Awake Prone Positioning for Non-Intubated COVID-19 Patients with Acute Respiratory Failure: A Meta-Analysis of Randomised Controlled Trials
Abstract
:1. Introduction
2. Methods
2.1. Data Sources and Search Strategy
2.2. Study Selection and Eligibility Criteria
2.3. Outcomes
2.4. Data Extraction
2.5. Risk of Bias and Certainty of Evidence Assessment
2.6. Data Synthesis
3. Results
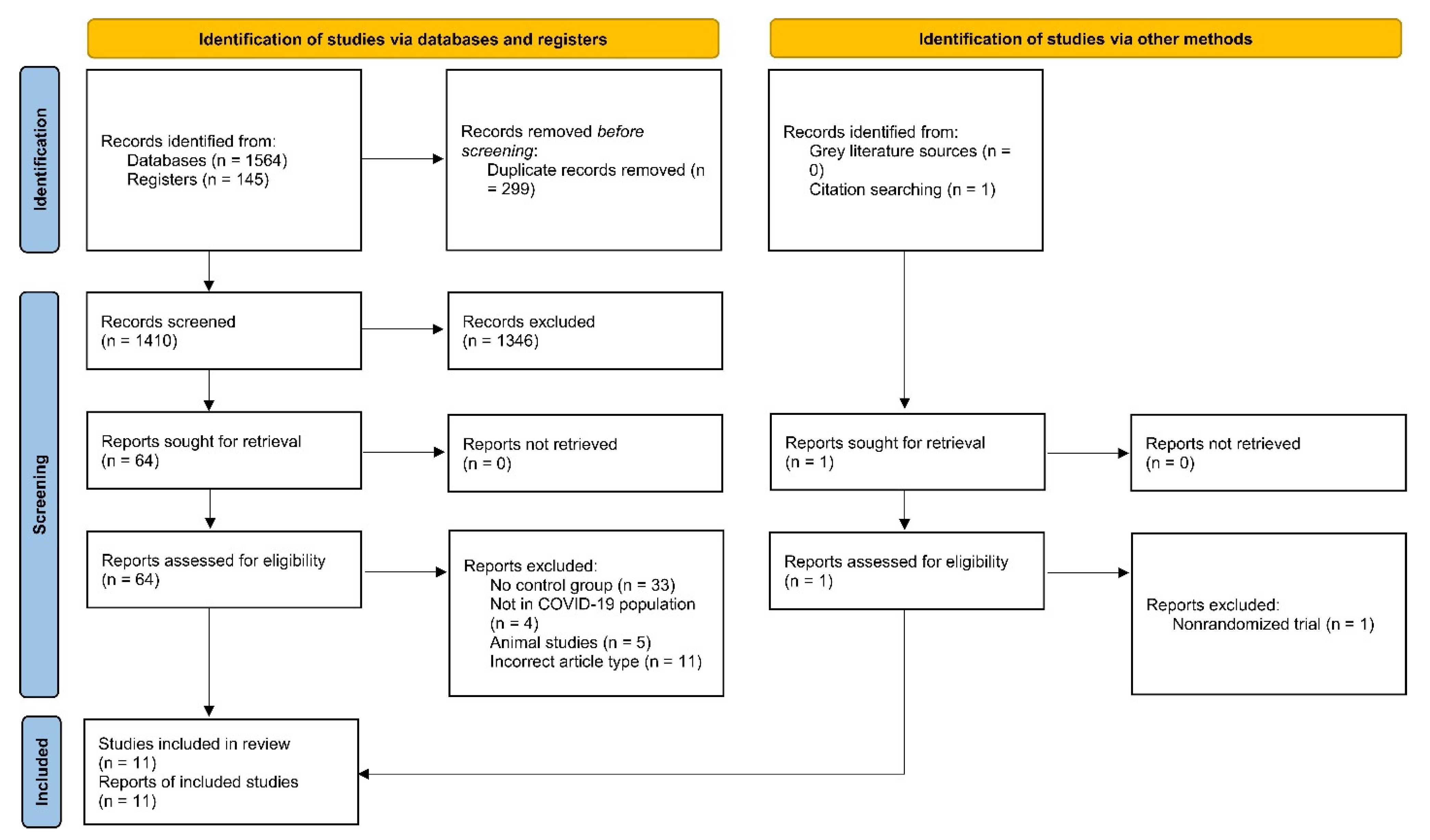
3.1. Search Results
3.2. Characteristics of Included Studies
3.3. Quality Assessment of Included Studies
3.4. Results of Meta-Analysis
3.4.1. Primary Outcomes
Risk of Intubation
Mortality
3.4.2. Secondary Outcomes
Length of ICU Stay
Length of Hospital Stay
Need for Escalating Respiratory Support
Ventilator-Free Days
Adverse Events
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Docherty, A.B.; Mulholland, R.H.; Lone, N.I.; Cheyne, C.P.; De Angelis, D.; Diaz-Ordaz, K.; Donegan, C.; Drake, T.M.; Dunning, J.; Funk, S.; et al. Changes in in-hospital mortality in the first wave of COVID-19: A multicentre prospective observational cohort study using the WHO Clinical Characterisation Protocol UK. Lancet Respir. Med. 2021, 9, 773–785. [Google Scholar] [CrossRef] [PubMed]
- Ferreyro, B.L.; Angriman, F.; Munshi, L.; Del Sorbo, L.; Ferguson, N.D.; Rochwerg, B.; Ryu, M.J.; Saskin, R.; Wunsch, H.; da Costa, B.R.; et al. Association of Noninvasive Oxygenation Strategies with All-Cause Mortality in Adults with Acute Hypoxemic Respiratory Failure. JAMA 2020, 324, 57. [Google Scholar] [CrossRef] [PubMed]
- Zayed, Y.; Barbarawi, M.; Kheiri, B.; Haykal, T.; Chahine, A.; Rashdan, L.; Dhillon, H.; Khaneki, S.; Bachuwa, G.; Seedahmed, E. Initial Noninvasive Oxygenation Strategies in Subjects With De Novo Acute Hypoxemic Respiratory Failure. Respir. Care 2019, 64, 1433–1444. [Google Scholar] [CrossRef] [PubMed]
- Koeckerling, D.; Barker, J.; Mudalige, N.L.; Oyefeso, O.; Pan, D.; Pareek, M.; Thompson, J.P.; Ng, G.A. Awake prone positioning in COVID-19. Thorax 2020, 75, 833–834. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Ma, Z.; Peppelenbosch, M.P.; Pan, Q. Potential association between COVID-19 mortality and health-care resource availability. Lancet Glob. Health 2020, 8, e480. [Google Scholar] [CrossRef] [Green Version]
