Microspheres as a Carrier System for Therapeutic Embolization Procedures: Achievements and Advances
Abstract
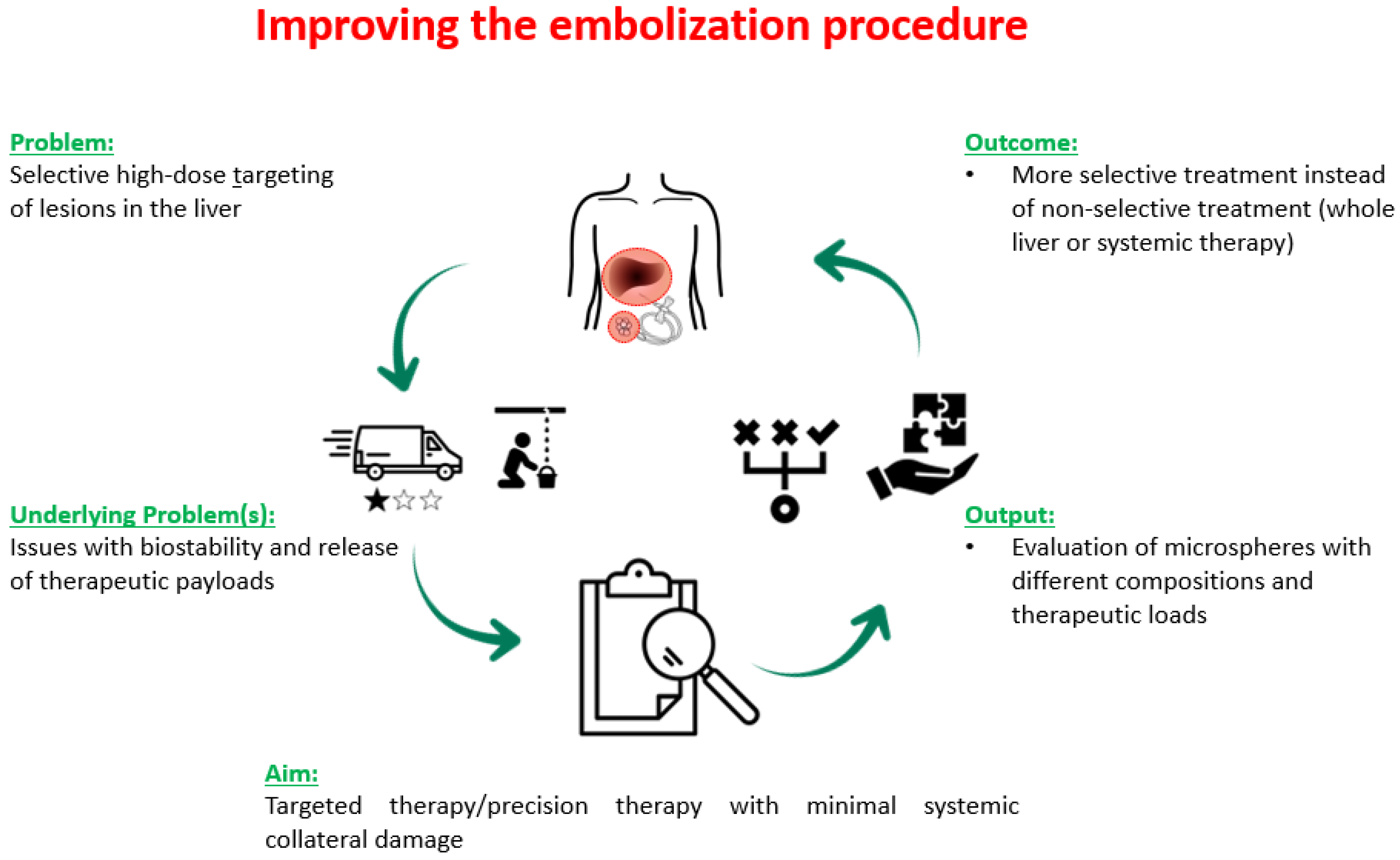
:1. Introduction
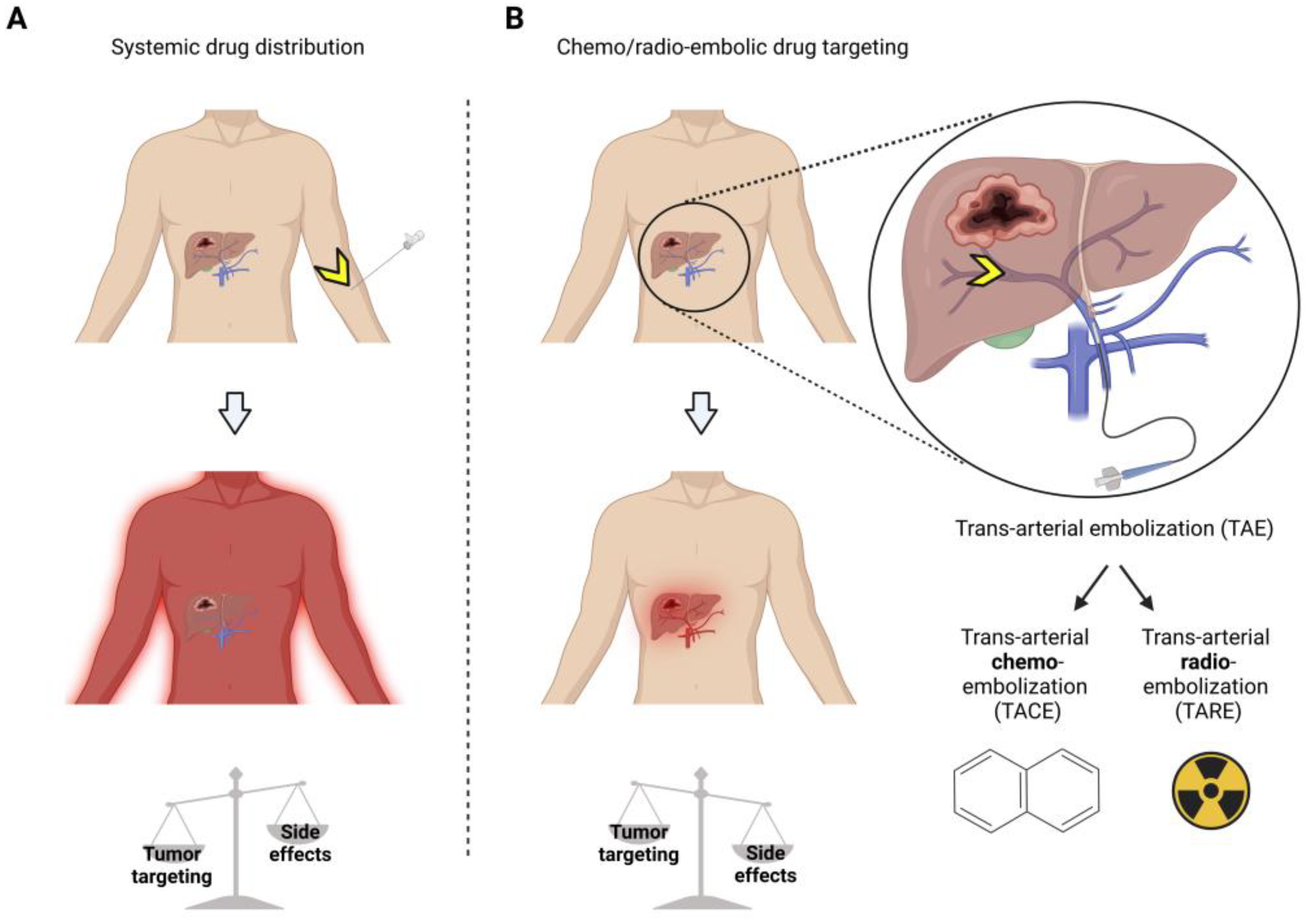
2. Trans-Arterial Embolization for Vascular Occlusion
3. Therapeutic Loads Employed during Microsphere-Trans-Arterial Embolization Therapy
3.1. Trans-Arterial Chemoembolization
3.2. Trans-Arterial Radioembolization (TARE)
3.3. TACE vs. TARE
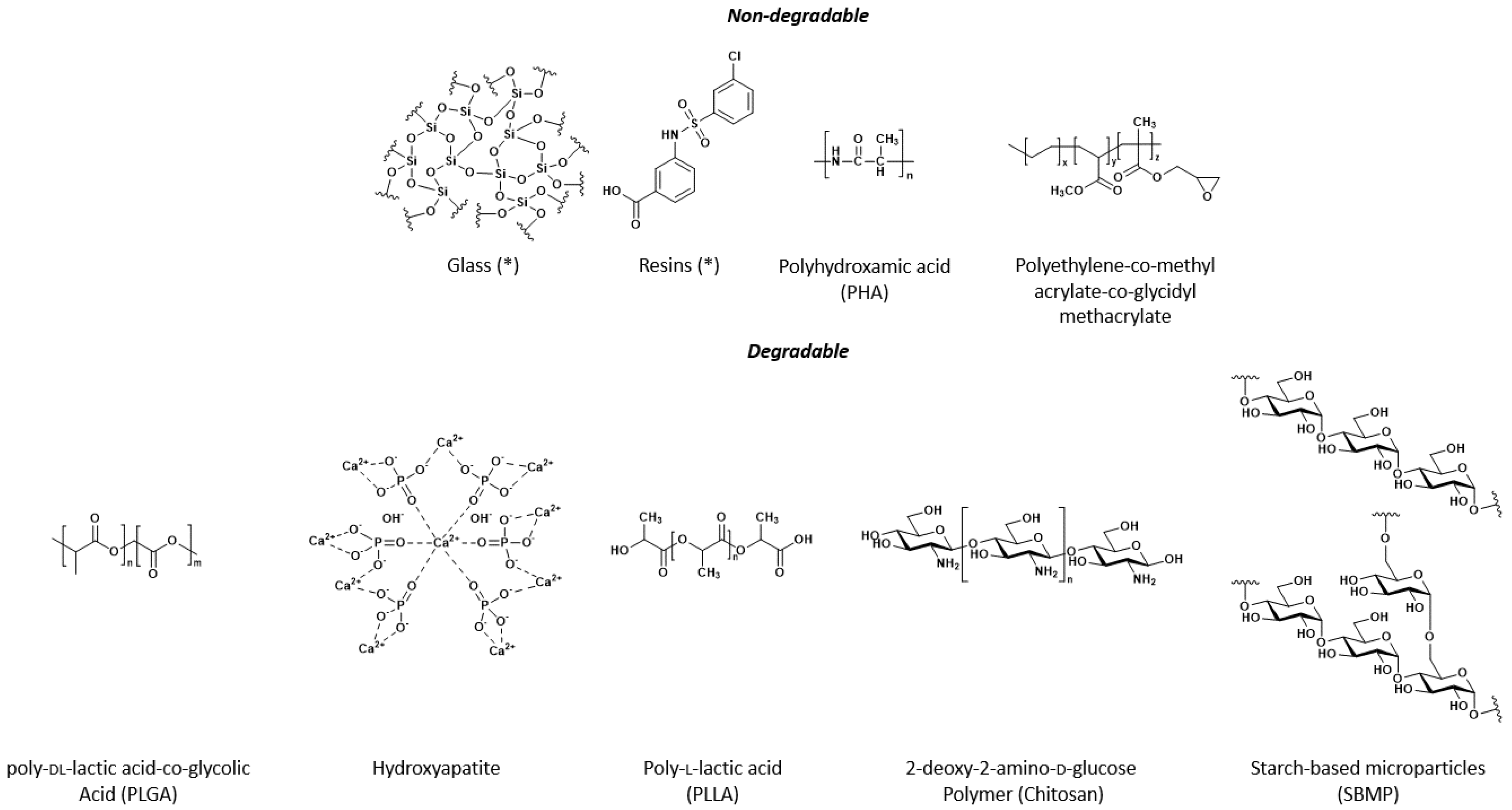
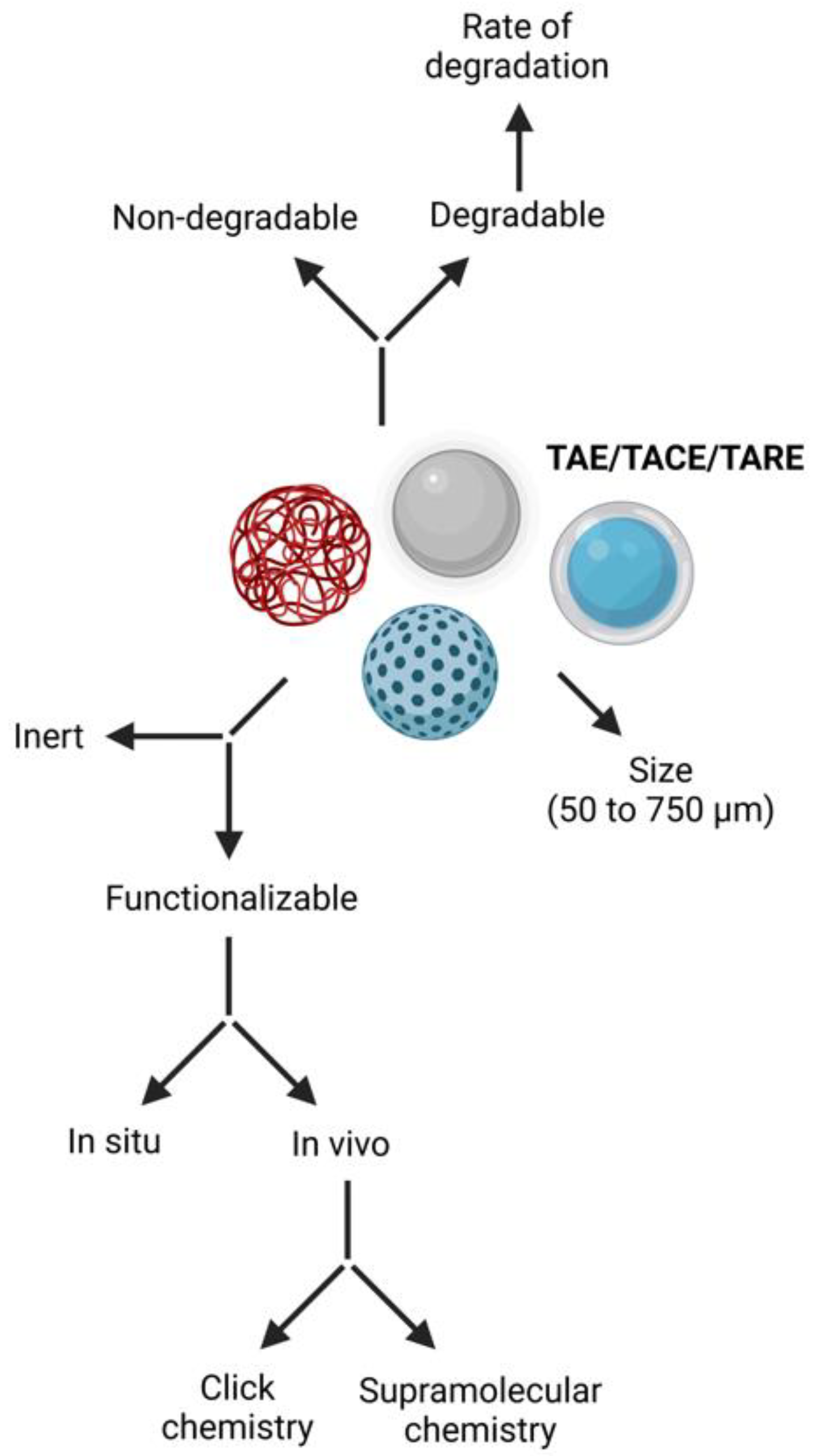
4. Chemical Properties and Advancements of Microsphere Carrier Systems
4.1. Non-Degradable Microspheres
- Chemically inert glass—microspheres are non-porous, do not induce immunological effects, and are FDA-approved for application in humans for embolization therapy. Glass microspheres have a primary yttria–alumina–silica system (YAS), and a ternary YAS composition (40% Y2O3, 40% SiO2, and 20% Al2O3) [66]. These microspheres, on average, have a diameter of 50–150 mm. Glass is used in clinical TAE and TARE therapies [115,116]. Radioisotopes can be embedded during preparation into the glass via thermal neutron irradiation in a nuclear reactor (Figure 2). In this process, irradiation of stable Y—through an 89Y (n, γ)90Y reaction—produces 90Y [43,117]. The amount can be fine-tuned between 0.5 and 11 GBq per treatment according to need [118]. These glass microspheres cannot be used for TACE as elution of embedded drugs and surface modifications are impossible.
- Ion-exchange resin-based microspheres SIR-Spheres® are porous, have a lower density/weight than glass, and are regularly used for TAE and TARE [54]. These polymers do not contain any groups amenable to covalent conjugation. Various resins were investigated for TARE only after including stable Y, Ho, or Sm, preparing microspheres during the synthesis process (Figure 2). As for glass microspheres, neutron-activated formulations in a research reactor after irradiation of stable Y, Ho, or SM isotopes through (n, γ) reaction procedures yielded 90Y, 166Ho, or 153Sm [43,54]. Bio-Rex 70 (Bio-Rad Inc. Veenendaal, The Netherlands) proved to have the best properties in stability, loading capacity, and sterilization [119]. Next to high-energy beta-radiation, 166Ho also emits gamma-radiation, which allows for imaging by gamma scintigraphy, thus helping determine treatment dose. 153Sm and 166Ho have a theragnostic advantage as they emit both therapeutic beta and diagnostic gamma radiations, allowing both imaging and therapy in one. In combination with 153Sm, this resin was pursued as an alternative microsphere in light of production time, stability, and costs [120,121].
- The discovery of the macroporous chelating ion exchanger G-Gel (Merck, Darmstad, Germany), consisting of poly(glycidyl methacrylate-co-ethylene dimethacrylate), helps provide a new class of 177Lu and 131I carriers [122]. Methacrylate is formed in beads that support high radionuclide loading due to macroporous structure and mechanically stable sphere-shaped particles of 20–40 μm. This concept is being implemented in G-Gel [43]. The material facilitates the conjugation of functional moieties, such as chelates and dyes, without negatively impacting the overall properties [70]. In this respect, DOTA and Quinoline-8-ol have been used as metal-binding ligands because they readily form stable complexes with nearly all therapeutically or diagnostically used metal ion radionuclides such as 90Y, 188Re, 166Ho, and 177Lu [54]. This stable complexation makes G-Gel less useful for drug release as with TACE.
