73 | −0.31 (−0.39, −0.24) | <0.0001 |
| BMI subgroups (kg/m2) | |||
| <18.5 | 87 (4.26%) | 0 | |
| [18.5, 25) | 1106 (54.11%) | −0.50 (−1.92, 0.91) | 0.486 |
| [25, 30) | 701 (34.30%) | −2.31 (−3.76, −0.87) | 0.0017 |
| ≥30 | 150 (7.34%) | −3.82 (−5.53, −2.10) | <0.0001 |
| Infertility duration (years) | 3.64 ± 2.53 | −0.28 (−0.39, −0.17) | <0.0001 |
| Infertility type | |||
| Primary infertility | 1384 (67.35%) | 0 | |
| Secondary infertility | 671 (32.65%) | −0.34 (−0.95, 0.27) | 0.272 |
| AMH (ng/mL) | 7.17 ± 3.23 | 0.94 (0.86, 1.01) | <0.0001 |
| Basal FSH (IU/L) | 6.87 ± 1.78 | −0.94 (−1.10, −0.78) | <0.0001 |
| Basal LH (IU/L) | 7.21 ± 5.02 | 0.28 (0.22, 0.34) | <0.0001 |
| Basal T (ng/mL) | 0.94 ± 0.52 | −2.31 (−2.85, −1.77) | <0.0001 |
| AFC | 19.68 ± 6.95 | 0.39 (0.36, 0.43) | <0.0001 |
| PCOS phenotypes | |||
| HA + OA + PCO | 372 (18.10%) | 0 | |
| HA + OA | 279 (13.58%) | −3.88 (−4.86, −2.89) | <0.0001 |
| HA + PCO | 583 (28.37%) | −3.71 (−4.54, −2.89) | <0.0001 |
| OA + PCO | 821 (39.95%) | −0.57 (−1.35, 0.21) | 0.150 |
| Blood pressure | |||
| Systolic (mmHg) | 113.16 ± 8.91 | 0.00 (−0.03, 0.04) | 0.844 |
| Diastolic (mmHg) | 73.46 ± 7.29 | −0.03 (−0.07, 0.01) | 0.128 |
| Triglyceride (mmol/L) | 1.30 ± 0.68 | −1.15 (−1.57, −0.73) | <0.0001 |
| Cholesterol (mmol/L) | 4.42 ± 0.67 | 0.03 (−0.39, 0.45) | 0.882 |
| Fasting glucose (mmol/L) | 5.30 ± 0.39 | −1.50 (−2.23, −0.77) | <0.0001 |
| Fasting insulin (mIU/L) | 13.87 ± 9.15 | −0.12 (−0.15, −0.09) | <0.0001 |
| HOMA-IR | 3.31 ± 2.29 | −0.50 (−0.62, −0.37) | <0.0001 |
| HOMA-IR Tercile | |||
| Low | 685 (33.33%) | 0 | |
| Middle | 685 (33.33%) | −1.03 (−1.71, −0.34) | 0.0035 |
| High | 685 (33.33%) | −2.52 (−3.21, −1.84) | <0.0001 |
| Protocol | |||
| GnRH-antagonist | 744 (36.20%) | 0 | |
| GnRH-antagonist | 1311 (63.80%) | −0.81 (−1.40, −0.22) | 0.0075 |
| FSH initial dosage (IU) | 197.44 ± 50.71 | −0.06 (−0.06, −0.05) | <0.0001 |
| Duration of COS (days) | 10.44 ± 2.58 | −0.69 (−0.80, −0.58) | <0.0001 |
| E2 on trigger day (pg/mL) | 4032.31 ± 1324.82 | 0.00 (0.00, 0.00) * | <0.0001 |
| Variable | All Patients | Lean (BMI < 25 kg/m2) | Overweight to Obese (BMI ≥ 25 kg/m2) | Test for Interaction | |||
|---|---|---|---|---|---|---|---|
| Adjusted β (95% CI) | p-Value | Adjusted β (95% CI) | p-Value | Adjusted β (95% CI) | p-Value | p-Value | |
| HOMA-IR | −0.24 (−0.35, −0.13) | <0.0001 | −0.33 (−0.51, −0.16) | <0.0001 | −0.12 (−0.27, 0.02) | 0.092 | 0.017 |
| HOMA-IR Tercile | 0.212 | ||||||
| Low | 0 | 0 | 0 | ||||
| Middle | −0.30 (−0.90, 0.30) | 0.326 | −0.63 (−1.46, 0.21) | 0.140 | −0.18 (−0.99, 0.62) | 0.654 | |
| High | −1.10 (−1.74, −0.46) | 0.0008 | −1.45 (−2.30, −0.61) | 0.0008 | −0.58 (−1.39, 0.23) | 0.163 | |
| HOMA-IR category | 0.090 | ||||||
| <2.56 | 0 | 0 | 0 | ||||
| ≥2.56 | −0.88 (−1.39, −0.36) | 0.0008 | −1.07 (−1.77, −0.37) | 0.003 | −0.53 (−1.27, 0.20) | 0.155 | |
| Variable | Non-Adjusted | Adjust I | Adjust II | |||
|---|---|---|---|---|---|---|
| Crude OR (95% CI) | p-Value | Adjusted OR (95% CI) | p-Value | Adjusted OR (95% CI) | p-Value | |
| biochemical pregnancy | ||||||
| HOMA-IR | 0.99 (0.94, 1.04) | 0.626 | 0.99 (0.93, 1.05) | 0.727 | 1.00 (0.94, 1.06) | 0.914 |
| HOMA-IR Tercile | ||||||
| Low | 1 | 1 | 1 | |||
