PLT Counts as a Predictive Marker after Plasma Exchange in Patients with Hepatitis B Virus-Related Acute-on-Chronic Liver Failure
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Observation Indicators
2.3. Statistical Analysis
3. Results
3.1. Characteristics and Outcomes of HBV-ACLF Patients after PE
3.2. Correlation Analysis of PLT with Liver Function Parameters and the MELD Series Score
3.3. Identification of Prognostic Risk Factors and Establishment of a New Prediction Model
3.4. Performance of the New Model
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PLT | Platelet |
| HBV-ACLF | Hepatitis B-related acute-on-chronic liver failure |
| PE | Plasma exchange |
| MELD | Model for end-stage liver disease score |
| ROC | Receiver operation characteristic |
| TBil | Total bilirubin |
| AUC | Area under the curve |
| ACLF | Acute-on-chronic liver failure |
| SMT | Standard medical treatment |
| ALSS | Artificial liver support system |
| LT | Liver transplantation |
| AFP | Alpha-fetoprotein |
| TPO | Thrombopoietin |
| PWR | PLT to WBC ratio |
| PT | Prothrombin time |
| INR | International normalized ratio |
| ALT | Alanine aminotransferase |
| AST | Aspartate aminotransferase |
| Alb | Albumin |
| Cre | Creatine |
| MELD | Model for end-stage liver disease |
| MELD-Na | MELD-sodium |
| iMELD | Integrated MELD |
| APASL | Asian Pacific Association for the Study of the Liver AARC APASL |
| CI | Confidence interval |
| 5-HT | 5-Hydroxytryptamine |
References
- Sarin, S.K.; APASL ACLF Research Consortium (AARC) for APASL ACLF Working Party; Choudhury, A.; Sharma, M.K.; Maiwall, R.; Al Mahtab, M.; Rahman, S.; Saigal, S.; Saraf, N.; Soin, A.S.; et al. Acute-on-chronic liver failure: Consensus recommendations of the Asian Pacific association for the study of the liver (APASL): An update. Hepatol. Int. 2019, 13, 353–390. [Google Scholar] [CrossRef] [Green Version]
- Weiss, E.M.; Saner, F.M.; Asrani, S.K.M.; Biancofiore, G.M.; Blasi, A.; Lerut, J.M.; Durand, F.M.; Fernandez, J.; Findlay, J.Y.M.; Fondevila, C.; et al. When Is a Critically Ill Cirrhotic Patient Too Sick to Transplant? Development of Consensus Criteria by a Multidisciplinary Panel of 35 International Experts. Transplantation 2021, 105, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Shang, D.B.; Xiang, X.G. Advances in the pathogenesis and treatment of acute-on-chronic liver failure. J. Clin. Hepatol. 2021, 37, 765–769. [Google Scholar]