- The interest in using cellulose for TARE comes from its nontoxicity, biocompatibility, biodegradability, and amenable chemistry for functionalization with, e.g., chelating groups. Polyhydroxyamic acid polyacrylamide (PHA) has been chosen for its capacity to form complexes with a wide range of metallic radionuclides [71,123]. In one study on the efficacy of a PHA loaded with 177Lu [123], PHA microspheres were synthesized starting from polyacrylamide. Incorporating isotopes such as 177Lu seems straightforward; modification of the polymers to incorporate dyes/chelates/adamantane seems impossible due to the complicated chemistry. Thus, PHA-functionalized microspheres were not applied for TACE, as is the case for resin microspheres. Subsequently, experimental variables such as reaction pH, amount of PHA microspheres, carrier 177Lu content, and incubation time were optimized for maximum uptake of 177Lu on PHA microspheres (median particle size to be 54 μm, which is still suitable for TARE, but relatively small for an effective TAE). Under optimized conditions, >99% loading of 177Lu on PHA microspheres with high stability could be achieved. 177Lu-PHA microspheres exhibited excellent in vitro stability in sodium phosphate solutions, saline, and serum for up to 5 days at 37 °C. In animal studies, 93% of 177Lu-PHA microspheres were retained in the liver at 96 h post-injection without significant leakage to other organs. Although the latter is encouraging, this set-up has not yet been evaluated in patients for HCC [71].
4.2. Bio-Degradable Particles
- Poly-DL-lactic-co-glycolic acid (PLGA) particles can be formed to the size of microspheres by employing emulsion or microemulsion polymerization, interfacial polymerization, and precipitation polymerization, and a monomer as a starting point [124]. Thereafter, they can be modified into biodegradable carriers for the controlled delivery of drugs and isotopes. PLGA has one reactive COOH group per polymer chain. Functionalization of PLGA microspheres should be possible, although the influence on the hydrogel formation in combination with PEG is unclear. PLGA is widely used for TACE [44,45,46], although the process of drug release is complex [125]. In general, drug release occurs mainly via diffusion through pores, osmotic pumping, degradation, or erosion. More recently, modifications for TARE have been initiated [126]. An application of PLGA in TARE is in Radiogel® (Vivos Inc., Richland, WA, USA), registered as a medical device under the FDA. InjecTable 90Y-Radiogel® comprises an insoluble 90Y-phosphate (YPO4) radiation source mixed within an injectable, thermally reversible, temperature-sensitive polymer solution that includes polylactide, polyglycolide, and polylactic-co-glycolic acid co-polymers, all embedded in a microsphere [126]. This hydrogel is a liquid at temperatures below body temperature but begins to gel and harden upon injection as the temperature increases to normal body temperature, thereby locking the particles in place. RadioGel® is drained within tumor extracellular spaces after injection when it warms to body temperature and has a short half-life, delivering more than 90% of its therapeutic radiation within 10 days. Over time, natural breakdown products of RadioGel® include lactic acid and glycolic acid (also known as non-toxic natural byproducts of the Krebs cycle), and the remaining radioactivity is excreted via urine [127].
- Hydroxyapatite (Ca10(PO4)6(OH)2) is a natural mineral constituent of bone matrix and, hence, is biocompatible. Hydroxyapatite particles can be easily synthesized in the desired particle-size range for embolization, and the abundant PO43− moieties can coordinate 166Ho. Earlier studies have investigated hydroxyapatite lanthanum oxide composites [128] and the effect of tissue engineering strategies on bone regeneration [129]. The synthesis of these particles requires heating in an oven at 1250 °C [130]. Hydroxyapatite particles were uniformly spherical and large (50 µm), with a high specific surface area, uniform mesopores, and a doxorubicin loading capacity of 460.8 µg mg−1. In vivo, hydroxyapatite particles could be smoothly delivered through an arterial catheter to achieve chemoembolization. Doxorubicin-loaded hydroxyapatite particles effectively inhibited liver cancer cell growth in a rabbit liver tumor model, demonstrating the efficacy of TACE [131]. Pre-clinical studies explored the possibility of using hydroxyapatite particles with a 20–60 μm size range for vascular occlusion [78,132]. After 6 weeks of therapy, the biodegradation of the hydroxyapatite particles was realized by metabolizing Ca2+ and PO43− ions [83,84].