| Middle | 0.90 (0.66, 1.21) | 0.475 | 0.87 (0.63, 1.22) | 0.426 | 0.91 (0.65, 1.27) | 0.573 |
| High | 0.94 (0.70, 1.27) | 0.687 | 0.95 (0.67, 1.33) | 0.749 | 1.00 (0.70, 1.43) | 0.992 |
| HOMA-IR category | ||||||
| <2.56 | 1 | 1 | ||||
| ≥2.56 | 0.95 (0.74, 1.21) | 0.656 | 0.93 (0.70, 1.22) | 0.584 | 0.97 (0.73, 1.29) | 0.829 |
| clinical pregnancy | ||||||
| HOMA-IR | 0.98 (0.93, 1.03) | 0.406 | 0.99 (0.93, 1.05) | 0.687 | 0.99 (0.94, 1.05) | 0.829 |
| HOMA-IR Tercile | ||||||
| Low | 1 | 1 | 1 | |||
| Middle | 0.85 (0.63, 1.15) | 0.285 | 0.83 (0.59, 1.15) | 0.258 | 0.85 (0.61, 1.19) | 0.343 |
| High | 0.89 (0.66, 1.19) | 0.432 | 0.93 (0.66, 1.31) | 0.687 | 0.97 (0.68, 1.38) | 0.876 |
| HOMA-IR category | ||||||
| <2.56 | 1 | 1 | 1 | |||
| ≥2.56 | 0.90 (0.70, 1.15) | 0.388 | 0.90 (0.69, 1.19) | 0.477 | 0.94 (0.71, 1.24) | 0.658 |
| early miscarriage | ||||||
| HOMA-IR | 1.13 (1.02, 1.26) | 0.023 | 1.08 (0.96, 1.22) | 0.204 | 1.08 (0.96, 1.22) | 0.190 |
| HOMA-IR Tercile | ||||||
| Low | 1 | 1 | 1 | |||
| Middle | 1.89 (0.87, 4.10) | 0.107 | 2.07 (0.88, 4.90) | 0.097 | 2.07 (0.88, 4.85) | 0.096 |
| High | 2.56 (1.23, 5.32) | 0.012 | 2.40 (1.03, 5.58) | 0.042 | 2.33 (1.00, 5.44) | 0.049 |
| HOMA-IR category | ||||||
| <2.56 | 1 | 1 | 1 | |||
| ≥2.56 | 2.30 (1.27, 4.16) | 0.006 | 2.21 (1.13, 4.33) | 0.020 | 2.16 (1.11, 4.20) | 0.024 |
| miscarriage | ||||||
| HOMA-IR | 1.11 (1.02, 1.22) | 0.019 | 1.04 (0.94, 1.16) | 0.407 | 1.05 (0.94, 1.16) | 0.376 |
| HOMA-IR Tercile | ||||||
| Low | 1 | 1 | 1 | |||
| Middle | 1.37 (0.76, 2.47) | 0.295 | 1.08 (0.56, 2.10) | 0.820 | 1.09 (0.56, 2.10) | 0.805 |
| High | 1.89 (1.08, 3.29) | 0.025 | 1.35 (0.71, 2.56) | 0.363 | 1.32 (0.69, 2.53) | 0.401 |
| HOMA-IR category | ||||||
| <2.56 | 1 | 1 | 1 | |||
| ≥2.56 | 1.80 (1.14, 2.86) | 0.013 | 1.38 (0.81, 2.33) | 0.234 | 1.37 (0.81, 2.32) | 0.240 |
| live birth | ||||||
| HOMA-IR | 0.95 (0.90, 1.00) | 0.045 | 0.97 (0.91, 1.03) | 0.380 | 0.98 (0.92, 1.04) | 0.457 |
| HOMA-IR Tercile | ||||||
| Low | 1 | 1 | 1 | |||
| Middle | 0.85 (0.63, 1.16) | 0.304 | 0.90 (0.64, 1.26) | 0.532 | 0.93 (0.66, 1.31) | 0.687 |
| High | 0.75 (0.56, 1.03) | 0.072 | 0.87 (0.61, 1.25) | 0.455 | 0.92 (0.64, 1.32) | 0.641 |
| HOMA-IR category | ||||||
| <2.56 | 1 | 1 | 1 | |||
| ≥2.56 | 0.77 (0.60, 0.99) | 0.044 | 0.85 (0.64, 1.12) | 0.249 | 0.88 (0.66, 1.18) | 0.383 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, Z.; Wang, L.; Wang, Y.; Fan, Y.; Jiang, L.; Xu, X.; Du, Y.; Hao, G. Impact of Insulin Resistance on Ovarian Sensitivity and Pregnancy Outcomes in Patients with Polycystic Ovary Syndrome Undergoing IVF. J. Clin. Med. 2023, 12, 818. https://doi.org/10.3390/jcm12030818
Luo Z, Wang L, Wang Y, Fan Y, Jiang L, Xu X, Du Y, Hao G. Impact of Insulin Resistance on Ovarian Sensitivity and Pregnancy Outcomes in Patients with Polycystic Ovary Syndrome Undergoing IVF. Journal of Clinical Medicine. 2023; 12(3):818. https://doi.org/10.3390/jcm12030818
Chicago/Turabian StyleLuo, Zhuoye, Lili Wang, Yizhuo Wang, Yanli Fan, Lei Jiang, Xin Xu, Yuanjie Du, and Guimin Hao. 2023. "Impact of Insulin Resistance on Ovarian Sensitivity and Pregnancy Outcomes in Patients with Polycystic Ovary Syndrome Undergoing IVF" Journal of Clinical Medicine 12, no. 3: 818. https://doi.org/10.3390/jcm12030818