- Angeli, P.; Rodríguez, E.; Piano, S.; Ariza, X.; Morando, F.; Solà, E.; Romano, A.; García, E.; Pavesi, M.; Risso, A.; et al. Acute kidney injury and acute-on- chronic liver failure classifications in prognosis assessment of patients with acute decompensation of cirrhosis. Gut 2015, 64, 1616–1622. [Google Scholar] [CrossRef] [Green Version]
- Yue-Meng, W.; Yang, L.-H.; Yang, J.-H.; Xu, Y.; Yang, J.; Song, G.-B. The effect of plasma exchange on entecavir-treated chronic hepatitis B patients with hepatic de-compensation and acute-on-chronic liver failure. Hepatol. Int. 2016, 10, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Liang, X.; Xu, S.; Xiong, Y.; Huang, J. A non-bioartificial liver support system combined with transplantation in HBV-related acute-on-chronic liver failure. Sci. Rep. 2021, 3, 2975. [Google Scholar] [CrossRef]
- Wang, X.; Sun, M.; Yang, X.; Gao, L.; Weng, M.; Yang, D.; Li, H.; Zhou, X.; Li, J.; Qin, S.; et al. Value of Liver Regeneration in Predicting Short-Term Prognosis for Patients with Hepatitis B-Related Acute-on-Chronic Liver Failure. BioMed Res. Int. 2020, 6, 5062873. [Google Scholar] [CrossRef]
- Starlinger, P.; Assinger, A. Importance of platelet-derived growth factors in liver regeneration. Expert Rev. Gastroenterol. Hepatol. 2016, 10, 557–559. [Google Scholar] [CrossRef] [Green Version]
- Aryal, B.; Yamakuchi, M.; Shimizu, T.; Kadono, J.; Furoi, A.; Gejima, K.; Komokata, T.; Hashiguchi, T.; Imoto, Y. Therapeutic implication of platelets in liver regeneration—Hopes and hues. Expert Rev. Gastroenterol. Hepatol. 2018, 12, 1219–1228. [Google Scholar] [CrossRef]
- Takahashi, K.; Nagai, S.; Collins, K.M.; Safwan, M.; Rizzari, M.D.; Schnickel, G.T.; Yoshida, A.; Abouljoud, M.S. Factors associated with low graft regeneration in the early phase after living donor liver transplantation. Clin. Transplant. 2019, 33, e13690. [Google Scholar] [CrossRef]
- Jie, Y.; Gong, J.; Xiao, C.; Zhu, S.; Zhou, W.; Luo, J.; Chong, Y.; Hu, B. Low Platelet to White Blood Cell Ratio Indicates Poor Prognosis for Acute-On-Chronic Liver Failure. BioMed Res. Int. 2018, 2018, 7394904. [Google Scholar] [CrossRef] [PubMed]
- Alsebaey, A.; Sabry, A.; Rashed, H.S.; Elsabaawy, M.M.; Ragab, A.; Aly, R.A.; Badran, H. MELD-Sarcopenia is Better than ALBI and MELD Score in Patients with Hepatocellular Carcinoma Awaiting Liver Transplantation. Asian Pac. J. Cancer Prev. 2021, 22, 2005–2009. [Google Scholar] [CrossRef]
- Qin, L.; Li, C.; Xie, F.; Wang, Z.; Wen, T. Combination of albumin-bilirubin grade and clinically significant portal hypertension predicts the prognosis of patients with hepatocellular carcinoma after liver resection. Biosci. Trends 2021, 15, 41–49. [Google Scholar] [CrossRef]
