Increased Hippocampal-Inferior Temporal Gyrus White Matter Connectivity following Donepezil Treatment in Patients with Early Alzheimer’s Disease: A Diffusion Tensor Probabilistic Tractography Study
Abstract
:1. Introduction
2. Subjects and Method
2.1. Participants
2.2. Image Acquisition
2.3. Data Processing and Analysis
2.3.1. Brain Volume Analysis
2.3.2. DTI Scalars and WM Connectivity Analyses
3. Results
3.1. Symptom Severity
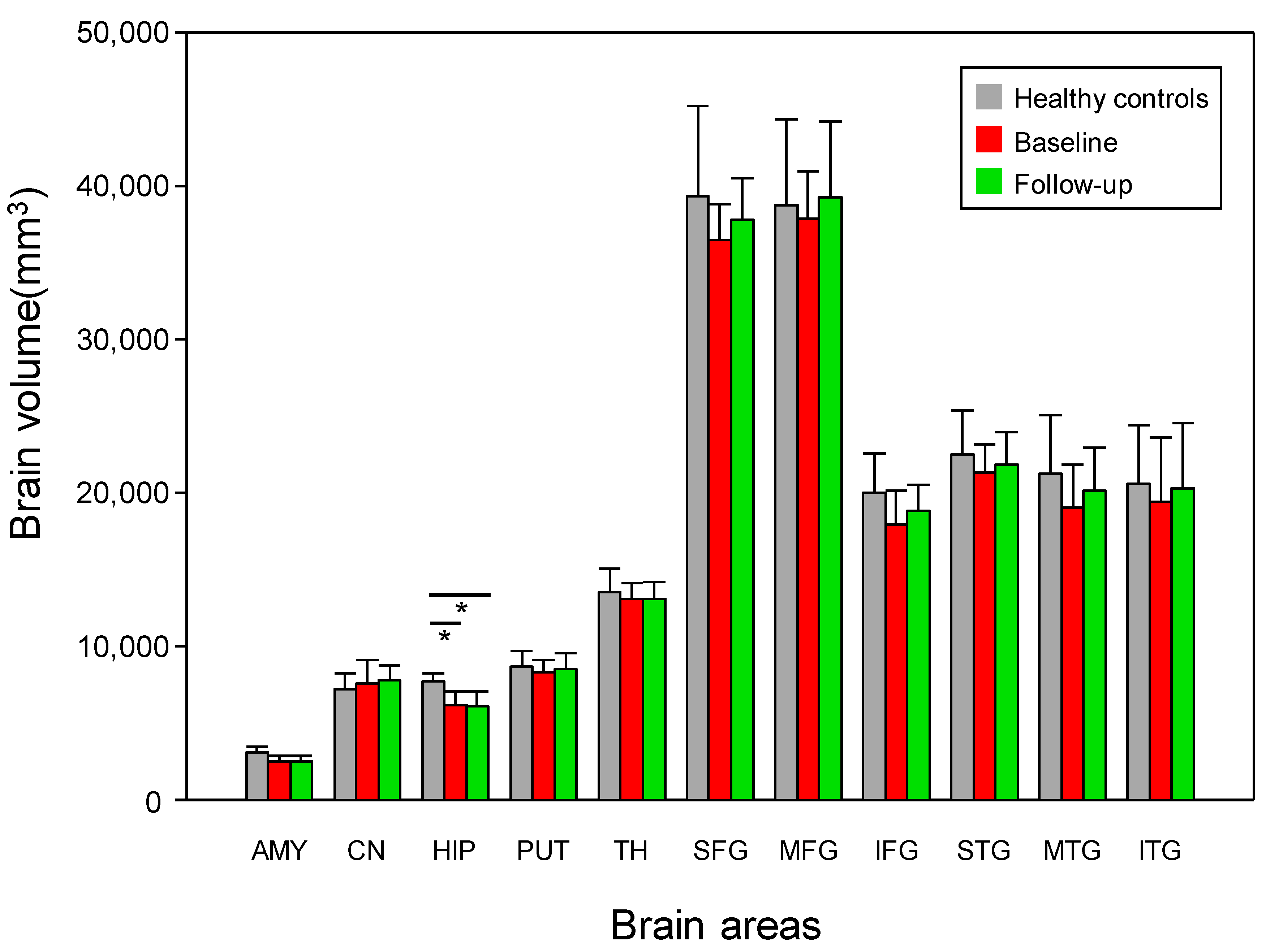
3.2. Changes in Brain Volume
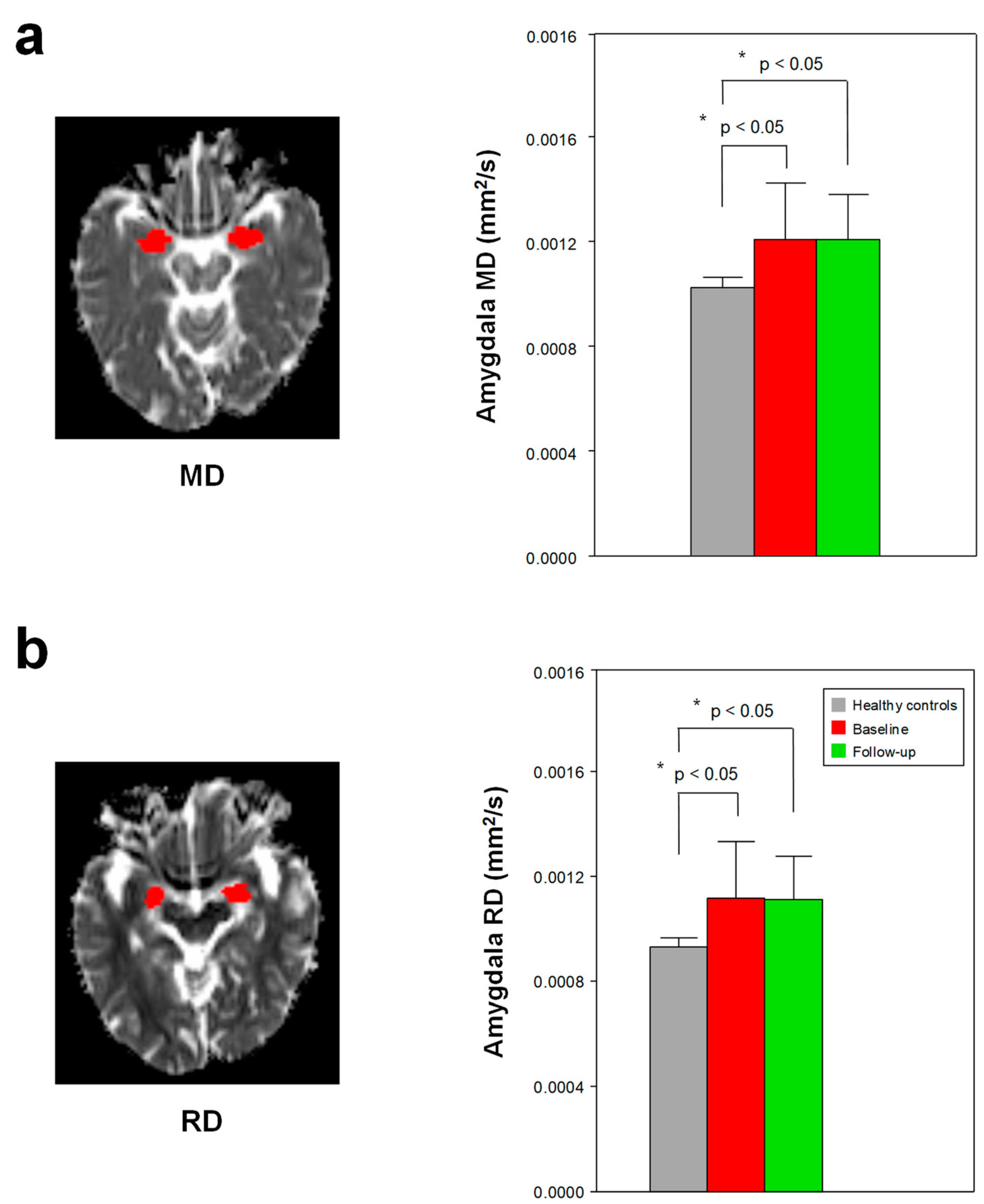
3.3. Changes in DTI Scalars
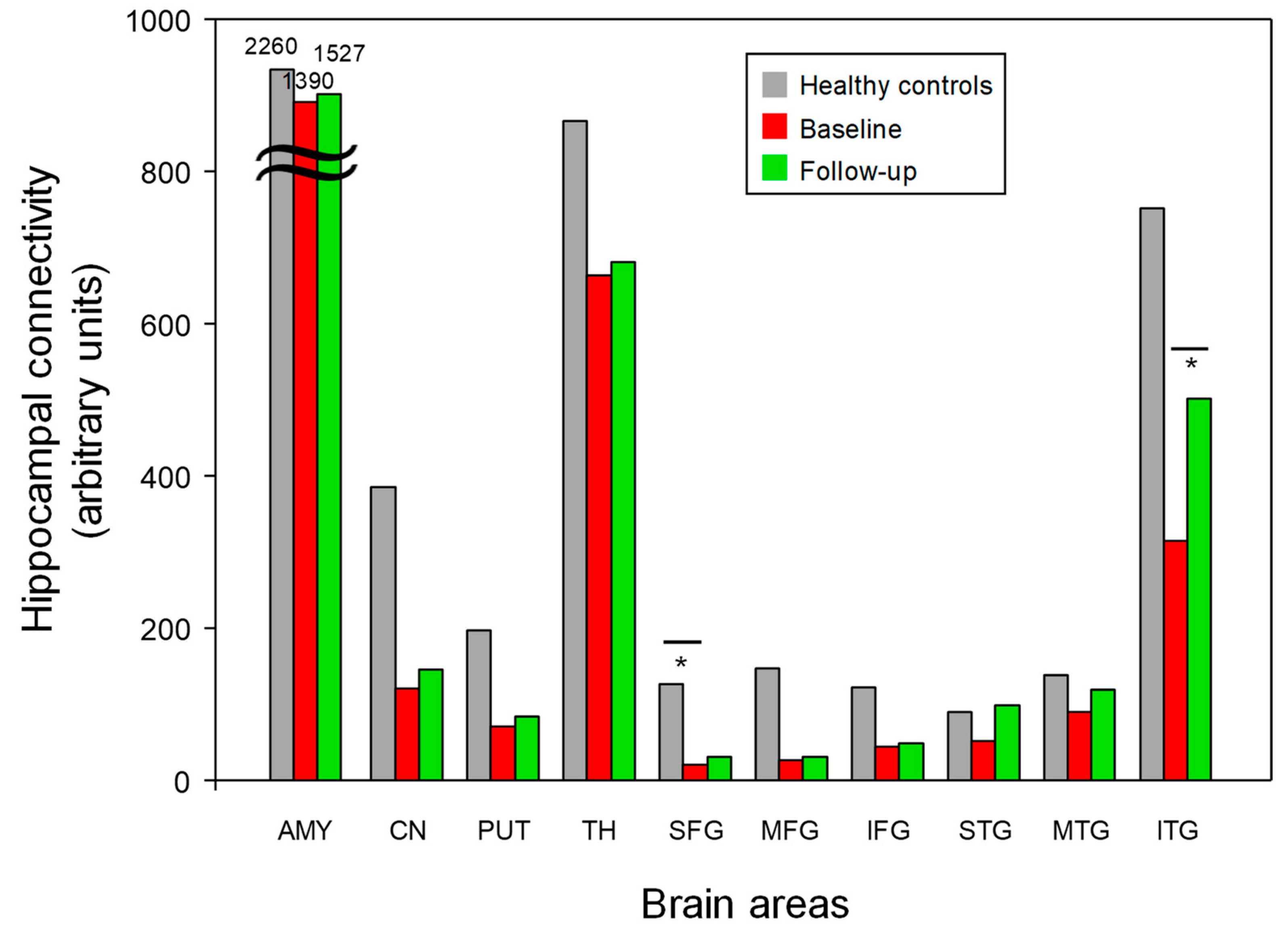
3.4. Hippocampal White Matter Connectivity
4. Discussion
4.1. Summary of Main Findings
4.2. Brain Volume and DTI Scalars in Early AD
4.3. Structural Connectivity in Early AD