- 166Ho-poly L-lactic acid (PLLA) microspheres have been developed as a possible alternative to TARE with glass- or resin-containing 90Y, as PLLA is biocompatible with the human body and its degradation reaction is mainly due to hydrolysis to lactic acid [75,77]. The chelated and stable form of Ho, Sm, or Y is added as an acetylacetonate compound and mixed with L-Lactic acid (LLA) polymer during microsphere polymerization [77,133]. When the particles are formed and isolated, they are irradiated with neutrons, which form the radioactive 166Ho, 153Sm, or 90Y [75].
- Macro-aggregate albumin particles from HSA-B20 (Rotop Pharmaka, Dresden, Germany), Vasculosis® (Global Medical Solutions, Auckland, New Zealand), MAA (DRAXIMAGE®, Kirkland, QC, Canada), Pulmocis® Curium (London, UK)) are prepared after heating albumin and can be labeled directly with 99mTc, a recipe routinely used for scout scans in TARE set-up [99,103,134]. Alternatively, different therapeutic approaches have been investigated for TACE [49] and TARE [86], but, to date, only 188Re-labeled human serum albumin (188Re-HSA) microspheres have made their way to the clinic [85]. One advantage of HSA is that it is an approved carrier molecule, with 99mTc-HSA (Vasculosis®, Nanocoll® (GE Healthcare Ltd., Milan, Italy), Nanoalbumon® (Radiopharmacy Laboratoy Ltd, Budaörs, Hungary), Magnevist® (Bayer Inc., Toronto, ON, Canada) routinely used in nuclear medicine centers, indicated for blood pool imaging, angiocardiography, and ventriculography [135]. Pre-clinical [87] and clinical feasibility studies with 188Re-MAA have been published [65,85]. Both clinical studies demonstrated high product stability, low urinary excretion, good tolerance, and acceptable toxicity. Larger cohorts are necessary to conclude the usefulness of this device, which seems to be the ideal match with 99mTc-MAA. More recently, 90Y-DTPA-HSA microspheres were successfully evaluated in rats [68]. In pre-clinical settings, MAA was functionalized with adamantane to allow a pre-targeting set-up in the liver of mice. With this, targeting and imaging with a radiolabeled CD-PIBMA-Cy5 polymer yielded uptake in the vasculature with the functionalized MAA in the liver of mice based on host–guest chemistry [136,137]. Recently, a similar pre-clinical setup was carried out using click chemistry based on the interaction between azide-functionalized MAA and a radiolabeled DBCO-carrying moiety [138]. Given these pre-clinical findings, forming HSA microspheres seems feasible and can be carried out at low costs in a GLP facility [85].
- Chitosan, a polymer of 2-deoxy-2-amino-d-glucose obtained from the exoskeletons of crustaceans such as crabs and shrimps, transforms from a liquid to a gel state above pH 6 [99]. Chitosan is also regularly used in nanoparticle vaccines [139]. The feasibility of chitosan for TAE was assessed in the renal arteries of a rabbit model [140]. The renal arteries were still completely occluded after 8 weeks, and no inflammatory reaction was observed. Several strategies are available to modify chitosan, which can be used to couple additional moieties of interest [141,142]; for example, pre-activation of the COOH-bearing label (e.g., adamantane) with DIC or EDC in (acidic) water can be followed by the addition of chitosan PyBOP base to subsequently conjugate the COOH-bearing label. Kim et al. studied doxorubicin-loaded chitosan microcapsules in TACE in rabbits [143]. In a recent study, biodegradable chitosan was used to deliver and retain 166Ho at the tumor site [31,79]. Chitosan was complexed with 166Ho after mixing 166HoCl3 or 166Ho(NO3)3 at pH 3 for 30 min [144,145]. The holmium/chitosan complex (Millican, Dong Wha Pharmaceutical Co., Seoul, Korea) was effective in treating small HCCs in a novel study based on 40 patients with single HCC < 3 cm in size with satisfactory response rates and survival rates of 87.2%, 71.8%, and 65.3% at 1, 2, and 3 years, respectively [79,89,90].
- Starch-based microparticles (SBMP) were proposed as a unique system for the pre-therapeutic step (scout scan) after 188Re or 68Ga radiolabeling and TARE after direct radiolabeling with 188Re using SnCl2 reduction with gluconate or with 68GaCl3 and sodium acetate [93]. SBMP appeared to be a promising theranostic agent for the internal radiation therapy of hepatocellular carcinoma. SBMP was first developed for lung perfusion scintigraphy and formulated as a ready-to-use 99mTc radiolabeling kit [91,92]. After selecting suitable size particles via mechanical filtration, an aldehyde is formed, followed by the attachment of a diamine-linker. This chemistry should be possible with an amine, for example, Ahx [91,92,93]. The in vivo stability of the compounds is of primary importance, especially considering the therapeutic one, i.e., the SBMP radiolabeled with 188Re, and further investigations in pre-clinical models are warranted.
5. Advancements and Future Perspectives for Therapeutic Loads
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pérez-López, A.; Martín-Sabroso, C.; Gómez-Lázaro, L.; Torres-Suárez, A.I.; Aparicio-Blanco, J. Embolization therapy with microspheres for the treatment of liver cancer: State-of-the-art of clinical translation. Acta Biomater. 2022, 149, 1–15. [Google Scholar] [CrossRef]
- Marchal, S.; El Hor, A.; Millard, M.; Gillon, V.; Bezdetnaya, L. Anticancer drug delivery: An update on clinically applied nanotherapeutics. Drugs 2015, 75, 1601–1611. [Google Scholar] [CrossRef] [PubMed]
- Lorscheider, M.; Gaudin, A.; Nakhlé, J.; Veiman, K.L.; Richard, J.; Chassaing, C. Challenges and opportunities in the delivery of cancer therapeutics: Update on recent progress. Ther. Deliv. 2021, 12, 55–76. [Google Scholar] [CrossRef]
- Gritzapis, A.D.; Mahaira, L.G.; Perez, S.A.; Cacoullos, N.T.; Papamichail, M.; Baxevanis, C.N. Vaccination with Human HER-2/neu (435-443) CTL Peptide Induces Effective Antitumor Immunity against HER-2/neu-Expressing Tumor Cells In vivo. Cancer Res. 2006, 66, 5452–5460. [Google Scholar] [CrossRef] [Green Version]
- Rajput, M.; Agrawal, P. Microspheres in cancer therapy. Indian J. Cancer 2010, 47, 458–468. [Google Scholar] [CrossRef] [PubMed]
- Sinha, V.R.; Goyal, V.; Bhinge, J.R.; Mittal, B.R.; Trehan, A. Diagnostic microspheres: An overview. Crit. Rev. Drug Carr. Syst. 2003, 20, 431–460. [Google Scholar] [CrossRef] [PubMed]
- Klibanov, A.L. Targeted delivery of gas-filled microspheres, contrast agents for ultrasound imaging. Adv. Drug Deliv. Rev. 1999, 37, 139–157. [Google Scholar] [CrossRef] [PubMed]
- Laeschke, K. Biocompatibility of microparticles into soft tissue fillers. Semin. Cutan Med. Surg. 2004, 23, 214–217. [Google Scholar] [CrossRef]
- Varde, N.K.; Pack, D.W. Microspheres for controlled release drug delivery. Expert Opin. Biol. Ther. 2004, 4, 35–51. [Google Scholar] [CrossRef] [PubMed]
- Niu, H.; Du, T.; Xiao, Q.; Hu, X.; Li, D.; Wang, C.; Gao, W.; Xing, T.; Xu, X. Application of embolization microspheres in interventional therapy of malignant non-hypervascular tumor of liver. Oncotarget 2017, 8, 55593–55599. [Google Scholar] [CrossRef] [Green Version]
- Osuga, K.; Nakajima, Y.; Sone, M.; Arai, Y.; Nambu, Y.; Hori, S. Transarterial embolization of hypervascular tumors using trisacryl gelatin microspheres (Embosphere): A prospective multicenter clinical trial in Japan. Jpn. J. Radiol. 2016, 34, 366–375. [Google Scholar] [CrossRef]
- Vaidya, S.; Tozer, K.R.; Chen, J. An overview of embolic agents. Semin. Interv. Radiol. 2008, 25, 204–215. [Google Scholar] [CrossRef] [Green Version]
- Granberg, D.; Eriksson, L.G.; Welin, S.; Kindmark, H.; Janson, E.T.; Skogseid, B.; Oberg, K.; Eriksson, B.; Nyman, R. Liver embolization with trisacryl gelatin microspheres (embosphere) in patients with neuroendocrine tumors. Acta Radiol. 2007, 48, 180–185. [Google Scholar] [CrossRef]
- Hiraki, T.; Koizumi, J.; Arai, Y.; Sakurai, Y.; Kumada, H.; Nambu, Y.; Hori, S. Transcatheter arterial embolization of hypervascular tumors with HepaSphere: Prospective multicenter open label clinical trial of microspheres in Japan. Jpn. J. Radiol. 2015, 33, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Kettenbach, J.; Stadler, A.; Katzler, I.V.; Schernthaner, R.; Blum, M.; Lammer, J.; Rand, T. Drug-loaded microspheres for the treatment of liver cancer: Review of current results. Cardiovasc. Interv. Radiol. 2008, 31, 468–476. [Google Scholar] [CrossRef] [PubMed]
- Caine, M.; Carugo, D.; Zhang, X.; Hill, M.; Dreher, M.R.; Lewis, A.L. Review of the development of methods for characterization of microspheres for use in embolotherapy: Translating bench to cathlab. Adv. Healthc. Mater. 2017, 6, 1601291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burrill, J.; Hafeli, U.; Liu, D. Advances in radioembolization—Embolics and isotopes. J. Nucl. Med. Radiat. Ther. 2011, 2, 1000107. [Google Scholar] [CrossRef]
- Duran, R.; Chapiro, J.; Schernthaner, R.E.; Geschwind, J.F. Systematic review of catheter-based intra-arterial therapies in hepatocellular carcinoma: State of the art and future directions. Br. J. Radiol. 2015, 88, 20140564. [Google Scholar] [CrossRef] [Green Version]