- Munshi, L.; Del Sorbo, L.; Adhikari, N.K.J.; Hodgson, C.L.; Wunsch, H.; Meade, M.O.; Uleryk, E.; Mancebo, J.; Pesenti, A.; Ranieri, V.M.; et al. Prone Position for Acute Respiratory Distress Syndrome. A Systematic Review and Meta-Analysis. Ann. Am. Thorac. Soc. 2017, 14, S280–S288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guérin, C.; Reignier, J.; Richard, J.-C.; Beuret, P.; Gacouin, A.; Boulain, T.; Mercier, E.; Badet, M.; Mercat, A.; Baudin, O.; et al. Prone Positioning in Severe Acute Respiratory Distress Syndrome. N. Engl. J. Med. 2013, 368, 2159–2168. [Google Scholar] [CrossRef] [PubMed]
- Coppo, A.; Bellani, G.; Winterton, D.; Di Pierro, M.; Soria, A.; Faverio, P.; Cairo, M.; Mori, S.; Messinesi, G.; Contro, E.; et al. Feasibility and physiological effects of prone positioning in non-intubated patients with acute respiratory failure due to COVID-19 (PRON-COVID): A prospective cohort study. Lancet Respir. Med. 2020, 8, 765–774. [Google Scholar] [CrossRef]
- Touchon, F.; Trigui, Y.; Prud’homme, E.; Lefebvre, L.; Giraud, A.; Dols, A.-M.; Martinez, S.; Bernardi, M.; Begne, C.; Granier, P.; et al. Awake prone positioning for hypoxaemic respiratory failure: Past, COVID-19 and perspectives. Eur. Respir. Rev. 2021, 30, 210022. [Google Scholar] [CrossRef]
- Perez-Nieto, O.R.; Escarraman-Martinez, D.; Guerrero-Gutierrez, M.A.; Zamarron-Lopez, E.I.; Mancilla-Galindo, J.; Kammar-García, A.; Martinez-Camacho, M.A.; Deloya-Tomás, E.; Sanchez-Díaz, J.S.; Macías-García, L.A.; et al. Awake prone positioning and oxygen therapy in patients with COVID-19: The APRONOX study. Eur. Respir. J. 2022, 59, 2100265. [Google Scholar] [CrossRef]
- Li, J.; Luo, J.; Pavlov, I.; Perez, Y.; Tan, W.; Roca, O.; Tavernier, E.; Kharat, A.; McNicholas, B.; Ibarra-Estrada, M.; et al. Awake prone positioning for non-intubated patients with COVID-19-related acute hypoxaemic respiratory failure: A systematic review and meta-analysis. Lancet Respir. Med. 2022, 10, 573–583. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Gu, X.; Tong, Z. Effect of Awake Prone Positioning in non-Intubated COVID-19 Patients with Acute Hypoxemic Respiratory Failure: A Systematic Review and Meta-Analysis. J. Intensive Care Med. 2022, 37, 1493–1503. [Google Scholar] [CrossRef] [PubMed]