4.4. Structural Connectivity after Donepezil Treatment in Early AD
4.5. Limitations and Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rasmussen, J.; Langerman, H. Alzheimer’s Disease-Why We Need Early Diagnosis. Degener. Neurol. Neuromuscul. Dis. 2019, 9, 123–130. [Google Scholar] [CrossRef] [Green Version]
- Gauthier, S.; Reisberg, B.; Zaudig, M.; Petersen, R.C.; Ritchie, K.; Broich, K.; Belleville, S.; Brodaty, H.; Bennett, D.; Chertkow, H.; et al. Mild cognitive impairment. Lancet 2006, 367, 1262–1270. [Google Scholar] [CrossRef] [PubMed]
- Rankin, D.; Black, M.; Flanagan, B.; Hughes, C.F.; Moore, A.; Hoey, L.; Wallace, J.; Gill, C.; Carlin, P.; Molloy, A.M.; et al. Identifying Key Predictors of Cognitive Dysfunction in Older People Using Supervised Machine Learning Techniques: Observational Study. JMIR Med. Inform. 2020, 8, e20995. [Google Scholar] [CrossRef] [PubMed]
- Lai, X.; Wen, H.; Li, Y.; Lu, L.; Tang, C. The Comparative Efficacy of Multiple Interventions for Mild Cognitive Impairment in Alzheimer’s Disease: A Bayesian Network Meta-Analysis. Front. Aging Neurosci. 2020, 12, 121. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.A.; Whittington, M.D.; Synnott, P.G.; McKenna, A.; Campbell, J.; Pearson, S.D.; Rind, D.M. Aducanumab for Alzheimer’s Disease: Effectiveness and Value; Final Evidence Report and Meeting Summary; Institute for Clinical and Economic Review: Boston, MA, USA, 2021; Available online: https://icer.org/wp-content/uploads/2020/10/ICER_ALZ_Draft_Evidence_Report_050521.pdf (accessed on 4 October 2021).