- Janevska, D.; Chaloska-Ivanova, V.; Janevski, V. Hepatocellular carcinoma: Risk factors, diagnosis and treatment. Open Access Maced. J. Med. Sci. 2015, 3, 732–736. [Google Scholar] [CrossRef] [Green Version]
- Coletta, M.; Nicolini, D.; Benedetti Cacciaguerra, A.; Mazzocato, S.; Rossi, R.; Vivarelli, M. Bridging patients with hepatocellular cancer waiting for liver transplant: All the patients are the same? Transl. Gastroenterol. Hepatol. 2017, 2, 78. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; Kang, J.; Golas, B.J.; Yeung, V.W.; Madoff, D.C. Minimally invasive local therapies for liver cancer. Cancer Biol. Med. 2014, 11, 217–236. [Google Scholar] [CrossRef] [PubMed]
- Freedman, J.; Nilsson, H.; Jonas, E. New horizons in ablation therapy for hepatocellular carcinoma. Hepat. Oncol. 2015, 2, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Saito, R.; Amemiya, H.; Hosomura, N.; Kawaida, H.; Shoda, K.; Furuya, S.; Akaike, H.; Kawaguchi, Y.; Inoue, S.; Kono, H.; et al. Intended preoperative trans-arterial embolization for large hepatocellular carcinoma: A retrospective cohort study. World J. Surg. Oncol. 2022, 20, 90. [Google Scholar] [CrossRef]
- Shimohira, M.; Sato, Y.; Yasumoto, T.; Kodama, Y.; Masada, T.; Inaba, Y.; Yamakado, K. Arterial embolization using microspheres for hypervascular liver metastases refractory to standard treatments: A multicenter prospective clinical trial. Cardiovasc. Interv. Radiol. 2021, 44, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Laurent, A. Microspheres and Nonspherical Particles for Embolization. Tech. Vasc. Interv. Radiol. 2007, 10, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.H.; Liapi, E.A.; Cornell, C.; Reb, P.; Buijs, M.; Vossen, J.A.; Ventura, V.P.; Geschwind, J.F. Doxorubicin-loaded QuadraSphere microspheres: Plasma pharmacokinetics and intratumoral drug concentration in an animal model of liver cancer. Cardiovasc. Interv. Radiol. 2010, 33, 576–582. [Google Scholar] [CrossRef] [Green Version]
- Hecq, J.D.; Lewis, A.L.; Vanbeckbergen, D.; Athanosopoulos, A.; Galanti, L.; Jamart, J.; Czuczman, P.; Chung, T. Doxorubicin-loaded drug-eluting beads (DC Bead®) for use in transarterial chemoembolization: A stability assessment. J. Oncol. Pharm. Pract. 2013, 19, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Lewis, A.L.; Gonzalez, M.V.; Lloyd, A.W.; Hall, B.; Tang, Y.; Willis, S.L.; Leppard, S.W.; Wolfenden, L.C.; Palmer, R.R.; Stratford, P.W.; et al. DC bead: In vitro characterization of a drug-delivery device for transarterial chemoembolization. J. Vasc. Interv. Radiol. 2006, 17, 335–342. [Google Scholar] [CrossRef]
- Wang, Y.; Molin, D.G.M.; Sevrin, C.; Grandfils, C.; van den Akker, N.M.S.; Gagliardi, M.; Knetsch, M.L.; Delhaas, T.; Koole, L.H. In vitro and in vivo evaluation of drug-eluting microspheres designed for transarterial chemoembolization therapy. Int. J. Pharm. 2016, 503, 150–162. [Google Scholar] [CrossRef]
- Liu, Y.S.; Lin, C.Y.; Chuang, M.T.; Lin, C.Y.; Tsai, Y.S.; Wang, C.K.; Ou, M.C. Five-year outcome of conventional and drug-eluting transcatheter arterial chemoembolization in patients with hepatocellular carcinoma. BMC Gastroenterol. 2018, 18, 124. [Google Scholar] [CrossRef]
- Ni, J.Y.; Xu, L.F.; Wang, W.D.; Sun, H.L.; Chen, Y.T. Conventional transarterial chemoembolization vs microsphere embolization in hepatocellular carcinoma: A meta-analysis. World J. Gastroenterol. 2014, 20, 17206–17217. [Google Scholar] [CrossRef]
- De Luis, E.; Bilbao, J.I.; de Ciercoles, J.A.; Martinez-Cuesta, A.; de Martino Rodriguez, A.; Lozano, M.D. In vivo evaluation of a new embolic spherical particle (HepaSphere) in a kidney animal model. Cardiovasc. Interv. Radiol. 2008, 31, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Kennoki, N.; Hori, S.; Hori, A.; Takeo, Y.; Oshiro, H. Transcatheter arterial chemoembolization with spherical embolic material for locally advanced breast cancer: First report of HepaSphere treatment for primary breast cancer. BJR Case Rep. 2016, 2, 20150417. [Google Scholar] [CrossRef] [PubMed]
- Sottani, C.; Leoni, E.; Porro, B.; Montagna, B.; Amatu, A.; Sottotetti, F.; Quaretti, P.; Poggi, G.; Minoia, C. Validation of an LC-MS/MS method for the determination of epirubicin in human serum of patients undergoing drug eluting microsphere-transarterial chemoembolization (DEM-TACE). J. Chromatogr. B 2009, 877, 3543–3548. [Google Scholar] [CrossRef] [PubMed]
- Poggi, G.; Quaretti, P.; Minoia, C.; Bernardo, G.; Bonora, M.R.; Gaggeri, R.; Ronchi, A.; Saluzzo, C.M.; Azzaretti, A.; Rodolico, G.; et al. Transhepatic arterial chemoembolization with oxaliplatin-eluting microspheres (OEM-TACE) for unresectable hepatic tumors. Anticancer Res. 2008, 28, 3835–3842. [Google Scholar] [PubMed]
- Poursaid, A.; Jensen, M.M.; Huo, E.; Ghandehari, H. Polymeric materials for embolic and chemoembolic applications. J. Control. Release 2016, 240, 414–433. [Google Scholar] [CrossRef] [Green Version]
- Duan, X.H.; Li, H.; Ren, J.Z.; Han, X.W.; Chen, P.F.; Li, F.Y.; Huang, G.H.; Ju, S.G. Hepatic Arterial Chemoembolization With Arsenic Trioxide Eluting CalliSpheres Microspheres Versus Lipiodol Emulsion: Pharmacokinetics And Intratumoral Concentration In A Rabbit Liver Tumor Model. Cancer Manag. Res. 2019, 11, 9979–9988. [Google Scholar] [CrossRef] [Green Version]
- Amrein, M.L.; Soong, C.; Liang, N. Upregulated Membrane Expression of a Conserved Voltage—Gated Sodium Channel, Nav1.4a, and Electrical Organ Discharge in Electric Mouse, P. pikachu. PLoS Biol. 2013, 11, 1001501. [Google Scholar] [CrossRef]
- Wu, B.; Zhou, J.; Ling, G.; Zhu, D.; Long, Q. CalliSpheres drug-eluting beads versus lipiodol transarterial chemoembolization in the treatment of hepatocellular carcinoma: A short-term efficacy and safety study. World J. Surg. Oncol. 2018, 16, 69. [Google Scholar] [CrossRef]
- Ma, Y.; Zhao, C.; Zhao, H.; Li, H.; Chen, C.; Xiang, H.; Zheng, C.; Ma, C.; Luo, C.; Qiu, H.; et al. Comparison of treatment efficacy and safety between drug-eluting bead transarterial chemoembolization with CalliSpheres® microspheres and conventional transarterial chemoembolization as first-line treatment in hepatocellular carcinoma patients. Am. J. Transl. Res. 2019, 11, 7456–7470. [Google Scholar]
- Zhang, S.; Huang, C.; Li, Z.; Yang, Y.; Bao, T.; Chen, H.; Zou, Y.; Song, L. Comparison of pharmacokinetics and drug release in tissues after transarterial chemoembolization with doxorubicin using diverse lipiodol emulsions and CalliSpheres Beads in rabbit livers. Drug Deliv. 2017, 24, 1011–1017. [Google Scholar] [CrossRef] [Green Version]
- Pelage, J.P.; Fohlen, A.; Mitry, E.; Lagrange, C.; Beauchet, A.; Rougier, P. Chemoembolization of neuroendocrine liver metastases using streptozocin and tris-acryl microspheres: Embozar (EMBOsphere + ZAnosaR) study. Cardiovasc. Interv. Radiol. 2017, 40, 394–400. [Google Scholar] [CrossRef] [Green Version]
- Beaujeux, R.; Laurent, A.; Wassef, M.; Casasco, A.; Gobin, Y.P.; Aymard, A.; Rufenacht, D.; Merland, J.J. Trisacryl gelatin microspheres for therapeutic embolization, II: Preliminary clinical evaluation in tumors and arteriovenous malformations. Am. J. Neuroradiol. 1996, 17, 541–548. [Google Scholar] [PubMed]
- Qian, J.; Truebenbach, J.; Graepler, F.; Pereira, P.; Huppert, P.; Eul, T.; Wiemann, G.; Claussen, C. Application of poly-lactide-co-glycolide-microspheres in the transarterial chemoembolization in an animal model of hepatocellular carcinoma. World J. Gastroenterol. 2003, 9, 94. [Google Scholar] [CrossRef] [PubMed]
- Makadia, H.K.; Siegel, S.J. Poly lactic-co-glycolic acid (PLGA) as biodegradable controlled drug delivery carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef]
- Blasi, P. Poly(lactic acid)/poly(lactic-co-glycolic acid)-based microparticles: An overview. J. Pharm. Investig. 2019, 49, 337–346. [Google Scholar] [CrossRef] [Green Version]
- Fuchs, K.; Duran, R.; Denys, A.; Bize, P.E.; Borchard, G.; Jordan, O. Drug-eluting embolic microspheres for local drug delivery-State of the art. J. Control. Release 2017, 262, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, S.; Miyazaki, M.; Endoh, F.; Takahashi, O.; Okui, K.; Morimoto, Y. Biodegradable mitomycin C microspheres given intra-arterially for inoperable hepatic cancer. With particular reference to a comparison with continuous infusion of mitomycin C and 5-fluorouracil. Cancer 1985, 56, 2404–2410. [Google Scholar] [CrossRef]
- Karimi, M.; Bahrami, S.; Ravari, S.B.; Zangabad, P.S.; Mirshekari, H.; Bozorgomid, M.; Shahreza, S.; Sori, M.; Hamblin, M.R. Albumin nanostructures as advanced drug delivery systems. Expert Opin. Drug Deliv. 2016, 13, 1609–1623. [Google Scholar] [CrossRef] [Green Version]
- Tsitskari, M.; Filippiadis, D.; Kostantos, C.; Palialexis, K.; Zavridis, P.; Kelekis, N.; Brountzos, E. The role of interventional oncology in the treatment of colorectal cancer liver metastases. Ann. Gastroenterol. 2019, 32, 147–155. [Google Scholar] [CrossRef]
- Forner, A.; Llovet, J.M.; Bruix, J. Hepatocellular carcinoma. Lancet 2012, 379, 1245–1255. [Google Scholar] [CrossRef] [PubMed]
