Radial and Circumferential CMR-Based RV Strain Predicts Low R Wave Amplitude after ICD Implantation in Patients with Arrhythmogenic Cardiomyopathy
Abstract
1. Introduction
2. Methods
2.1. Study Population and Definitions
2.2. Device Implantation and Interrogation
2.3. CMR Protocol
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. CMR-Based Structural and Functional Characteristics
3.3. The Clinical, Operation, and Imaging Correlates of RWA among ACM Patients
3.4. CMR Imaging Predictors for Identifying Low RWA Risk
3.5. Association of Pre-Procedure CMR Parameters and RWA at 2–6-Month Follow-Up
3.6. Intra- and Interobserver Variability
4. Discussion
4.1. Issue of Low RWA after ICD Implantation in ACM
4.2. Relationship between RWA and ACM
5. Clinical Implications
6. Limitations
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Corrado, D.; Link, M.S.; Calkins, H. Arrhythmogenic Right Ventricular Cardiomyopathy. N. Engl. J. Med. 2017, 376, 1489–1490. [Google Scholar] [CrossRef] [PubMed]
- Towbin, J.A.; McKenna, W.J.; Abrams, D.J.; Ackerman, M.J.; Calkins, H.; Darrieux, F.C.; Zareba, W. 2019 HRS expert consensus statement on evaluation, risk stratification, and management of arrhythmogenic cardiomyopathy: Executive summary. Heart Rhythm. 2019, 16, e373–e407. [Google Scholar] [CrossRef]
- Corrado, D.; Wichter, T.; Link, M.S.; Hauer, R.N.; Marchlinski, F.E.; Anastasakis, A.; Calkins, H. Treatment of Arrhythmogenic Right Ventricular Cardiomyopathy/Dysplasia: An International Task Force Consensus Statement. Circulation 2015, 132, 441–453. [Google Scholar] [CrossRef] [PubMed]
- Al-Khatib, S.M.; Stevenson, W.G.; Ackerman, M.J.; Bryant, W.J.; Callans, D.J.; Curtis, A.B.; Page, R.L. 2017 AHA/ACC/HRS Guideline for Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation 2018, 138, e272–e391. [Google Scholar] [CrossRef] [PubMed]
- Wichter, T.; Paul, M.; Wollmann, C.; Acil, T.; Gerdes, P.; Ashraf, O.; Tjan, T.D.T.; Soeparwata, R.; Block, M.; Borggrefe, M.; et al. Implantable Cardioverter/Defibrillator Therapy in Arrhythmogenic Right Ventricular Cardiomyopathy: Single-Center Experience of Long-Term Follow-Up and Complications in 60 Patients. Circulation 2004, 109, 1503–1508. [Google Scholar] [CrossRef] [PubMed]