- Fazzini, B.; Page, A.; Pearse, R.; Puthucheary, Z. Prone positioning for non-intubated spontaneously breathing patients with acute hypoxaemic respiratory failure: A systematic review and meta-analysis. Br. J. Anaesth. 2022, 128, 352–362. [Google Scholar] [CrossRef] [PubMed]
- Alhazzani, W.; Parhar, K.K.S.; Weatherald, J.; Al Duhailib, Z.; Alshahrani, M.; Al-Fares, A.; Buabbas, S.; Cherian, S.V.; Munshi, L.; Fan, E.; et al. Effect of Awake Prone Positioning on Endotracheal Intubation in Patients With COVID-19 and Acute Respiratory Failure: A Randomized Clinical Trial. JAMA 2022, 327, 2104–2113. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Wan, X.; Wang, W.; Liu, J.; Tong, T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 2014, 14, 135. [Google Scholar] [CrossRef] [Green Version]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [Green Version]
- Guyatt, G.H.; Oxman, A.D.; Vist, G.E.; Kunz, R.; Falck-Ytter, Y.; Alonso-Coello, P.; Schünemann, H.J. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008, 336, 924–926. [Google Scholar] [CrossRef] [Green Version]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions, 2nd ed.; Wiley Blackwell: Hoboken, NJ, USA, 2019. [Google Scholar]
- Viechtbauer, W. Conducting Meta-Analyses in R with the metafor Package. J. Stat. Softw. 2010, 36, 1–48. [Google Scholar] [CrossRef] [Green Version]
- Richardson, M.; Garner, P.; Donegan, S. Interpretation of subgroup analyses in systematic reviews: A tutorial. Clin. Epidemiol. Glob. Health 2019, 7, 192–198. [Google Scholar] [CrossRef]
- Rosén, J.; von Oelreich, E.; Fors, D.; Jonsson Fagerlund, M.; Taxbro, K.; Skorup, P.; Eby, L.; Campoccia Jalde, F.; Johansson, N.; Bergström, G.; et al. Awake prone positioning in patients with hypoxemic respiratory failure due to COVID-19: The PROFLO multicenter randomized clinical trial. Crit. Care 2021, 25, 209. [Google Scholar] [CrossRef] [PubMed]
- Awake Prone Positioning in COVID-19 Suspects with Hypoxemic Respiratory Failure (NCT04853979). 2021. Available online: https://clinicaltrials.gov/show/NCT04853979 (accessed on 26 June 2022).