- Ha, J.M.; Sohn, W.; Cho, J.Y.; Pyo, J.H.; Choi, K.; Sinn, D.H.; Gwak, G.-Y.; Choi, M.S.; Lee, J.H.; Koh, K.C.; et al. Static and dynamic prognostic factors for hepatitis-B-related acute-on-chronic liver failure. Clin. Mol. Hepatol. 2015, 21, 232–241. [Google Scholar] [CrossRef] [Green Version]
- Biggins, S.W.; Kim, W.R.; Terrault, N.A.; Saab, S.; Balan, V.; Schiano, T.; Benson, J.; Therneau, T.; Kremers, W.; Wiesner, R.; et al. Evidence-Based Incorporation of Serum Sodium Concentration Into MELD. Gastroenterology 2006, 130, 1652–1660. [Google Scholar] [CrossRef] [PubMed]
- Luca, A.; Angermayr, B.; Bertolini, G.; Koenig, F.; Vizzini, G.; Ploner, M.; Peck-Radosavljevic, M.; Gridelli, B.; Bosch, J. An integrated MELD model including serum sodium and age improves the prediction of early mortality in patients with cirrhosis. Liver Transplant. 2007, 13, 1174–1180. [Google Scholar] [CrossRef] [PubMed]
- Kurokawa, T.; Ohkohchi, N. Platelets in liver disease, cancer and regeneration. World J. Gastroenterol. 2017, 23, 3228–3239. [Google Scholar] [CrossRef]
- Takahashi, K.; Liang, C.; Oda, T.; Ohkohchi, N. Platelet and liver regeneration after liver surgery. Surg. Today 2019, 50, 974–983. [Google Scholar] [CrossRef]
- Padickakudy, R.; Pereyra, D.; Offensperger, F.; Jonas, P.; Oehlberger, L.; Schwarz, C.; Haegele, S.; Assinger, A.; Brostjan, C.; Gruenberger, T.; et al. Bivalent role of intra-platelet serotonin in liver regeneration and tumor recurrence in humans. J. Hepatol. 2017, 67, 1243–1252. [Google Scholar] [CrossRef]
- Greco, E.; Lupia, E.; Bosco, O.; Vizio, B.; Montrucchio, G. Platelets and Multi-Organ Failure in Sepsis. Int. J. Mol. Sci. 2017, 18, 2200. [Google Scholar] [CrossRef] [Green Version]
- Ling, Q.; Xu, X.; Wei, Q.; Liu, X.; Guo, H.; Zhuang, L.; Chen, J.; Xia, Q.; Xie, H.; Wu, J.; et al. Downgrading MELD Improves the Outcomes after Liver Transplantation in Patients with Acute-on-Chronic Hepatitis B Liver Failure. PLoS ONE 2012, 7, e30322. [Google Scholar] [CrossRef]
- Li, X.; Zhang, L.; Pu, C.; Tang, S. Liver transplantation in Acute-on-Chronic liver failure: Timing of transplantation and selection of patient population. Front. Med. 2022, 8, 1030336. [Google Scholar] [CrossRef]
- Larsen, F.S. Artificial liver support in acute and acute-on-chronic liver failure. Curr. Opin. Crit. Care 2019, 25, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Han, W.; Su, R.; Chen, J.; Zong, W.; Wang, Y.; Wang, W.; Cheng, G.; Ou, L.; Yu, Y. Non-ionic macroporous polystyrene adsorbents for removal of serum toxins in liver failure by hemoperfusion. Artif. Cells Nanomed. Biotechnol. 2017, 45, 174–183. [Google Scholar] [CrossRef] [Green Version]