- Kossaify, A. Sensing and Detection Functions in Implantable Cardioverter Defibrillators: The Good, the Bad and the Ugly. Acta Cardiol. Sin. 2020, 36, 308–317. [Google Scholar] [CrossRef]
- Hsu, S.S.; Mohib, S.; Schroeder, A.; Deger, F.T. T wave oversensing in implantable cardioverter defibrillators. J. Interv. Card. Electrophysiol. 2004, 11, 67–72. [Google Scholar] [CrossRef]
- Watanabe, H.; Chinushi, M.; Izumi, D.; Sato, A.; Okada, S.; Okamura, K.; Aizawa, Y. Decrease in Amplitude of Intracardiac Ventricular Electrogram and Inappropriate Therapy in Patients with an Implantable Cardioverter Defibrillator. Int. Heart J. 2006, 47, 363–370. [Google Scholar] [CrossRef]
- Mugnai, G.; Tomei, R.; Dugo, C.; Tomasi, L.; Morani, G.; Vassanelli, C. Implantable cardioverter-defibrillators in patients with arrhythmogenic right ventricular cardiomyopathy: The course of electronic parameters, clinical features, and complications during long-term follow-up. J. Interv. Card. Electrophysiol. 2014, 41, 23–29. [Google Scholar] [CrossRef]
- Christensen, A.H.; Platonov, P.G.; Svensson, A.; Jensen, H.K.; Rootwelt-Norberg, C.; Dahlberg, P.; Svendsen, J.H. Complications of implantable cardioverter-defibrillator treatment in arrhythmogenic right ventricular cardiomyopathy. EP Eur. 2022, 24, 306–312. [Google Scholar] [CrossRef]
- Lillo-Castellano, J.M.; Marina-Breysse, M.; Gómez-Gallanti, A.; Martinez-Ferrer, J.B.; Alzueta, J.; Pérez-Álvarez, L.; Filgueiras-Rama, D. Safety threshold of R-wave amplitudes in patients with implantable cardioverter defibrillator. Heart 2016, 102, 1662–1670. [Google Scholar] [CrossRef] [PubMed]
- Aquaro, G.D.; De Luca, A.; Cappelletto, C.; Raimondi, F.; Bianco, F.; Botto, N.; Sinagra, G. Prognostic value of magnetic resonance phenotype in patients with arrhythmogenic right ventricular cardiomyopathy. J. Am. Coll. Cardiol. 2020, 75, 2753–2765. [Google Scholar] [CrossRef]
- Farrelly, C.; Shah, S.; Davarpanah, A.; Keeling, A.N.; Carr, J.C. ECG-gated multiecho Dixon fat-water separation in cardiac MRI: Advantages over conventional fat-saturated imaging. Am. J. Roentgenol. 2012, 199, W74–W83. [Google Scholar] [CrossRef] [PubMed]