- Kharat, A.; Dupuis-Lozeron, E.; Cantero, C.; Marti, C.; Grosgurin, O.; Lolachi, S.; Lador, F.; Plojoux, J.; Janssens, J.-P.; Soccal, P.M.; et al. Self-proning in COVID-19 patients on low-flow oxygen therapy: A cluster randomised controlled trial. ERJ Open Res. 2021, 7, 00692–02020. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.A.; Horton, D.J.; Fuller, M.J.; Yee, J.; Aliyev, N.; Boltax, J.P.; Chambers, J.H.; Lanspa, M.J. Patient-directed Prone Positioning in Awake Patients with COVID-19 Requiring Hospitalization (PAPR). Ann. Am. Thorac. Soc. 2021, 18, 1424–1426. [Google Scholar] [CrossRef] [PubMed]
- Jayakumar, D.; Ramachandran, D.N.B.P.; Rabindrarajan, D.N.B.E.; Vijayaraghavan, M.D.B.K.T.; Ramakrishnan, A.B.N.; Venkataraman, A.B.R. Standard Care Versus Awake Prone Position in Adult Nonintubated Patients With Acute Hypoxemic Respiratory Failure Secondary to COVID-19 Infection—A Multicenter Feasibility Randomized Controlled Trial. J. Intensive Care Med. 2021, 36, 918–924. [Google Scholar] [CrossRef]
- Rampon, G.; Jia, S.; Agrawal, R.; Arnold, N.; Martín-Quirόs, A.; Fischer, E.A.; Malatack, J.; Jagan, N.; Sergew, A.; Case, A.H.; et al. Smartphone-Guided Self-prone Positioning vs Usual Care in Nonintubated Hospital Ward Patients with COVID-19. Chest 2022, 162, 782–791. [Google Scholar] [CrossRef]
- Gad, G.S. Awake prone positioning versus non invasive ventilation for COVID-19 patients with acute hypoxemic respiratory failure. Egypt. J. Anaesth. 2021, 37, 85–90. [Google Scholar] [CrossRef]
- Fralick, M.; Colacci, M.; Munshi, L.; Venus, K.; Fidler, L.; Hussein, H.; Britto, K.; Fowler, R.; Da Costa, B.; Dhalla, I.; et al. Prone positioning of patients with moderate hypoxia due to COVID-19: A multicenter pragmatic randomized trial [COVID-PRONE]. medRxiv 2021. medRxiv:2021.11.05.21264590. [Google Scholar]
- Ehrmann, S.; Li, J.; Ibarra-Estrada, M.; Perez, Y.; Pavlov, I.; McNicholas, B.; Roca, O.; Mirza, S.; Vines, D.; Garcia-Salcido, R.; et al. Awake prone positioning for COVID-19 acute hypoxaemic respiratory failure: A randomised, controlled, multinational, open-label meta-trial. Lancet Respir. Med. 2021, 9, 1387–1395. [Google Scholar] [CrossRef]
- Taylor, S.P.; Bundy, H.; Smith, W.M.; Skavroneck, S.; Taylor, B.; Kowalkowski, M.A. Awake Prone Positioning Strategy for Nonintubated Hypoxic Patients with COVID-19: A Pilot Trial with Embedded Implementation Evaluation. Ann. Am. Thorac. Soc. 2021, 18, 1360–1368. [Google Scholar] [CrossRef]
- Rahmani, F.; Salmasi, S.; Rezaeifar, P. Prone Position Effects in the Treatment of Covid-19 Patients. Casp. J. Intern. Med. 2020, 11, 580. [Google Scholar]
- Bloomfield, R.; Noble, D.W.; Sudlow, A. Prone position for acute respiratory failure in adults. Cochrane Database Syst. Rev. 2015, 2020, CD008095. [Google Scholar]