- Hoffmann, K.; Nagel, A.J.; Tanabe, K.; Fuchs, J.; Dehlke, K.; Ghamarnejad, O.; Lemekhova, A.; Mehrabi, A. Markers of liver regeneration—The role of growth factors and cytokines: A systematic review. BMC Surg. 2020, 20, 31. [Google Scholar] [CrossRef] [Green Version]
- Kakisaka, K.; Kataoka, K.; Onodera, M.; Suzuki, A.; Endo, K.; Tatemichi, Y.; Kuroda, H.; Ishida, K.; Takikawa, Y. Alpha-fetoprotein: A biomarker for the recruitment of progenitor cells in the liver in patients with acute liver injury or failure. Hepatol. Res. 2015, 45, E12–E20. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Shen, C.; Yang, J.; Yang, X.; Qin, S.; Zeng, H.; Wu, X.; Tang, S.; Zeng, W. Alpha-Fetoprotein as a Predictive Marker for Patients with Hepatitis B-Related Acute-on-Chronic Liver Failure. Can. J. Gastroenterol. Hepatol. 2018, 2018, 1232785. [Google Scholar] [CrossRef]
- Qin, S.; Tang, S.H.; Wang, X.H.; Wang, X.P.; Sun, M.Y.; Wu, X.L.; Zeng, W.Z. Value of serum alpha-fetoprotein for the prognostic evaluation of hepatitis B virus-related acute-on-chronic liver failure treated with artificial liver. Chin. J. Hepatol. 2020, 28, 69–72. [Google Scholar]

| Parameter | Survival (n = 129) | Non-Survival (n = 41) | p Value |
|---|---|---|---|
| Age (years) | 42.80 ± 10.95 | 49.46 ± 8.38 | 0.000 |
| Sex (M:F) | 114/15 | 36/5 | 0.922 |
| PLT (109/L) | 113.00 (85.00, 154.00) | 93.00 (55.00, 93.00) | 0.006 |
| PT (s) | 18.30 (15.60, 22.00) | 21.10 (17.00, 25.00) | 0.017 |
| INR | 1.60 (1.37, 1.97) | 1.89 (1.52, 2.67) | 0.011 |
| AFP (ng/mL) | 88.58 (26.73, 260.05) | 48.07 (17.09, 141.82) | 0.042 |
| ALT (IU/L) | 771.10 (219.05, 1658.65) | 461.50 (150.50, 1440.25) | 0.261 |
| AST (IU/L) | 436.20 (142.60, 1207.85) | 376.20 (163.65, 1068.60) | 0.736 |
| Alb (g/L) | 33.70 (31.50, 37.40) | 32.60 (28.95, 36.70) | 0.173 |
| TBil (umol/L) | 313.77 ± 129.44 | 378.95 ± 156.44 | 0.011 |
| Cre (umol/L) | 72.20 (61.00, 82.00) | 76.00 (64.50, 86.50) | 0.273 |
| MELD | 19.62 (17.77, 23.12) | 22.59 (20.46, 25.27) | 0.000 |
| MELD-Na | 17.22 (12.60, 20.53) | 21.64 (17.56, 24.48) | 0.001 |
| iMELD | 36.76 (33.25, 40.63) | 41.66 (38.89, 45.66) | 0.000 |
| Parameter | PLT | |
|---|---|---|
| r Value | p Value | |
| ALT | 0.227 | 0.003 |
| AST | 0.084 | 0.278 |
| TBil | 0.015 | 0.847 |
| AFP | 0.063 | 0.413 |
| Alb | 0.219 | 0.004 |