- Oda, S.; Morita, K.; Kidoh, M.; Nagayama, Y.; Nakaura, T.; Shirahama, Y.; Hirai, T. Three-Dimensional Modified Dixon ECG-Gated Cardiac Magnetic Resonance Imaging in Arrhythmogenic Right Ventricular Cardiomyopathy/Dysplasia. Circ. Cardiovasc. Imaging 2021, 14, e012745. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Tandri, H.; Calkins, H.; Bluemke, D.A. Role of cardiovascular magnetic resonance imaging in arrhythmogenic right ventricular dysplasia. J. Cardiovasc. Magn. Reson. 2008, 10, 32. [Google Scholar] [CrossRef]
- Chen, X.; Li, L.; Cheng, H.; Song, Y.; Ji, K.; Chen, L.; Zhao, S. Early left ventricular involvement detected by cardiovascular magnetic resonance feature tracking in arrhythmogenic right ventricular cardiomyopathy: The effects of left ventricular late gadolinium enhancement and right ventricular dysfunction. J. Am. Heart Assoc. 2019, 8, e012989. [Google Scholar] [CrossRef]
- Durbin, J.; Watson, G.S. Testing for serial correlation in least squares regression. III. Biometrika 1971, 58, 1–19. [Google Scholar] [CrossRef]
- Bosman, L.P.; Nielsen Gerlach, C.L.; Cadrin-Tourigny, J.; Orgeron, G.; Tichnell, C.; Murray, B.; Te Riele, A.S. Comparing clinical performance of current implantable cardioverter-defibrillator implantation recommendations in arrhythmogenic right ventricular cardiomyopathy. EP Eur. 2022, 24, 296–305. [Google Scholar] [CrossRef]
- Link, M.S.; Wang, P.J.; Haugh, C.J.; Homoud, M.K.; Foote, C.B.; Costeas, X.B.; Estes, N.A., III. Arrhythmogenic right ventricular dysplasia: Clinical results with implantable cardioverter defibrillators. J. Interv. Card. Electrophysiol. 1997, 1, 41–48. [Google Scholar] [CrossRef]
- Breithardt, G.; Wichter, T.; Haverkamp, W.; Borggrefe, M.; Block, M.; Hammel, D.; Scheld, H.H. Implantable cardioverter defibrillator therapy in patients with arrhythmogenic right ventricular cardiomyopathy, long QT syndrome, or no structural heart disease. Am. Heart J. 1994, 127, 1151–1158. [Google Scholar] [CrossRef]