- Gattinoni, L.; Marini, J.J.; Pesenti, A.; Quintel, M.; Mancebo, J.; Brochard, L. The “baby lung” became an adult. Intensive Care Med. 2016, 42, 663–673. [Google Scholar] [CrossRef] [PubMed]
- Scaravilli, V.; Grasselli, G.; Castagna, L.; Zanella, A.; Isgrò, S.; Lucchini, A.; Patroniti, N.; Bellani, G.; Pesenti, A. Prone positioning improves oxygenation in spontaneously breathing nonintubated patients with hypoxemic acute respiratory failure: A retrospective study. J. Crit. Care 2015, 30, 1390–1394. [Google Scholar] [CrossRef] [PubMed]
- Tonelli, R.; Fantini, R.; Tabbì, L.; Castaniere, I.; Pisani, L.; Pellegrino, M.R.; Della Casa, G.; D’Amico, R.; Girardis, M.; Nava, S.; et al. Early Inspiratory Effort Assessment by Esophageal Manometry Predicts Noninvasive Ventilation Outcome in De Novo Respiratory Failure. A Pilot Study. Am. J. Respir. Crit. Care Med. 2020, 202, 558–567. [Google Scholar] [CrossRef] [Green Version]
- Vieillard-Baron, A.; Charron, C.; Caille, V.; Belliard, G.; Page, B.; Jardin, F. Prone Positioning Unloads the Right Ventricle in Severe ARDS. Chest 2007, 132, 1440–1446. [Google Scholar] [CrossRef] [Green Version]
- Ng, Z.; Tay, W.C.; Ho, C.H.B. Awake prone positioning for non-intubated oxygen dependent COVID-19 pneumonia patients. Eur. Respir. J. 2020, 56, 2001198. [Google Scholar] [CrossRef] [PubMed]
- Busana, M.; Giosa, L.; Cressoni, M.; Gasperetti, A.; Di Girolamo, L.; Martinelli, A.; Sonzogni, A.; Lorini, L.; Palumbo, M.M.; Romitti, F.; et al. The impact of ventilation–perfusion inequality in COVID-19: A computational model. J. Appl. Physiol. 2021, 130, 865–876. [Google Scholar] [CrossRef] [PubMed]
- Brochard, L.; Slutsky, A.; Pesenti, A. Mechanical Ventilation to Minimize Progression of Lung Injury in Acute Respiratory Failure. Am. J. Respir. Crit. Care Med. 2017, 195, 438–442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cruces, P.; Retamal, J.; Hurtado, D.E.; Erranz, B.; Iturrieta, P.; González, C.; Díaz, F. A physiological approach to understand the role of respiratory effort in the progression of lung injury in SARS-CoV-2 infection. Crit. Care 2020, 24, 494. [Google Scholar] [CrossRef] [PubMed]
- Beran, A.; Mhanna, M.; Srour, O.; Ayesh, H.; Sajdeya, O.; Ghazaleh, S.; Mhanna, A.; Ghazaleh, D.; Khokher, W.; Maqsood, A.; et al. Effect of Prone Positioning on Clinical Outcomes of Non-Intubated Subjects With COVID-19. Respir. Care 2022, 67, 471–479. [Google Scholar] [CrossRef]
- Rossi, S.; Palumbo, M.M.; Sverzellati, N.; Busana, M.; Malchiodi, L.; Bresciani, P.; Ceccarelli, P.; Sani, E.; Romitti, F.; Bonifazi, M.; et al. Mechanisms of oxygenation responses to proning and recruitment in COVID-19 pneumonia. Intensive Care Med. 2022, 48, 56–66. [Google Scholar] [CrossRef]