- Kloeckner, R.; Weinmann, A.; Prinz, F.; Pinto dos Santos, D.; Ruckes, C.; Dueber, C.; Pitton, M.B. Conventional transarterial chemoembolization versus drug-eluting bead transarterial chemoembolization for the treatment of hepatocellular carcinoma. BMC Cancer 2015, 15, 465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sangro, B.; Carpanese, L.; Cianni, R.; Golfieri, R.; Gasparini, D.; Ezziddin, S.; Paprottka, P.M.; Fiore, F.; van Buskirk, M.; Ignacio Bilbao, J.; et al. Survival after Yttrium-90 resin microsphere radioembolization of hepatocellular carcinoma across Barcelona clinic liver cancer stages: A European evaluation. Hepatology 2011, 54, 868–878. [Google Scholar] [CrossRef] [PubMed]
- Bouvry, C.; Palard, X.; Edeline, J.; Ardisson, V.; Loyer, P.; Garin, E.; Lepareur, N. Transarterial radioembolization (TARE) agents beyond (90)Y-microspheres. Biomed. Res. Int. 2018, 2018, 1435302. [Google Scholar] [CrossRef] [Green Version]
- Prince, J.F.; van den Bosch, M.A.A.J.; Nijsen, J.F.W.; Smits, M.L.J.; van den Hoven, A.F.; Nikolakopoulos, S.; Wessels, F.J.; Bruijnen, R.C.G.; Braat, M.N.G.J.A.; Zonnenberg, B.A.; et al. Efficacy of Radioembolization with166Ho-Microspheres in Salvage Patients with Liver Metastases: A Phase 2 Study. J. Nucl. Med. 2018, 59, 582–588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Filippi, L.; Schillaci, O.; Cianni, R.; Bagni, O. Yttrium-90 resin microspheres and their use in the treatment of intrahepatic cholangiocarcinoma. Future Oncol. 2018, 14, 809–818. [Google Scholar] [CrossRef]
- Robinson, T.J.; Du, L.; Matsuoka, L.; Sze, D.Y.; Kennedy, A.S.; Gandhi, R.T.; Kouri, B.E.; Collins, Z.S.; Kokabi, N.; Grilli, C.J.; et al. Survival and toxicities after Yttrium-90 transarterial radioembolization of Cholangiocarcinoma in the RESiN registry. J. Vasc. Interv. Radiol. 2022, in press. [CrossRef]
- Bargellini, I.; Bozzi, E.; Lorenzoni, G.; Boni, G.; Bianchi, F.; Traino, C.A.; Masi, G.; Cioni, R.; Crocetti, L. Role of Transhepatic Arterial Radioembolization in Metastatic Colorectal Cancer. Cardiovasc. Interv. Radiol. 2022, 45, 1579–1589. [Google Scholar] [CrossRef]
- Ingenerf, M.K.; Karim, H.; Fink, N.; Ilhan, H.; Ricke, J.; Treitl, K.M.; Schmid-Tannwald, C. Apparent diffusion coefficients (ADC) in response assessment of transarterial radioembolization (TARE) for liver metastases of neuroendocrine tumors (NET): A feasibility study. Acta Radiol. 2022, 63, 877–888. [Google Scholar] [CrossRef]
- Raoul, J.L.; Guyader, D.; Bretagne, J.F.; Heautot, J.F.; Duvauferrier, R.; Bourguet, P.; Bekhechi, D.; Deugnier, Y.M.; Gosselin, M. Prospective randomized trial of chemoembolization versus intra-arterial injection of 131I-labeled-iodized oil in the treatment of hepatocellular carcinoma. Hepatology 1997, 26, 1156–1161. [Google Scholar] [CrossRef]
- Pirayesh, E.; Amoui, M.; Akhlaghpoor, S.; Tolooee, S.; Khorrami, M.; Poorbeigi, H.; Sheibani, S.; Assadi, M. Technical considerations of phosphorous-32 Bremsstrahlung SPECT imaging after radioembolization of hepatic tumors: A clinical assessment with a review of imaging parameters. Radiol. Res. Pract. 2014, 2014, 407158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raoul, J.L.; Duvauferrier, R.; Bourguet, P.; Bretagne, J.F.; Coornaert, S.; Darnault, P.; Deugnier, Y.; Herry, J.Y.; Gastard, J. Lipiodolized angiography in hepatocellular carcinomas. Contribution of iodine-131-labelled lipiodol. J. Radiol. 1986, 67, 797–801. [Google Scholar] [PubMed]
- Hafeli, U.O.; Casillas, S.; Dietz, D.W.; Pauer, G.J.; Rybicki, L.A.; Conzone, S.D.; Day, D.E. Hepatic tumor radioembolization in a rat model using radioactive rhenium (186Re/188Re) glass microspheres. Int. J. Radiat. Oncol. Biol. Phys. 1999, 44, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Lepareur, N.; Lacoeuille, F.; Bouvry, C.; Hindre, F.; Garcion, E.; Cherel, M.; Noiret, N.; Garin, E.; Knapp, F.F.R., Jr. Rhenium-188 labeled radiopharmaceuticals: Current clinical applications in oncology and promising perspectives. Front. Med. 2019, 6, 00132. [Google Scholar] [CrossRef] [Green Version]
- Liepe, K.; Brogsitter, C.; Leonhard, J.; Wunderlich, G.; Hliscs, R.; Pinkert, J.; Folprecht, G.; Kotzerke, J. Feasibility of high activity rhenium-188-microsphere in hepatic radioembolization. Jpn. J. Clin. Oncol. 2007, 37, 942–950. [Google Scholar] [CrossRef] [Green Version]
- Poorbaygi, H.; Aghamiri, S.M.R.; Sheibani, S.; Kamali-asl, A.; Mohagheghpoor, E. Production of glass microspheres comprising 90Y and 177Lu for treating of hepatic tumors with SPECT imaging capabilities. Appl. Radiat. Isot. 2011, 69, 1407–1414. [Google Scholar] [CrossRef]
- Akram, A.R.; Avlonitis, N.; Scholefield, E.; Vendrell, M.; McDonald, N.; Aslam, T.; Craven, T.H.; Gray, C.; Collie, D.S.; Fisher, A.J.; et al. Enhanced avidity from a multivalent fluorescent antimicrobial peptide enables pathogen detection in a human lung model. Sci. Rep. 2019, 9, 8422. [Google Scholar] [CrossRef] [Green Version]
- Vukadinović, V.; Janković, D.; Radović, M.; Milanović, Z.; Mirković, M.; Stanković, D.; Vranješ-Đurić, S. Optimization of the radiolabelling method for improved in vitro and in vivo stability of 90Y-albumin microspheres. Appl. Radiat. Isot. 2020, 156, 108984. [Google Scholar] [CrossRef]
- Hashikin, N.A.A.; Yeong, C.-H.; Abdullah, B.J.J.; Ng, K.-H.; Chung, L.-Y.; Dahalan, R.; Perkins, A.C. Neutron activated samarium-153 microparticles for transarterial radioembolization of liver tumour with post-procedure imaging capabilities. PLoS ONE 2015, 10, 0138106. [Google Scholar] [CrossRef] [Green Version]
- Hruby, M.; Skodova, M.; Mackova, H.; Skopal, J.; Tomes, M.; Kropacek, M.; Zimova, J.; Kucka, J. Lutetium-177 and iodine-131 loaded chelating polymer microparticles intended for radioembolization of liver malignancies. React. Funct. Polym. 2011, 71, 1155–1159. [Google Scholar] [CrossRef]
- Pandey, U.; Subramanian, S.; Shaikh, S.; Gamre, N.; Kumar, S.; Dash, A. Synthesis and preliminary biological evaluation of 177Lu-labeled polyhydroxamic acid microparticles toward therapy of hepatocellular carcinoma. Cancer Biother. Radiopharm. 2019, 34, 306–315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saxena, S.K.; Kumar, Y.; Shaikh, S.H.; Pandey, U.; Kumar, S.A.; Dash, A. Preparation of radioactive skin patches using polyhydroxamic acid-grafted cellulose films toward applications in treatment of superficial tumors. Cancer Biother. Radiopharm. 2017, 32, 364–370. [Google Scholar] [CrossRef] [PubMed]
- Wong, Y.H.; Tan, H.Y.; Kasbollah, A.; Abdullah, B.J.J.; Yeong, C.H. Preparation and in vitro evaluation of neutron-activated, theranostic samarium-153-labeled microspheres for transarterial radioembolization of hepatocellular carcinoma and liver metastasis. Pharmaceutics 2019, 11, 596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fisher, D.; Fidel, J.; Maitz, C. Direct interstitial treatment of solid tumors using an injectable yttrium-90-polymer composite. Cancer Biother. Radiopharm. 2020, 35, 2947. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mumper, R.J.; Ryo, U.Y.; Jay, M. Neutron-activated holmium-166-poly (L-lactic acid) microspheres: A potential agent for the internal radiation therapy of hepatic tumors. J. Nucl. Med. 1991, 32, 2139–2143. [Google Scholar]
- Vente, M.A.; Nijsen, J.F.; de Roos, R.; van Steenbergen, M.J.; Kaaijk, C.N.; Koster-Ammerlaan, M.J.; de Leege, P.F.; Hennink, W.E.; van Het Schip, A.D.; Krijger, G.C. Neutron activation of holmium poly(L-lactic acid) microspheres for hepatic arterial radio-embolization: A validation study. Biomed. Microdevices 2009, 11, 763–772. [Google Scholar] [CrossRef] [Green Version]
- Mumper, R.J.; Jay, M. Biodegradable radiotherapeutic polyester microspheres: Optimization and in-vitro/in-vivo evaluation. J. Control. Release 1992, 18, 193–203. [Google Scholar] [CrossRef]
- Das, T.; Chakraborty, S.; Sarma, H.D.; Venkatesh, M.; Banerjee, S. 166Ho-labeled hydroxyapatite particles: A possible agent for liver cancer therapy. Cancer Biother. Radiopharm. 2009, 24, 7–14. [Google Scholar] [CrossRef]
- Kim, J.K.; Han, K.H.; Lee, J.T.; Paik, Y.H.; Ahn, S.H.; Lee, J.D.; Lee, K.S.; Chon, C.Y.; Moon, Y.M. Long-term clinical outcome of phase IIb clinical trial of percutaneous injection with holmium-166/chitosan complex (Milican) for the treatment of small hepatocellular carcinoma. Clin. Cancer Res. 2006, 12, 543–548. [Google Scholar] [CrossRef] [Green Version]
- De la Vega, J.C.; Esquinas, P.L.; Rodríguez-Rodríguez, C.; Bokharaei, M.; Moskalev, I.; Liu, D.; Saatchi, K.; Häfeli, U.O. Radioembolization of hepatocellular carcinoma with built-in dosimetry: First in vivo results with uniformly-sized, biodegradable microspheres labeled with188re. Theranostics 2019, 9, 868–883. [Google Scholar] [CrossRef]
- Jamre, M.; Shamsaei, M.; Erfani, M.; Sadjadi, S.; Maragheh, M.G. Preparation and evaluation of 188Re sulfide colloidal nanoparticles loaded biodegradable poly (L-lactic acid) microspheres for radioembolization therapy. J. Label. Compd. Radiopharm. 2018, 61, 586–594. [Google Scholar] [CrossRef] [PubMed]
- Hrubý, M.; Hradil, J.; Beneš, M.J. Interactions of phenols with silver(I), copper(II) and iron(III) complexes of chelating methacrylate-based polymeric sorbent containing quinolin-8-ol groups. React. Funct. Polym. 2004, 59, 105–118. [Google Scholar] [CrossRef]