- Herman, A.R.; Gardner, M.; Steinberg, C.; Yeung-Lai-Wah, J.A.; Healey, J.S.; Leong-Sit, P.; Chakrabarti, S. Long-term right ventricular implantable cardioverter-defibrillator lead performance in arrhythmogenic right ventricular cardiomyopathy. Heart Rhythm. 2016, 13, 1964–1970. [Google Scholar] [CrossRef]
- Bonou, M.; Mavrogeni, S.; Kapelios, C.J.; Markousis-Mavrogenis, G.; Aggeli, C.; Cholongitas, E.; Barbetseas, J. Cardiac Adiposity and Arrhythmias: The Role of Imaging. Diagnostics 2021, 11, 362. [Google Scholar] [CrossRef] [PubMed]
- Bucius, P.; Erley, J.; Tanacli, R.; Zieschang, V.; Giusca, S.; Korosoglou, G.; Kelle, S. Comparison of feature tracking, fast-SENC, and myocardial tagging for global and segmental left ventricular strain. ESC Heart Fail. 2019, 7, 523–532. [Google Scholar] [CrossRef]
- Bourfiss, M.; Prakken, N.H.J.; James, C.A.; Planken, R.N.; Boekholdt, S.M.; Ahmetagic, D.; Te Riele, A.S.J.M. Prognostic value of strain by feature-tracking cardiac magnetic resonance in arrhythmogenic right ventricular cardiomyopathy. Eur. Heart J. Cardiovasc. Imaging 2022, 24, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Kuschyk, J.; Müller-Leisse, J.; Duncker, D.; Tülümen, E.; Fastenrath, F.; Fastner, C.; Rudic, B. Comparison of transvenous vs. subcutaneous defibrillator therapy in patients with cardiac arrhythmia syndromes and genetic cardiomyopathies. Int. J. Cardiol. 2021, 323, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Kempa, M.; Łaskawski, G.; Budrejko, S.; Królak, T.; Kozłowski, D.; Rogowski, J.; Raczak, G. Epicardial screw-in sensing lead on the left ventricle to treat undersensing of ventricular arrhythmias in a patient with arrhythmogenic right ventricular cardiomyopathy. Cardiol. J. 2017, 24, 710–711. [Google Scholar] [CrossRef]
- Taleski, J. Left Ventricular Lead Placement for Pacing and Sensing in a Patient with Arrhythmogenic Right Ventricular Cardiomyopathy Undergoing ICD Implantation. Acta Clin. Croat. 2019, 58, 391–393. [Google Scholar] [CrossRef] [PubMed]
- Lochy, S.; Francois, B.; Hollanders, G.; Provenier, F. Left ventricular sensing and pacing for sensing difficulties in internal cardioverter defibrillator therapy for arrhythmogenic right ventricular cardiomyopathy. Europace 2010, 12, 1195–1196. [Google Scholar] [CrossRef] [PubMed]