- Ibarra-Estrada, M.; Li, J.; Pavlov, I.; Perez, Y.; Roca, O.; Tavernier, E.; McNicholas, B.; Vines, D.; Marín-Rosales, M.; Vargas-Obieta, A.; et al. Factors for success of awake prone positioning in patients with COVID-19-induced acute hypoxemic respiratory failure: Analysis of a randomized controlled trial. Crit. Care 2022, 26, 84. [Google Scholar] [CrossRef] [PubMed]
- Guérin, C.; Albert, R.K.; Beitler, J.; Gattinoni, L.; Jaber, S.; Marini, J.J.; Munshi, L.; Papazian, L.; Pesenti, A.; Vieillard-Baron, A.; et al. Prone position in ARDS patients: Why, when, how and for whom. Intensive Care Med. 2020, 46, 2385–2396. [Google Scholar] [CrossRef] [PubMed]
- Shoults, B.; Barber, M.; Millham, L.; Mulla, M.; Nanji, N.; Steele, G.; Peck, T.; Smithedajkul, P.; Worsham, C.; Currier, P.; et al. Feasibility and Limitations of Proning Protocol for Nonintubated Patients With COVID-19. J. Patient Exp. 2021, 8, 237437352098148. [Google Scholar] [CrossRef]
- Tuzun, S.; Keles, A.; Okutan, D.; Yildiran, T.; Palamar, D. Assessment of musculoskeletal pain, fatigue and grip strength in hospitalized patients with Covid-19. Eur. J. Phys. Rehabil. Med. 2021, 57, 653–662. [Google Scholar] [CrossRef] [PubMed]
- Klaiman, T.; Silvestri, J.A.; Srinivasan, T.; Szymanski, S.; Tran, T.; Oredeko, F.; Sjoding, M.W.; Fuchs, B.D.; Maillie, S.; Jablonski, J.; et al. Improving Prone Positioning for Severe Acute Respiratory Distress Syndrome during the COVID-19 Pandemic. An Implementation-Mapping Approach. Ann. Am. Thorac. Soc. 2021, 18, 300–307. [Google Scholar] [CrossRef]
| Study | Country | Setting | Participants | Age * | Gender, n (%) | BMI * | Baseline P/F (S/F) * | Baseline Corticosteroid Use, n (%) | Duration of APP in the Intervention Group (hours) * | Follow-up | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | APP | Control | APP | Control | APP | Control | APP | Control | APP | Control | APP | |||||
| Alhazzani, W., 2022 [14] | Canada, Kuwait, Saudi Arabia, and USA | (ICU) or a monitored acute care unit. | 400 | 58.3 ± 13.2 | 56.8 ± 12.5 | 61 (31) Female | 56 (27) Female | 29.5 ± 4.9 | 29.7 ± 4.7 | 136 (110–181) | 132 (103–174) | 186 (95) | 194 (95) | Daily: 5 (2.6–8) | At 30 and 60 days | |
| 195 | 205 | 134 (69) Male | 149 (73) Male | |||||||||||||
| Ehrmann, S., 2021 [30] | Mexico, France, USA, Spain, Ireland, and Canada | ICU, intermediate care unit, emergency department, general ward | 1126 | 60.7 ± 14 | 61.5 ± 13.3 | 191 (34) Female | 184 (33) Female | 29.7 ± 4.6 | 29.7 ± 4.6 | 148.6 ± 43.1 | 147.9 ± 43.9 | 492 (88%) | 494 (88%) | Daily: 5 (1.6–8.8) | 28 days | |
| 557 | 564 | 366 (66) Male | 380 (67) Male | |||||||||||||
| Fralick, M., 2021 [29] | USA and Canada | General ward | 257 | 54 (44–62) | 59.5 (45–68) | 45 (36.9) Female | 44 (34.9) Female | NR | 305 (267, 339) | 303 (261, 336) | 119 (97.5) | 117 (92.9) | Total: 6 (1.5–12.8) | Up until mortality, hospital discharge or 30 days | ||
| Daily: 2.5 | ||||||||||||||||
| 122 | 126 | |||||||||||||||