- Chinol, M.; Vallabhajosula, S.; Goldsmith, S.J.; Klein, M.J.; Deutsch, K.F.; Chinen, L.K.; Brodack, J.W.; Deutsch, E.A.; Watson, B.A.; Tofe, A.J.; et al. Chemistry and biological behavior of samarium-153 and rhenium-186-labeled hydroxyapatite particles: Potential radiopharmaceuticals for radiation synovectomy. J. Nucl. Med. 1993, 34, 1536–1542. [Google Scholar] [PubMed]
- Unni, P.R.; Chaudhari, P.R.; Venkatesh, M.; Ramamoorthy, N.; Pillai, M.R. Preparation and bioevaluation of 166Ho labelled hydroxyapatite (HA) particles for radiosynovectomy. Nucl. Med. Biol. 2002, 29, 199–209. [Google Scholar] [CrossRef]
- Nowicki, M.L.; Cwikla, J.B.; Sankowski, A.J.; Shcherbinin, S.; Grimmes, J.; Celler, A.; Buscombe, J.R.; Bator, A.; Pech, M.; Mikolajczak, R.; et al. Initial study of radiological and clinical efficacy radioembolization using 188Re-human serum albumin (HSA) microspheres in patients with progressive, unresectable primary or secondary liver cancers. Med. Sci. Monit. 2014, 20, 1353–1362. [Google Scholar] [CrossRef] [Green Version]
- Bhushan, B.; Khanadeev, V.; Khlebtsov, B.; Khlebtsov, N.; Gopinath, P. Impact of albumin based approaches in nanomedicine: Imaging, targeting and drug delivery. Adv. Colloid Interface Sci. 2017, 246, 13–39. [Google Scholar] [CrossRef]
- Chen, L.-C.; Lee, W.-C.; Ho, C.-L.; Chang, Y.-J.; Chen, S.-J.; Chang, C.-H. Biodistribution, pharmacokinetics and efficacy of (188)re(i)-tricarbonyl-labeled human serum albumin microspheres in an orthotopic hepatoma rat model. In Vivo 2018, 32, 567–573. [Google Scholar] [CrossRef]
- Cremonesi, M.; Chiesa, C.; Strigari, L.; Ferrari, M.; Botta, F.; Guerriero, F.; de Cicco, C.; Bonomo, G.; Orsi, F.; Bodei, L.; et al. Radioembolization of hepatic lesions from a radiobiology and dosimetric perspective. Front. Oncol. 2014, 4, 210. [Google Scholar] [CrossRef] [Green Version]
- Memon, K.; Lewandowski, R.J.; Kulik, L.; Riaz, A.; Mulcahy, M.F.; Salem, R. Radioembolization for primary and metastatic liver cancer. Semin. Radiat. Oncol. 2011, 21, 294–302. [Google Scholar] [CrossRef] [Green Version]
- Sohn, J.H.; Choi, H.J.; Lee, J.T.; Lee, J.D.; Kim, J.H.; Moon, Y.M.; Park, K.; Park, K.B.; Kim, E.; Yoo, N.C.; et al. Phase II study of transarterial holmium-166-chitosan complex treatment in patients with a single, large hepatocellular carcinoma. Oncology 2009, 76, 1–9. [Google Scholar] [CrossRef]
- Lacoeuille, F.; Hindré, F.; Denizot, B.; Bouchet, F.; Legras, P.; Couturier, O.; Askiénazy, S.; Benoit, J.P.; le Jeune, J.J. New starch-based radiotracer for lung perfusion scintigraphy. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lacoeuille, F.; Hindré, F.; Venier-Julienne, M.C.; Sergent, M.; Bouchet, F.; Jouaneton, S.; Denizot, B.; Askienazy, S.; Benoit, J.P.; Couturier, O.F.; et al. A starch-based microparticulate system dedicated to diagnostic and therapeutic nuclear medicine applications. Biomaterials 2011, 32, 7999–8009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verger, E.; Drion, P.; Meffre, G.; Bernard, C.; Duwez, L.; Lepareur, N.; Couturier, O.; Hindré, F.; Hustinx, R.; Lacoeuille, F.; et al. 68Ga and 188Re starch-based microparticles as theranostic tool for the hepatocellular carcinoma: Radiolabeling and preliminary in vivo rat studies. PLoS ONE 2016, 11, e0164626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braat, A.J.A.T.; Smits, M.L.J.; Braat, M.N.G.J.A.; van den Hoven, A.F.; Prince, J.F.; de Jong, H.W.A.M.; van den Bosch, M.A.A.J.; Lam, M.G.E.H. 90Y hepatic radioembolization: An update on current practice and recent developments. J. Nucl. Med. 2015, 56, 1079–1087. [Google Scholar] [CrossRef] [Green Version]
- Salem, R.; Lewandowski, R.J.; Gates, V.L.; Nutting, C.W.; Murthy, R.; Rose, S.C.; Soulen, M.C.; Geschwind, J.-F.H.; Kulik, L.; Kim, Y.H.; et al. Research reporting standards for radioembolization of hepatic malignancies. J. Vasc. Interv. Radiol. 2011, 22, 265–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hickey, R.; Lewandowski, R.J.; Prudhomme, T.; Ehrenwald, E.; Baigorri, B.; Critchfield, J.; Kallini, J.; Gabr, A.; Gorodetski, B.; Geschwind, J.-F.; et al. 90Y radioembolization of colorectal hepatic metastases using glass microspheres: Safety and survival outcomes from a 531-patient multicenter study. J. Nucl. Med. 2016, 57, 665–671. [Google Scholar] [CrossRef] [Green Version]
- Uliel, L.; Royal, H.D.; Darcy, M.D.; Zuckerman, D.A.; Sharma, A.; Saad, N.E. From the angio suite to the γ-camera: Vascular mapping and 99mTc-MAA hepatic perfusion imaging before liver radioembolization—A comprehensive pictorial review. J. Nucl. Med. 2012, 53, 1736–1747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riaz, A.; Awais, R.; Salem, R. Side effects of Yttrium-90 radioembolization. Front. Oncol. 2014, 4, 00198. [Google Scholar] [CrossRef] [Green Version]
- Xing, M.; Lahti, S.; Kokabi, N.; Schuster, D.M.; Camacho, J.C.; Kim, H.S. 90Y Radioembolization lung shunt fraction in primary and metastatic liver cancer as a biomarker for survival. Clin. Nucl. Med. 2016, 41, 21–27. [Google Scholar] [CrossRef]
- Stella, M.; Braat, A.; van Rooij, R.; de Jong, H.; Lam, M. Holmium-166 Radioembolization: Current Status and Future Prospective. Cardiovasc. Interv. Radiol. 2022, 45, 1634–1645. [Google Scholar] [CrossRef]
- Deidda, D.; Denis-Bacelar, A.M.; Fenwick, A.J.; Ferreira, K.M.; Heetun, W.; Hutton, B.F.; Robinson, A.P.; Scuffham, J.; Thielemans, K. Hybrid kernelised expectation maximisation for Bremsstrahlung SPECT reconstruction in SIRT with (90)Y micro-spheres. EJNMMI Phys. 2022, 9, 25. [Google Scholar] [CrossRef]
- D’Arienzo, M.; Pimpinella, M.; Capogni, M.; de Coste, V.; Filippi, L.; Spezi, E.; Patterson, N.; Mariotti, F.; Ferrari, P.; Chiaramida, P.; et al. Phantom validation of quantitative Y-90 PET/CT-based dosimetry in liver radioembolization. EJNMMI Res. 2017, 7, 94. [Google Scholar] [CrossRef] [Green Version]
- Ilhan, H.; Goritschan, A.; Paprottka, P.; Jakobs, T.F.; Fendler, W.P.; Todica, A.; Bartenstein, P.; Hacker, M.; Haug, A.R. Predictive value of 99mTc-MAA SPECT for 90Y-labeled resin microsphere distribution in radioembolization of primary and secondary hepatic tumors. J. Nucl. Med. 2015, 56, 1654–1660. [Google Scholar] [CrossRef] [Green Version]
- Bult, W.; Vente, M.A.; Zonnenberg, B.A.; van Het Schip, A.D.; Nijsen, J.F. Microsphere radioembolization of liver malignancies: Current developments. Q. J. Nucl. Med. Mol. Imaging 2009, 53, 325–335. [Google Scholar]
- Elschot, M.; Nijsen, J.F.; Lam, M.G.; Smits, M.L.; Prince, J.F.; Viergever, M.A.; van den Bosch, M.A.; Zonnenberg, B.A.; de Jong, H.W. 99mTc-MAA overestimates the absorbed dose to the lungs in radioembolization: A quantitative evaluation in patients treated with 166Ho-microspheres. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 1965–1975. [Google Scholar] [CrossRef]
- She, W.H.; Cheung, T.T.; Yau, T.C.; Chan, A.C.; Chok, K.S.; Chu, F.S.; Liu, R.K.; Poon, R.T.; Chan, S.C.; Fan, S.T.; et al. Survival analysis of transarterial radioembolization with yttrium-90 for hepatocellular carcinoma patients with HBV infection. Hepatobiliary Surg. Nutr. 2014, 3, 185–193. [Google Scholar] [CrossRef] [Green Version]
- Kwon, J.H.; Kim, G.M.; Han, K.; Won, J.Y.; Kim, M.D.; Lee, D.Y.; Lee, J.; Choi, W.; Kim, Y.S.; Kim, D.Y.; et al. Safety and efficacy of transarterial radioembolization combined with chemoembolization for bilobar hepatocellular carcinoma: A single-center retrospective study. Cardiovasc. Interv. Radiol. 2018, 41, 459–465. [Google Scholar] [CrossRef]
- Kim, D.Y.; Han, K.H. Transarterial chemoembolization versus transarterial radioembolization in hepatocellular carcinoma: Optimization of selecting treatment modality. Hepatol. Int. 2016, 10, 883–892. [Google Scholar] [CrossRef]
- Moreno-Luna, L.E.; Yang, J.D.; Sanchez, W.; Paz-Fumagalli, R.; Harnois, D.M.; Mettler, T.A.; Gansen, D.N.; de Groen, P.C.; Lazaridis, K.N.; Narayanan Menon, K.V.; et al. Efficacy and safety of transarterial radioembolization versus chemoembolization in patients with hepatocellular carcinoma. Cardiovasc. Interv. Radiol. 2013, 36, 714–723. [Google Scholar] [CrossRef] [Green Version]
- Lobo, L.; Yakoub, D.; Picado, O.; Ripat, C.; Pendola, F.; Sharma, R.; ElTawil, R.; Kwon, D.; Venkat, S.; Portelance, L.; et al. Unresectable hepatocellular carcinoma: Radioembolization versus chemoembolization: A systematic review and meta-analysis. Cardiovasc. Interv. Radiol. 2016, 39, 1580–1588. [Google Scholar] [CrossRef]
- Marcacuzco Quinto, A.; Nutu, O.A.; San Román Manso, R.; Justo Alonso, I.; Calvo Pulido, J.; Manrique Municio, A.; García-Sesma, Á.; Loinaz Segurola, C.; Martínez Caballero, J.; Jiménez Romero, L.C. Complications of transarterial chemoembolization (TACE) in the treatment of liver tumors. Cir. Esp. 2018, 96, 560–567. [Google Scholar] [CrossRef]
- Kim, H.C. Radioembolization for the treatment of hepatocellular carcinoma. Clin. Mol. Hepatol. 2017, 23, 109–114. [Google Scholar] [CrossRef] [Green Version]
- Kang, Y.J.; Lee, B.C.; Kim, J.K.; Yim, N.Y.; Kim, H.O.; Cho, S.B.; Jeong, Y.Y. Conventional versus small doxorubicin-eluting bead transcatheter arterial chemoembolization for treating barcelona clinic liver cancer stage 0/A hepatocellular carcinoma. Cardiovasc. Interv. Radiol. 2020, 43, 55–64. [Google Scholar] [CrossRef]