- Augustine, D.; Lewandowski, A.J.; Lazdam, M.; Rai, A.; Francis, J.; Myerson, S.; Leeson, P. Global and regional left ventricular myocardial deformation measures by magnetic resonance feature tracking in healthy volunteers: Comparison with tagging and relevance of gender. J. Cardiovasc. Magn. Reson. 2013, 15, 8. [Google Scholar] [CrossRef]
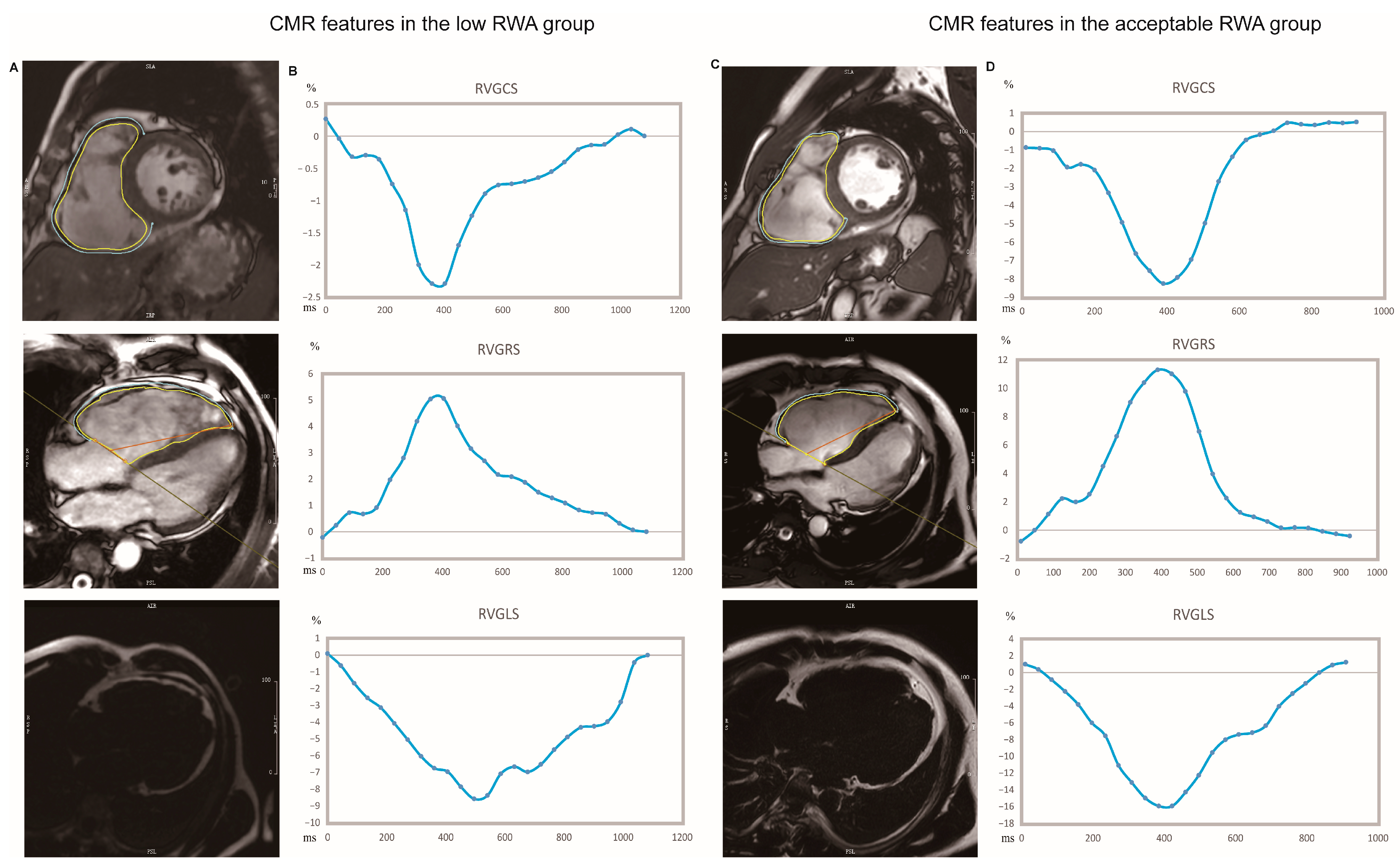

| All (n = 87) | Acceptable RWA (n = 68) | Low RWA (n = 19) | p-Value | |
|---|---|---|---|---|
| Age at implantation, yrs | 43.8 ± 14.3 | 43.3 ± 13.3 | 41.0 ±17.8 | 0.542 |
| Male, n (%) | 61 (70.1%) | 47 (69.1%) | 14 (73.7%) | 0.701 |
| BMI, kg/m2 | 24.1 ± 3.6 | 24.2 ± 3.8 | 24.0 ± 3.1 | 0.845 |
| NYHA Class, n (%) | 0.006 | |||
| I | 2 (2.3%) | 2 (2.9%) | 0 (0.00%) | |
| II | 34 (39.1%) | 20 (29.4%) | 14 (73.7%) | |
| III | 38 (43.7%) | 34 (50.0%) | 4 (21.0%) | |
| IV | 13 (14.9%) | 12 (17.7%) | 1 (5.3%) | |
| Family History of ACM, n (%) | 12 (13.8%) | 9 (13.24%) | 3 (15.8%) | 0.775 |
| Symptoms | ||||
| Syncope, n (%) | 44 (50.6%) | 34 (50.0%) | 10 (52.6%) | 0.839 |
| Amaurosis, n (%) | 31 (35.6%) | 22 (32.4%) | 9 (47.4%) | 0.227 |
| Chest pain, n (%) | 7 (8.1%) | 7 (10.3%) | 0 (0.00%) | 0.339 |
| Fatigue, n (%) | 24 (27.6%) | 20 (29.4%) | 4 (21.1%) | 0.471 |
| Stuffy, n (%) | 46 (52.9%) | 36 (52.9%) | 10 (52.6%) | 0.981 |
| Palpation, n (%) | 66 (75.9%) | 51 (75.0%) | 15 (79.0%) | 0.722 |
| Oedema, n (%) | 6 (6.9%) | 5 (7.4%) | 1 (5.3%) | 1.000 |
| ECG Characteristics | ||||
| TWI: V1-V3, n (%) | 56 (64.4%) | 38 (55.9%) | 18 (94.7%) | 0.002 |
| TWI: V4-V6, n (%) | 34 (39.1%) | 26 (38.2%) | 8 (42.1%) | 0.760 |
| RBBB pattern, n (%) | 28 (32.2%) | 20 (29.4%) | 8 (42.1%) | 0.295 |
| LBBB pattern, n (%) | 0 (0%) | 0 (0%) | 0 (0%) | - |
| Epsilon Wave, n (%) | 23 (26.4%) | 15 (22.1%) | 8 (42.1%) | 0.080 |
| Arrhythmia Characteristics | ||||
| History of sustained SVT/VF, n (%) | 67 (77.0%) | 53 (77.9%) | 14 (73.7%) | 0.697 |
| NS-VT, n (%) | 56 (64.4%) | 38 (55.8%) | 18 (94.7%) | 0.002 |