| Gad, G.S., 2021 [28] | Egypt | Critical care isolation | 30 | 46 (33–51) | 49 (38–62) | Male:Female | NR | 11 (97–175) | 126 (88–164) | NR | NR | NR | ||||
| 15 | 15 | 08:07 | 09:06 | |||||||||||||
| Rampon, G., 2022 [27] | USA | General ward | 134 | 159 | 54 (43–63) | 52 (39–62) | 80 (59.7) Male | 96 (60.4) Male | NR | 402 (311–457) | 396 (308–457) | NR | NR | 14 days | ||
| Harris, unpublished | Qatar | General ward | 30 | 31 | 40 (36–45) | 41 (35–50) | 25 (83.3) Male | 29 (93.5) Male | 27.2 ± 4.6 | 28.4 ± 3.7 | 196 (182–240) | 196 (165–245 | 30 (100) | 31 (100) | NR | 30 days |
| Jayakumar, D., 2021 [26] | India | ICU | 30 | 30 | 57.3 ± 12.1 | 54.8 ± 11.1 | 25 (83.3) Male | 25 (83.3) Male | 25.8 ± 2.6 | 28.2 ± 5.7 | P/F 185.6 ± 126.1 | P/F 201.4 ± 118.8 | 30 (100) | 30 (100) | NR | Until Discharge |
| Johnson, S.A., 2021 [25] | USA | General ward | 15 | 15 | 46 (33–51) | 49 (38–62) | 8 (53.3) Male | 9 (60) Male | 29.3 (24.4–32.9) | 32.9 (27.5–39.4) | NR | NR | Total: 1.6 (0.2–3.1) | 28 days | ||
| Kharat, A., 2021 [24] | Switzerland | General ward | 27 | 58 ± 12 | 17 (63) Total | 28.2 ± 4.7 | 336 (303–388) | 318 (284–341) | NR | Total: 4.9 ± 3.6 | 1 day (24 h) | |||||
| 17 | 10 | 11 (65) Male | 6 (60) Male | 27.3 ± 4.2 | 29.7 ± 5.3 | |||||||||||
| Rosén, J., 2021 [22] | Sweden | ICU and ward | 75 | 65 (55–70) | 66 (53–74) | 32 (82) Male | 23 (64) Male | 29 (27–33) | 28 (25–30) | 115.5 (93.75–129.75) | 115.5 (86.25–130.5) | NR | Daily: 9 (4.4–10.6) | 30 days | ||
| Taylor, S.P., 2021 [31] | USA | General ward | 41 | 60 (54–63) | 56 (45–66) | 3 (23) Female | 10 (37) Female | 31 (28–38) | 29 (26–39) | NR | NR | NR | Until discharge | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheema, H.A.; Siddiqui, A.; Ochani, S.; Adnan, A.; Sukaina, M.; Haider, R.; Shahid, A.; Rehman, M.E.U.; Awan, R.U.; Singh, H.; et al. Awake Prone Positioning for Non-Intubated COVID-19 Patients with Acute Respiratory Failure: A Meta-Analysis of Randomised Controlled Trials. J. Clin. Med. 2023, 12, 926. https://doi.org/10.3390/jcm12030926
Cheema HA, Siddiqui A, Ochani S, Adnan A, Sukaina M, Haider R, Shahid A, Rehman MEU, Awan RU, Singh H, et al. Awake Prone Positioning for Non-Intubated COVID-19 Patients with Acute Respiratory Failure: A Meta-Analysis of Randomised Controlled Trials. Journal of Clinical Medicine. 2023; 12(3):926. https://doi.org/10.3390/jcm12030926
Chicago/Turabian StyleCheema, Huzaifa Ahmad, Amna Siddiqui, Sidhant Ochani, Alishba Adnan, Mahnoor Sukaina, Ramsha Haider, Abia Shahid, Mohammad Ebad Ur Rehman, Rehmat Ullah Awan, Harpreet Singh, and et al. 2023. "Awake Prone Positioning for Non-Intubated COVID-19 Patients with Acute Respiratory Failure: A Meta-Analysis of Randomised Controlled Trials" Journal of Clinical Medicine 12, no. 3: 926. https://doi.org/10.3390/jcm12030926