- Fidelman, N.; Kerlan, R.K., Jr. Transarterial chemoembolization and (90)Y radioembolization for hepatocellular carcinoma: Review of current applications beyond intermediate-stage disease. Am. J. Roentgenol. 2015, 205, 742–752. [Google Scholar] [CrossRef]
- Chauhan, N.; Bukovcan, J.; Boucher, E.; Cosgrove, D.; Edeline, J.; Hamilton, B.; Kulik, L.; Master, F.; Salem, R. Intra-arterial TheraSphere Yttrium-90 glass microspheres in the treatment of patients with unresectable hepatocellular carcinoma: Protocol for the STOP-HCC Phase 3 randomized controlled trial. JMIR Res. Protoc. 2018, 7, e11234. [Google Scholar] [CrossRef]
- Salem, R.; Padia, S.A.; Lam, M.; Chiesa, C.; Haste, P.; Sangro, B.; Toskich, B.; Fowers, K.; Herman, J.M.; Kappadath, S.C.; et al. Clinical, dosimetric, and reporting considerations for Y-90 glass microspheres in hepatocellular carcinoma: Updated 2022 recommendations from an international multidisciplinary working group. Eur. J. Nucl. Med. Mol. Imaging 2022, 50, 328–343. [Google Scholar] [CrossRef]
- Pham, T.M.; Duong, V.D.; Doan, V.-D.; Vo, V.T.; Le, V.T. Design synthesis of Y-90 glass microspheres and study of their therapeutic effects on mouse liver cancer cell line Hep3B. Chemosphere 2022, 299, 134431. [Google Scholar] [CrossRef]
- James, T.; Hill, J.; Fahrbach, T.; Collins, Z. Differences in radiation activity between glass and resin 90Y microspheres in treating unresectable hepatic cancer. Health Phys. 2017, 112, 300–304. [Google Scholar] [CrossRef]
- Schubiger, P.A.; Beer, H.F.; Geiger, L.; Rösler, H.; Zimmermann, A.; Triller, J.; Mettler, D.; Schilt, W. 90Y-resin particles—Animal experiments on pigs with regard to the introduction of superselective embolization therapy. Int. J. Radiat. Appl. Instrum. B. Nucl. Med. Biol. 1991, 18, 305–311. [Google Scholar] [CrossRef]
- Carmona, M.; Warchoł, J.; Lucas, A.D.; Rodriguez, J.F. Ion-Exchange equilibria of Pb2+, Ni2+, and Cr3+ ions for H+ on Amberlite IR-120 Resin. J. Chem. Engin. Data 2008, 53, 1325–1331. [Google Scholar] [CrossRef]
- Mondal, A.; De, S.; Maiti, S.; Sarkar, B.; Sk, A.K.; Jacob, R.; Moorthy, A.; Paira, P. Amberlite IR-120 (H) mediated “on water” synthesis of fluorescent Ruthenium(II)-arene 8-hydroxyquinoline complexes for cancer therapy and live cell imaging. J. Photochem. Photobiol. B 2018, 178, 380–394. [Google Scholar] [CrossRef]
- Švec, F.; Hradil, J.; Čoupek, J.; Kálal, J. Reactive polymers I. Macroporous methacrylate copolymers containing epoxy groups. Die Angew. Makromol. Chem. Appl. Macromol. Chem. Phys. 1975, 48, 135–143. [Google Scholar] [CrossRef]
- Saxena, S.; Gomber, C. Surmounting antimicrobial resistance in the millennium superbug: Staphylococcus aureus. Cent. Eur. J. Med. 2010, 5, 12–29. [Google Scholar] [CrossRef]
- Astete, C.E.; Sabliov, C.M. Synthesis and characterization of PLGA nanoparticles. J. Biomater. Sci. Polym. 2006, 17, 247–289. [Google Scholar] [CrossRef]
- Fredenberg, S.; Wahlgren, M.; Reslow, M.; Axelsson, A. The mechanisms of drug release in poly(lactic-co-glycolic acid)-based drug delivery systems—A review. Int. J. Pharm. 2011, 415, 34–52. [Google Scholar] [CrossRef]
- Fisher, D.R. Radiation safety for yttrium-90-polymer composites (radiogel™) in therapy of solid tumors. Health Phys. 2021, 120, 510–516. [Google Scholar] [CrossRef]
- Lambert, B.; Mertens, J.; Ravier, M.; Blanken, T.; Defreyne, L.; van Vlierberghe, H.; D’Asseler, Y.; Oltenfreiter, R. Urinary excretion of Yttrium-90 following intra-arterial microsphere treatment for liver tumours. J. Nucl. Med. 2011, 52, 1744. [Google Scholar]
- Bozkurt, Y.; Pazarlioglu, S.; Gokce, H.; Gurler, I.; Salman, S. Hydroxyapatite lanthanum oxide composites. Acta Phys. Pol. A 2015, 127, 1407–1409. [Google Scholar] [CrossRef]
- Wieszczycka, K.; Staszak, K.; Woźniak-Budych, M.J.; Jurga, S. Lanthanides and tissue engineering strategies for bone regeneration. Coord. Chem. Rev. 2019, 388, 248–267. [Google Scholar] [CrossRef]
- Kang, N.H.; Kim, S.J.; Song, S.H.; Choi, S.; Choi, S.Y.; Kim, Y.J. Hydroxyapatite synthesis using EDTA. J. Craniofac. Surg. 2013, 24, 1042–1045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, L.; Liang, X.; Xu, X.; Zhang, X.; Wen, J.; Chen, K.; Su, X.; Teng, Z.; Lu, G.; Xu, J.; et al. Magnetic mesoporous embolic microspheres in transcatheter arterial chemoembolization for liver cancer. Acta Biomater. 2021, 130, 374–384. [Google Scholar] [CrossRef]
- Kubo, M.; Kuwayama, N.; Hirashima, Y.; Takaku, A.; Ogawa, T.; Endo, S. Hydroxyapatite ceramics as a particulate embolic material: Report of the physical properties of the hydroxyapatite particles and the animal study. Am. J. Neuroradiol. 2003, 24, 1540–1544. [Google Scholar] [PubMed]
- Smits, M.L.J.; Nijsen, J.F.W.; van den Bosch, M.A.A.J.; Lam, M.G.E.H.; Vente, M.A.D.; Huijbregts, J.E.; van Het Schip, A.D.; Elschot, M.; Bult, W.; de Jong, H.W.A.M.; et al. Holmium-166 radioembolization for the treatment of patients with liver metastases: Design of the phase I HEPAR trial. J. Exp. Clin. Cancer Res. 2010, 29, 70. [Google Scholar] [CrossRef] [Green Version]
- Grosser, O.S.; Ruf, J.; Kupitz, D.; Pethe, A.; Ulrich, G.; Genseke, P.; Mohnike, K.; Pech, M.; Richter, W.S.; Ricke, J.; et al. Pharmacokinetics of 99mtc-maa- and 99mtc-hsa-microspheres used in preradioembolization dosimetry: Influence on the liver-lung shunt. J. Nucl. Med. 2016, 57, 925–927. [Google Scholar] [CrossRef] [Green Version]
- Wunderlich, G.; Pinkert, J.; Andreeff, M.; Stintz, M.; Knapp, F.F., Jr.; Kropp, J.; Franke, W.G. Preparation and biodistribution of rhenium-188 labeled albumin microspheres B 20: A promising new agent for radiotherapy. Appl. Radiat. Isot. 2000, 52, 63–68. [Google Scholar] [CrossRef]
- Spa, S.J.; Welling, M.M.; van Oosterom, M.N.; Rietbergen, D.D.D.; Burgmans, M.C.; Verboom, W.; Huskens, J.; Buckle, T.; van Leeuwen, F.W.B. A supramolecular approach for liver radioembolization. Theranostics 2018, 8, 2377–2386. [Google Scholar] [CrossRef] [Green Version]
- Chung, A.S.; Niesen, M.C.; Graber, T.J.; Schwartz, A.J.; Beauchamp, C.P.; Clarke, H.D.; Spangehl, M.J. Two-stage debridement with prosthesis retention for acute periprosthetic joint infections. J. Arthroplast. 2019, 34, 1207–1213. [Google Scholar] [CrossRef]
- Welling, M.M.; Duszenko, N.; van Willigen, D.M.; Smits, W.K.; Buckle, T.; Roestenberg, M.; van Leeuwen, F.W.B. Cyclodextrin/Adamantane-mediated targeting of inoculated bacteria in mice. Bioconjug. Chem. 2021, 32, 607–614. [Google Scholar] [CrossRef]
- Yu, S.; Hao, S.; Sun, B.; Zhao, D.; Yan, X.; Jin, Z.; Zhao, K. Quaternized chitosan nanoparticles in vaccine applications. Curr. Med. Chem. 2020, 27, 4932–4944. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, N.; Luo, Q.; Li, Y.; Sun, L.; Wang, H.; Xu, K.; Wang, B.; Zhen, Y. In vivo assessment of chitosan/β-glycerophosphate as a new liquid embolic agent. Interv. Neuroradiol. 2011, 17, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Mourya, V.K.; Inamdar, N.N. Chitosan-modifications and applications: Opportunities galore. React. Funct. Polym. 2008, 68, 1013–1051. [Google Scholar] [CrossRef]
- Tan, M.L.; Choong, P.F.M.; Dass, C.R. Review: Doxorubicin delivery systems based on chitosan for cancer therapy. J. Pharm. Pharmacol. 2010, 61, 131–142. [Google Scholar] [CrossRef]
- Kim, J.S.; Kwak, B.K.; Shim, H.J.; Lee, Y.C.; Baik, H.W.; Lee, M.J.; Han, S.M.; Son, S.H.; Kim, Y.B.; Tokura, S.; et al. Preparation of doxorubicin-containing chitosan microspheres for transcatheter arterial chemoembolization of hepatocellular carcinoma. J. Microencapsul. 2007, 24, 408–419. [Google Scholar] [CrossRef]
- Park, K.B.; Kim, Y.M.; Shin, B.C.; Kim, J.R.; Ryu, J.M.; Lim, S.M. Therapeutic Application of New Holmium-166 Chitosan Complex in Malignant and Benign Diseases; International Atomic Energy Agency: Vienna, Austria, 1998; pp. 569–580. [Google Scholar]
- Lohar, S.; Jadhav, S.; Chakravarty, R.; Chakraborty, S.; Sarma, H.D.; Dash, A. A kit based methodology for convenient formulation of 166Ho-Chitosan complex for treatment of liver cancer. Appl. Radiat. Isot. 2020, 161, 109161. [Google Scholar] [CrossRef]
- Bruix, J.; Sala, M.; Llovet, J.M. Chemoembolization for hepatocellular carcinoma. Gastroenterology 2004, 127, S179–S188. [Google Scholar] [CrossRef]
- Lewis, A.L.; Holden, R.R. DC Bead embolic drug-eluting bead: Clinical application in the locoregional treatment of tumours. Expert Opin. Drug Deliv. 2011, 8, 153–169. [Google Scholar] [CrossRef]
- Gewirtz, D. A critical evaluation of the mechanisms of action proposed for the antitumor effects of the anthracycline antibiotics adriamycin and daunorubicin. Biochem. Pharm. 1999, 57, 727–741. [Google Scholar] [CrossRef]
- Hagan, A.; Phillips, G.J.; Macfarlane, W.M.; Lloyd, A.W.; Czuczman, P.; Lewis, A.L. Preparation and characterisation of vandetanib-eluting radiopaque beads for locoregional treatment of hepatic malignancies. Eur. J. Pharm. Sci. 2017, 101, 22–30. [Google Scholar] [CrossRef] [Green Version]