| 24 h PVC counts, n (n = 72) | 880 (87–3550) | 754 (1–3158) | 3056 (405–10,000) | 0.067 |
| RV originated /LBBB morphology VT, n (%) | 51 (58.6%) | 32 (47.1%) | 19 (100.00%) | <0.001 |
| LV originated/RBBB morphology VT, n (%) | 19 (21.8%) | 16 (23.5%) | 3 (15.8%) | 0.470 |
| Multi-morphology PVC, n (%) (n = 72) | 49 (68.1%) | 35 (63.6%) | 14 (82.4%) | 0.148 |
| ICD Brand | 0.631 | |||
| Biotronik, n (%) | 34 (39.1%) | 26 (38.2%) | 8 (42.1%) | |
| Boston Scientific, n (%) | 12 (13.8%) | 11 (16.2%) | 1 (5.3%) | |
| Medtronic, n (%) | 19 (21.8%) | 15 (22.1%) | 4 (21.1%) | |
| Abbott, n (%) | 22 (25.3%) | 16 (23.5%) | 6 (31.6%) | |
| ICD Type | 0.391 | |||
| Single-chamber, n (%) | 66 (75.9%) | 53 (77.9%) | 13 (68.4%) | |
| Dual-chamber, n (%) | 21 (24.1%) | 15 (22.1%) | 6 (31.6%) | |
| Ventricular Lead position | 0.164 | |||
| Apex, n (%) | 57 (65.5%) | 42 (61.8%) | 15 (78.9%) | |
| Septum, n (%) | 30 (34.5%) | 26 (38.2%) | 4 (21.1%) | |
| Pacing parameters | ||||
| R wave amplitude, mV | 8.0 (5.1–11.8) | 9.7 (7.0–13.6) | 3.1 (2.7–4.2) | <0.001 |
| Impedance, ohms | 595.0 (509.5–774.0) | 595.5 (520.0–781.0) | 560.0 (493.0–753.5) | 0.332 |
| Pacing threshold, V @0.5 ms | 0.5 (0.5–0.8) | 0.5 (0.5–0.8) | 0.5 (0.5–0.7) | 0.645 |
| Medication Treatment | ||||
| Diuretic agent, n (%) | 42 (48.3%) | 35 (51.5%) | 7 (36.8%) | 0.259 |
| ACEI/ARB/ARNI, n (%) | 47 (54.0%) | 40 (58.8%) | 7 (36.8%) | 0.089 |
| AAD, n (%) | 58 (66.7%) | 43 (63.2%) | 15 (78.9%) | 0.199 |
| Beta Blocker, n (%) | 77 (88.5%) | 59 (86.8%) | 18 (94.7%) | 0.335 |
| All (n = 87) | Acceptable RWA (n = 68) | Low RWA (n = 19) | p-Value | |
|---|---|---|---|---|
| Right Ventricular | ||||
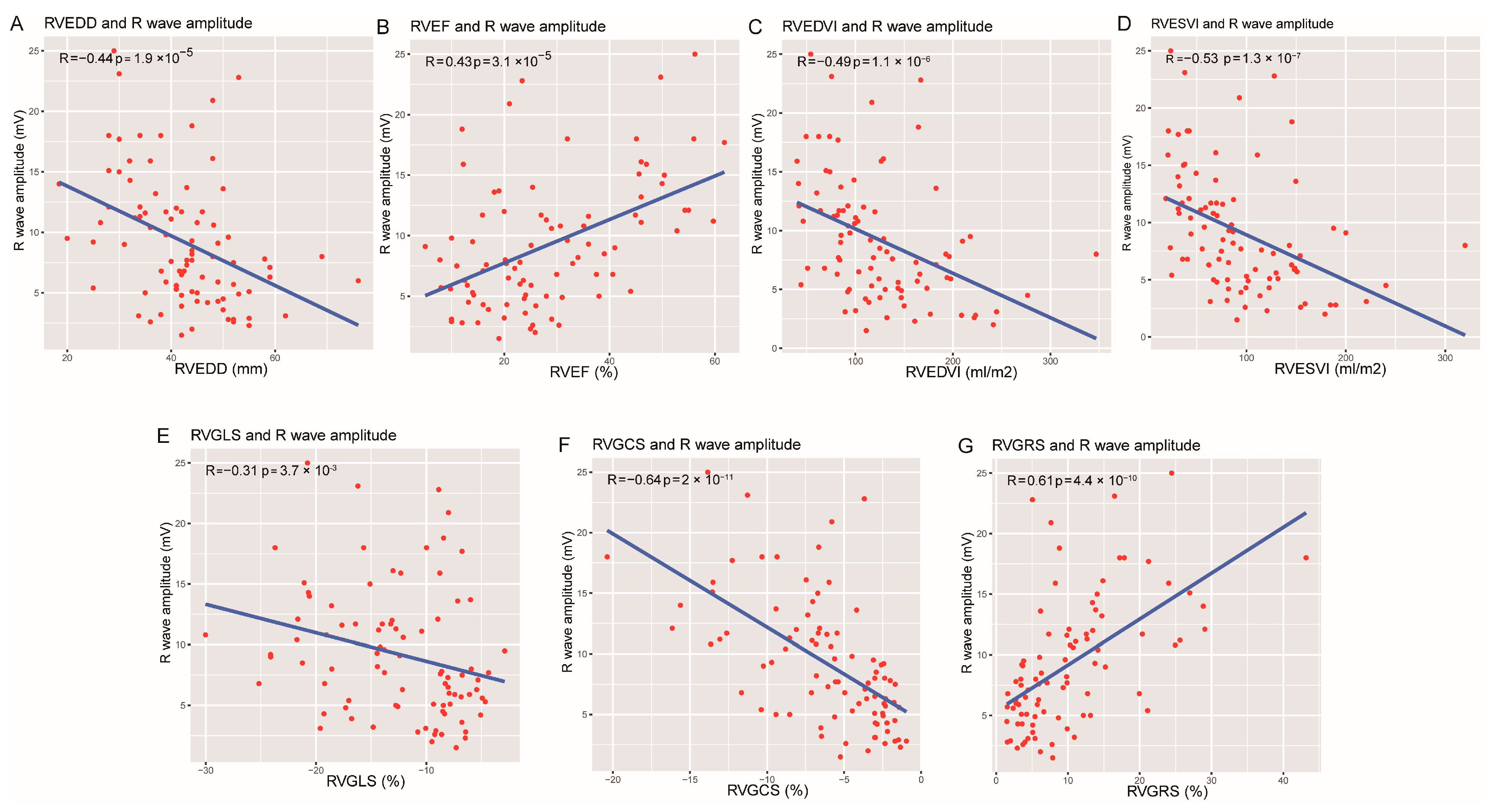
| RVEDD, mm | 43.40 ± 10.25 | 41.03 ± 10.59 | 47.30 ± 7.23 | 0.018 |
| RVEF, % | 28.24 ± 14.19 | 30.35 ± 15.03 | 20.70 ± 6.74 | 0.008 |
| RVEDVi, mL/m2 | 115.33 (83.50–161.51) | 102.32 (80.42–139.41) | 147.08 (112.48–215.41) | 0.001 |
| RVESVi, mL/m2 | 81.81 (46.70–125.07) | 70.14 (40.52–111.97) | 113.54 (92.36–168.94) | <0.001 |
| RVGLS, % | −12.09 (−16.59-−8.00) | −12.58 (−17.19-−8.01) | −9.23 (−13.70–−7.81) | 0.299 |