| PT | −0.073 | 0.347 |
| INR | −0.057 | 0.461 |
| MELD | −0.042 | 0.583 |
| MELD-Na | −0.089 | 0.249 |
| iMELD | −0.155 | 0.043 |
| Parameter | β | Univariate HR (95% CI) | p | β | Multivariate HR (95% CI) | p |
|---|---|---|---|---|---|---|
| Age (years) | 0.049 | 1.050 (1.020–1.081) | 0.001 | 0.043 | 1.044 (1.012–1.077) | 0.007 |
| PLT (109/L) | −0.009 | 0.991 (0.984–0.997) | 0.005 | −0.009 | 0.990 (0.984–0.997) | 0.021 |
| PT (s) | 0.004 | 1.004 (0.985–1.023) | 0.672 | |||
| INR | 0.463 | 1.589 (0.995–2.539) | 0.053 | |||
| AFP (ng/mL) | −0.001 | 0.999 (0.997–1.000) | 0.136 | |||
| ALT (IU/L) | 0.000 | 1.000 (0.999–1.000) | 0.148 | |||
| AST (IU/L) | 0.000 | 1.000 (0.999–1.000) | 0.416 | |||
| Alb (g/L) | −0.062 | 0.940 (0.875–1.011) | 0.094 | |||
| Tbil (umol/L) | 0.003 | 1.003 (1.001–1.005) | 0.012 | 0.002 | 1.002 (1.000–1.004) | 0.042 |
| Cre (umol/L) | 0.000 | 1.000 (0.986–1.021) | 0.177 | |||
| MELD | 0.029 | 1.029 (1.004–1.055) | 0.021 | 0.006 | 1.006 (0.897–1.127) | 0.310 |
| MELD-Na | 0.031 | 1.031 (1.010–1.053) | 0.003 | 0.009 | 1.009 (0.928–1.097) | 0.458 |
| iMELD | 0.034 | 1.035 (1.015–1.054) | 0.000 | 0.142 | 1.037 (1.016–1.059) | 0.032 |
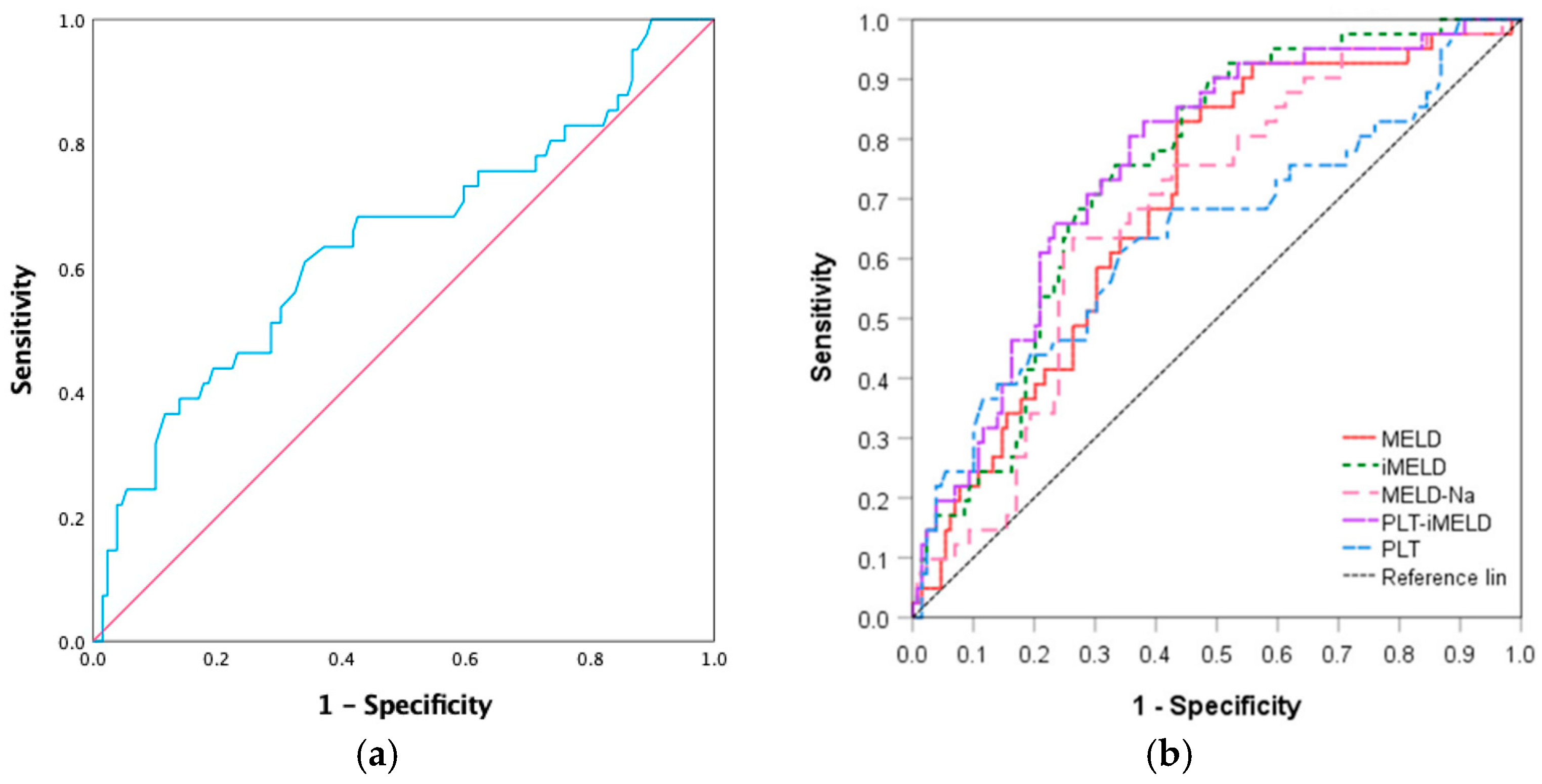
| ROC | AUC | 95%CI | Sensitivity | Specificity | Youden Index | Cut Off Value | p Value |
|---|---|---|---|---|---|---|---|
| Area | |||||||
| PLT | 0.643 | 0.539–0.747 | 61.00% | 65.90% | 0.269 | 99.50 | 0.006 |
| MELD | 0.695 | 0.609–0.782 | 82.90% | 56.60% | 0.395 | 20.20 | 0.001 |
| MELD-Na | 0.679 | 0.590–0.767 | 63.40% | 73.60% | 0.371 | 19.87 | 0.001 |
| iMELD | 0.745 | 0.666–0.823 | 75.60% | 66.70% | 0.423 | 39.30 | 0.001 |
| PLT-iMELD | 0.758 | 0.678–0.838 | 82.90% | 62.00% | 0.449 | 4.50 | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Li, H.; Zhu, Y.; Xu, H.; Tang, S. PLT Counts as a Predictive Marker after Plasma Exchange in Patients with Hepatitis B Virus-Related Acute-on-Chronic Liver Failure. J. Clin. Med. 2023, 12, 851. https://doi.org/10.3390/jcm12030851
Li X, Li H, Zhu Y, Xu H, Tang S. PLT Counts as a Predictive Marker after Plasma Exchange in Patients with Hepatitis B Virus-Related Acute-on-Chronic Liver Failure. Journal of Clinical Medicine. 2023; 12(3):851. https://doi.org/10.3390/jcm12030851
Chicago/Turabian StyleLi, Xue, Hao Li, Yucui Zhu, Huaqian Xu, and Shanhong Tang. 2023. "PLT Counts as a Predictive Marker after Plasma Exchange in Patients with Hepatitis B Virus-Related Acute-on-Chronic Liver Failure" Journal of Clinical Medicine 12, no. 3: 851. https://doi.org/10.3390/jcm12030851