- Liang, B.; Zheng, C.-S.; Feng, G.-S.; Wu, H.-P.; Wang, Y.; Zhao, H.; Qian, J.; Liang, H.-M. Correlation of hypoxia-inducible factor 1α with angiogenesis in liver tumors after transcatheter arterial embolization in an animal model. Cardiovasc. Interv. Radiol. 2010, 33, 806–812. [Google Scholar] [CrossRef]
- Rhee, T.K.; Young, J.Y.; Larson, A.C.; Haines, G.K.; Sato, K.T.; Salem, R.; Mulcahy, M.F.; Kulik, L.M.; Paunesku, T.; Woloschak, G.E.; et al. Effect of transcatheter arterial embolization on levels of hypoxia-inducible factor-1alpha in rabbit VX2 liver tumors. J. Vasc. Interv. Radiol. 2007, 18, 639–645. [Google Scholar] [CrossRef]
- Gomes, J.; Gang, G.J.; Mathews, A.; Stayman, J.W. An investigation of low-dose 3D scout scans for computed tomography. Proc. Int. Soc. Opt. Eng. 2017, 10132, 677–682. [Google Scholar] [CrossRef] [Green Version]
- Welling, M.M.; Spa, S.J.; van Willigen, D.M.; Rietbergen, D.D.D.; Roestenberg, M.; Buckle, T.; van Leeuwen, F.W.B. In vivo stability of supramolecular host-guest complexes monitored by dual-isotope multiplexing in a pre-targeting model of experimental liver radioembolization. J. Control. Release 2019, 293, 126–134. [Google Scholar] [CrossRef]
- Duszenko, N.; van Willigen, D.M.; Welling, M.M.; de Korne, C.M.; van Schuijlenburg, R.; Winkel, B.M.F.; van Leeuwen, F.W.B.; Roestenberg, M. A supramolecular platform technology for bacterial cell surface modification. ACS Infect. Dis. 2020, 6, 1734–1744. [Google Scholar] [CrossRef] [PubMed]
- Welling, M.M.; Duszenko, N.; van Willigen, D.M.; Hensbergen, A.W.; Buckle, T.; Rietbergen, D.D.D.; Roestenberg, M.; van Leeuwen, F.W.B. Interventional nuclear medicine: “click” chemistry as an in vivo targeting strategy for imaging microspheres and bacteria. Biomater. Sci. 2021, 9, 1683–1690. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Zhang, J.; Liao, Y.; Alakpa, E.V.; Bunpetch, V.; Zhang, J.; Ouyang, H. Current advances in microsphere based cell culture and tissue engineering. Biotechnol. Adv. 2020, 39, 107459. [Google Scholar] [CrossRef]
- Guerra Liberal, F.D.C.; O’Sullivan, J.M.; McMahon, S.J.; Prise, K.M. Targeted alpha therapy: Current clinical applications. Cancer Biother. Radiopharm. 2020, 35, 404–417. [Google Scholar] [CrossRef]
- Vaidyanathan, G.; Zalutsky, M.R. Applications of 211At and 223Ra in targeted alpha-particle radiotherapy. Curr. Radiopharm. 2011, 4, 283–294. [Google Scholar] [CrossRef] [Green Version]
- Silindir-Gunay, M.; Karpuz, M.; Ozer, A.Y. Targeted alpha therapy and nanocarrier approach. Cancer Biother. Radiopharm. 2020, 35, 446–458. [Google Scholar] [CrossRef]
- Chow, R.; Simone, C.B., 2nd; Jairam, M.P.; Swaminath, A.; Boldt, G.; Lock, M. Radiofrequency ablation vs radiation therapy vs transarterial chemoembolization vs yttrium 90 for local treatment of liver cancer—A systematic review and network meta-analysis of survival data. Acta Oncol. 2022, 61, 484–494. [Google Scholar] [CrossRef]
| Property | Importance |
|---|---|
| Specific gravity (particle density) | Dispersion in other media or occlusion of the micro-vasculature |
| Size | Particle size (diameter = 50–750 mm) that allows occlusion of the microvasculature |
| Durability | Strength during production, solvent resistance, sterilization, chemical stability, or biodegradation, the release of the therapeutical payload |
| Biocompatibility | Safety, toxicity, stability, suitable for intra-arterial delivery |
| Pharmacology | Controlled dosimetry and dosing, full control over release profile by diffusion, zero-order kinetics |
| Surface properties | Hydrophobic vs. hydrophilic surface, surface area, and porosity, ability to coat or functionalize the spheres |
| Microspheres Composition | Product Name | Particle Size Range (⌀ μm) | PC/H (FDA Clearance) | Biodegradable | References |
|---|---|---|---|---|---|
| Tris acryl gelatin microspheres (TAGM) | Embosphere® (Merit Medical Systems, South Jordan, UT, USA) | 100–300, 300–500 | H (FDA) | No | [24] |
| Polyvinyl alcohol (PVA) sodium acrylate co-polymer microspheres | Contour SE® (Boston Scientific, Marlborough, MA, USA), Bead Block® (Boston Scientific, Marlborough, MA, USA) Embozene® (Varian Medical Systems, Palo Alto, CA, USA) | Irregular: 150–250, 250–355, 355–500, 500–710 | H (FDA) | No | [25] |
| Polyvinyl alcohol-based hydrogel microspheres with sulphonate groups | LC Bead® (Boston Scientific, Marlborough, MA, USA) | 75–150, 100–300, 300–500, 500–700 | H (FDA) | No | [26,27,28,29] |
| Co-polymer of PEG and diacrylamide | Hydropearl® (Terumo Medical Co., Somerset, NJ, USA) | 75–1100 | H (FDA) | No | [1] |
| Starch microspheres | Embocept® (Pharmacept, Berlin, Germany), Spherex® (Magle Life Sciences, Lund, Sweden) | 50 | PC | Yes | [1] |
| Gelatin microspheres | Gel-Bead (Teleflex, Morrisville, NC, USA) | 100–300, 300–500, 500–700, 7000–1000 | H (FDA) | Yes | [1] |
| Collagen-coated poly-(DL-lactic acid-co-glycolic acid (PLGA) microspheres | Occlusin500® (IMBiotechnologies, Edmonton, AL, Canada) | 150–210 | H | Yes | [1] |
| Microspheres Composition | Product Name | Particle Size Range (⌀ µm) | Drug Load | PC/H (FDA) | Biodegradable | References |
|---|---|---|---|---|---|---|
| Polyvinyl alcohol (PVA) sodium acrylate co-polymer microspheres | QuadraSphere® and HepaSphere™ (Merit Medical Systems, Inc., South Jordan, UT, USA) DC Bead® (Boston Scientific, Marlborough, MA, USA), LC Bead®, and Bead Block® (Boston Scientific, Marlborough, MA, USA) | 50–100, 100–300, 200–400 | Doxorubicin, irinotecan, epirubicin, oxaliplatin | PC/H (FDA) | No | [14,26,32,33,34,35,36] |
| Ion-exchange microspheres | CalliSpheres® Beads (Jiangsu Hengrui Medicine Co. Ltd. Jiangsu, China) | 100–300 | Doxorubicin, pirarubicin, oxaliplatin | PC/H | No | [37,38,39,40,41] |
| Tris acryl gelatin microspheres (TAGM) | Embosphere (Merit Medical Systems, South Jor-dan, UT, USA), Embozene®, and Oncozene™ (Varian Medical Systems, Palo Alto, CA, USA) | 40–120, 100–300 | Doxorubicin and Irinotecan | H (FDA) | No | [13,36,42,43] |
| Poly-lactide-co-glycolide (PLGA) | Dexon®, Vicryl®, PerserisTM, Indivior (Indivior Inc. North Chesterfiled, VI, USA), Risperdal Consta® | 20–100 | Mitomycin, doxorubicin, irinotecan, sunitinib, cisplatin | PC/H (FDA) | Yes | [44,45,46,47] |
| Albumin microspheres | Nab-paclitaxel | 10–220 | Mitomycin C, doxorubicin, paclitaxel | PC/H (FDA) | Yes | [48,49] |
| Microspheres Composition | Product Name | Particle Size Range (⌀ µm) | Radioisotope Load | Pre-Clinical/ Human Use (FDA Clearance) | Biodegradable | References |
|---|---|---|---|---|---|---|
| Glass | Lipiocis, TheraSphere® (Boston Scientific, Marlborough, MA, USA) | 50–150, 20–30, 25–32 | 32P, 90Y, 177lu, 186Re, 188Re | PC/H (FDA for 90Y, 186Re, and 188Re) | No | [60,61,62,63,64,65] |
| Resin | SIR-Spheres® (Sirtex Medical Inc. Woburn, MA, USA), Amberlite IR-120 (Thermo Fisher Scientific, Landau, Germany) | 20–60 | 90Y, 153Sm | PC/H (FDA for 90Y) | No | [66,67,68,69] |
| Polyhydroxyamic acid polyacrylamide (PHA) | Experimental | 54 | 177lu, 131I | PC | No | [70,71,72] |
| Styrene divinylbenzene | Amberlite IR-120 (Thermo Fisher Scientific, Landau, Germany) | 20–40 | 152Sm | PC | No | [69,73] |
| Poly- DL-lactic acid-co-glycolic acid (PLGA) | YPO4 crystalline particles Radiogel® (Vivos Inc., Richland, WA, USA) | 0.5–2 | 90Y | PC (FDA-approved as a medical device) | Yes | [74] |
| Poly (L-lactic acid) PLLA | Resomer® L104 (Merck, Darmstad, Germany) | 10–45, 20–40 | 188Re/166Ho/175Yb | PC/C | Yes | [75,76,77,78,79,80,81] |
| Poly (glycidyl methacrylate-co-ethylene dimethacrylate & Quinoline-8-ol | G-Gel (Merck, Darmstad, Germany) | 20–40 | 131I, 177lu | PC | No | [66,70,71,72,82] |
| Hydroxyapatite | QuiremSpheres (Quirem Medical, Deventer, The Netherlands) | 20–60 | 166Ho | PC | No | [78,83,84] |
| Albumin | HSA-B20 (Rotop Pharmaka, Dresden, Germany) Vasculosis® (Global Medical Solutions, Auckland, New Zealand) MAA (DRAXIMAGE®, Kirkland, QC, Canada), Pulmocis® (Curium, London, UK) | 25–35, 15–37 | 90Y, 186Re, 188Re | PC/C | Yes | [63,64,65,85,86,87] |
| Chitosan | Millican (Dong Wha Pharmaceutical Co., Soeul, South Korea) | 5–20 | 166Ho | PC/C | Yes | [75,76,77,78,79,88,89,90] |
| Starch-based microparticles (SBMP) | Experimental Kit | 18–42 | 188Re | PC | No | [91,92,93] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Welling, M.M.; Duszenko, N.; van Meerbeek, M.P.; Molenaar, T.J.M.; Buckle, T.; van Leeuwen, F.W.B.; Rietbergen, D.D.D. Microspheres as a Carrier System for Therapeutic Embolization Procedures: Achievements and Advances. J. Clin. Med. 2023, 12, 918. https://doi.org/10.3390/jcm12030918
Welling MM, Duszenko N, van Meerbeek MP, Molenaar TJM, Buckle T, van Leeuwen FWB, Rietbergen DDD. Microspheres as a Carrier System for Therapeutic Embolization Procedures: Achievements and Advances. Journal of Clinical Medicine. 2023; 12(3):918. https://doi.org/10.3390/jcm12030918
Chicago/Turabian StyleWelling, Mick. M., Nikolas Duszenko, Maarten P. van Meerbeek, Tom J. M. Molenaar, Tessa Buckle, Fijs W. B. van Leeuwen, and Daphne D. D. Rietbergen. 2023. "Microspheres as a Carrier System for Therapeutic Embolization Procedures: Achievements and Advances" Journal of Clinical Medicine 12, no. 3: 918. https://doi.org/10.3390/jcm12030918