| RVGCS, % | −5.56 (−8.31-−2.95) | −6.08 (−9.42–−3.52) | −2.86 (−4.17–1.95) | <0.001 |
| RVGRS, % | 7.88 (4.17–13.61) | 9.87 (5.40–14.76) | 5.10 (3.31–6.98) | 0.002 |
| RVLGE presence, n (%) | 54 (62.07%) | 36 (52.94%) | 18 (94.74%) | <0.001 |
| RV-FAT infiltration, n (%) | 50 (57.47%) | 32 (47.06%) | 18 (94.74%) | <0.001 |
| Left Ventricular | ||||
| LVEDD, mm | 52.90 ± 9.40 | 53.38 ± 10.30 | 51.17 ± 3.00 | 0.367 |
| LVEF, % | 46.62 ± 13.24 | 45.65 ± 13.76 | 50.09 ±10.83 | 0.198 |
| LVEDVi, mL/m2 | 82.70 (63.25–105.70) | 83.04 (66.91–110.22) | 79.87 (59.92–98.55) | 0.377 |
| LVESVi, mL/m2 | 42.31 (26.31–64.91) | 42.34 (26.44–68.66) | 36.61 (25.60–58.81) | 0.309 |
| LVGLS, % | −11.49 ± 3.40 | −11.50 ± 3.47 | −11.46 ± 3.21 | 0.964 |
| LVGCS, % | −15.14 (−17.50-−10.91) | −14.87 (−17.01-−10.49) | −15.98 (−18.31-−13.82) | 0.169 |
| LVGRS, % | 23.03 ± 9.56 | 22.33 ± 9.63 | 25.52 ± 9.12 | 0.200 |
| Positive LV-LGE, n (%) | 44 (50.57%) | 34 (50.00%) | 10 (52.63%) | 0.839 |
| LV-LGE extend (%) | 6.72 (0.00–18.47) | 3.36 (0.00–18.80) | 7.22 (0.00–14.21) | 0.751 |
| Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|
| Beta (SE) | p-Value | Beta (SE) | p-Value | |
| Age, yrs | 0.048 (0.040) | 0.229 | ||
| Male, (vs. female) | 0.229 (1.253) | 0.855 | ||
| BMI, kg/m2 | 0.083 (0.159) | 0.602 | ||
| Epsilon | −2.436 (1.274) | 0.059 | ||
| RBBB | −1.654 (1.215) | 0.177 | ||
| TWI: V1-V3 | −3.530 (1.135) | 0.003 | ||
| LBBB-VT | −3.992 (1.081) | <0.001 | ||
| LVEF, % | −0.048 (0.043) | 0.267 | ||
| LVEDD, mm | 0.130 (0.060) | 0.033 | ||
| LVESVi, mL/m2 | 0.037 (0.020) | 0.067 | ||
| LVEDVi, mL/m2 | 0.034 (0.018) | 0.063 | ||
| RVEF, % | 0.180 (0.036) | <0.001 | ||
| RVEDD, mm | −0.205 (0.052) | <0.001 | ||
| RVESVi, mL/m2 | −0.040 (0.009) | <0.001 | ||
| RVEDVi, mL/m2 | −0.038 (0.009) | <0.001 | ||
| CMR-FT and tissue characterization | ||||
| LVGLS, % | 0.049 (0.170) | 0.772 | ||
| LVGCS, % | 0.159 (0.128) | 0.217 | ||
| LVGRS, % | −0.055 (0.060) | 0.362 | ||
| LV-LGE | 1.002 (1.142) | 0.383 | ||
| LV-LGE extent, % | 0.061 (0.038) | 0.109 | ||
| RVGLS, % | −0.379 (0.061) | 0.015 | −0.085 (0.106) | 0.455 a |
| RVGCS, % | −0.771 (0.115) | <0.001 | −0.608 (0.183) | 0.001 b |
| RVGRS, % | 0.356 (0.062) | <0.001 | 0.291 (0.097) | 0.004 c |
| RV-LGE | −4.115 (1.095) | <0.001 | −0.558 (1.466) | 0.705 d |
| RV-FAT | −4.022 (1.075) | <0.001 | −1.363 (1.289) | 0.294 e |
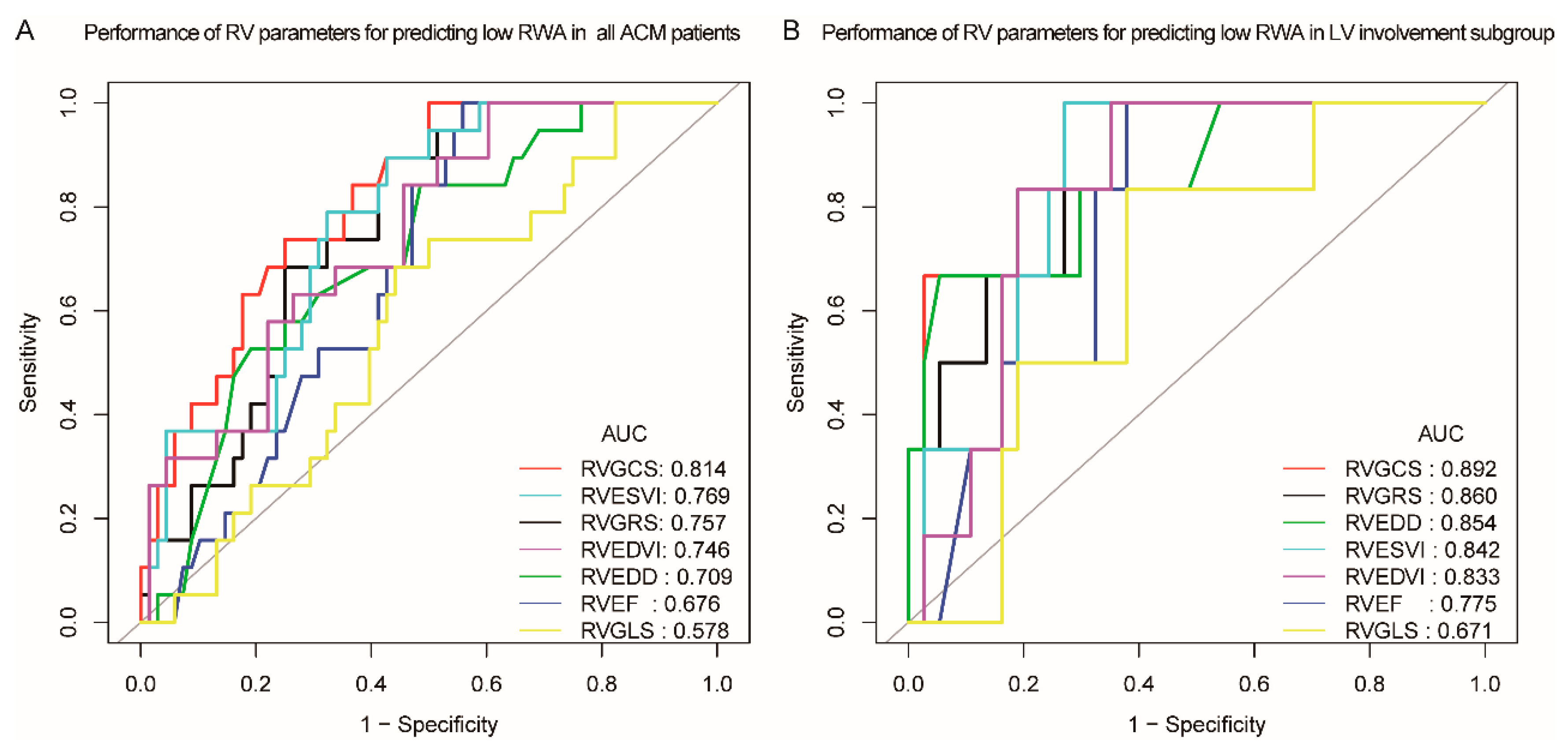
| Variables | ROC Area (AUC) | 95% CI Low | 95% CI Upper | Best Cutoff | Sensitivity | Specificity |
|---|---|---|---|---|---|---|
| RVEDD, mm | 0.709 | 0.585 | 0.832 | 41.8 | 0.842 | 0.515 |
| RVEF, % | 0.677 | 0.563 | 0.790 | 31.5 | 1.000 | 0.441 |
| RVEDVi, ml/m2 | 0.746 | 0.631 | 0.861 | 88.5 | 1.000 | 0.397 |
| RVESVi, mL/m2 | 0.769 | 0.664 | 0.875 | 78.4 | 0.895 | 0.574 |
| RVGRS, % | 0.757 | 0.653 | 0.861 | 8.75 | 0.895 | 0.574 |
| RVGCS, % | 0.814 | 0.718 | 0.910 | −6.55 | 1.000 | 0.500 |
| RVGLS, % | 0.578 | 0.443 | 0.713 | −11.43 | 0.684 | 0.559 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Z.; Song, Y.; Chen, L.; Ma, X.; Dai, Y.; Zhao, S.; Chen, K.; Zhang, S. Radial and Circumferential CMR-Based RV Strain Predicts Low R Wave Amplitude after ICD Implantation in Patients with Arrhythmogenic Cardiomyopathy. J. Clin. Med. 2023, 12, 886. https://doi.org/10.3390/jcm12030886
Chen Z, Song Y, Chen L, Ma X, Dai Y, Zhao S, Chen K, Zhang S. Radial and Circumferential CMR-Based RV Strain Predicts Low R Wave Amplitude after ICD Implantation in Patients with Arrhythmogenic Cardiomyopathy. Journal of Clinical Medicine. 2023; 12(3):886. https://doi.org/10.3390/jcm12030886
Chicago/Turabian StyleChen, Zhongli, Yanyan Song, Liang Chen, Xuan Ma, Yan Dai, Shihua Zhao, Keping Chen, and Shu Zhang. 2023. "Radial and Circumferential CMR-Based RV Strain Predicts Low R Wave Amplitude after ICD Implantation in Patients with Arrhythmogenic Cardiomyopathy" Journal of Clinical Medicine 12, no. 3: 886. https://doi.org/10.3390/jcm12030886
APA StyleChen, Z., Song, Y., Chen, L., Ma, X., Dai, Y., Zhao, S., Chen, K., & Zhang, S. (2023). Radial and Circumferential CMR-Based RV Strain Predicts Low R Wave Amplitude after ICD Implantation in Patients with Arrhythmogenic Cardiomyopathy. Journal of Clinical Medicine, 12(3), 886. https://doi.org/10.3390/jcm12030886