- Robinson, R.L.; Rentz, D.M.; Andrews, J.S.; Zagar, A.; Kim, Y.; Bruemmer, V.; Schwartz, R.L.; Ye, W.; Fillit, H.M. Costs of Early Stage Alzheimer’s Disease in the United States: Cross-Sectional Analysis of a Prospective Cohort Study (GERAS-US)1. J. Alzheimers Dis. 2020, 75, 437–450. [Google Scholar] [CrossRef] [Green Version]
- Assuncao, S.S.; Sperling, R.A.; Ritchie, C.; Kerwin, D.R.; Aisen, P.S.; Lansdall, C.; Atri, A.; Cummings, J. Meaningful benefits: A framework to assess disease-modifying therapies in preclinical and early Alzheimer’s disease. Alzheimers Res. Ther. 2022, 14, 54. [Google Scholar] [CrossRef]
- Tahami Monfared, A.A.; Houghton, K.; Zhang, Q.; Mauskopf, J.; Alzheimer’s Disease Neuroimaging, I. Staging Disease Severity Using the Alzheimer’s Disease Composite Score (ADCOMS): A Retrospective Data Analysis. Neurol. Ther. 2022, 11, 413–434. [Google Scholar] [CrossRef]
- Seltzer, B.; Zolnouni, P.; Nunez, M.; Goldman, R.; Kumar, D.; Ieni, J.; Richardson, S.; Donepezil “402” Study, G. Efficacy of donepezil in early-stage Alzheimer disease: A randomized placebo-controlled trial. Arch. Neurol. 2004, 61, 1852–1856. [Google Scholar] [CrossRef] [Green Version]
- Misra, C.; Fan, Y.; Davatzikos, C. Baseline and longitudinal patterns of brain atrophy in MCI patients, and their use in prediction of short-term conversion to AD: Results from ADNI. Neuroimage 2009, 44, 1415–1422. [Google Scholar] [CrossRef] [Green Version]
- Xue, J.; Guo, H.; Gao, Y.; Wang, X.; Cui, H.; Chen, Z.; Wang, B.; Xiang, J. Altered Directed Functional Connectivity of the Hippocampus in Mild Cognitive Impairment and Alzheimer’s Disease: A Resting-State fMRI Study. Front. Aging Neurosci. 2019, 11, 326. [Google Scholar] [CrossRef] [Green Version]
- Tabatabaei-Jafari, H.; Shaw, M.E.; Cherbuin, N. Cerebral atrophy in mild cognitive impairment: A systematic review with meta-analysis. Alzheimers Dement. 2015, 1, 487–504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frisoni, G.B.; Fox, N.C.; Jack, C.R., Jr.; Scheltens, P.; Thompson, P.M. The clinical use of structural MRI in Alzheimer disease. Nat. Rev. Neurol. 2010, 6, 67–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, G.P.; Feng, Z.; He, F.P.; Chen, Z.Q.; Liu, X.Y.; Liu, P.; Luo, B.Y. Correlation of hippocampal volume and cognitive performances in patients with either mild cognitive impairment or Alzheimer’s disease. CNS Neurosci. Ther. 2015, 21, 15–22. [Google Scholar] [CrossRef]
- Rose, S.E.; Janke, A.L.; Chalk, J.B. Gray and white matter changes in Alzheimer’s disease: A diffusion tensor imaging study. J. Magn. Reson. Imaging 2008, 27, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Caso, F.; Agosta, F.; Mattavelli, D.; Migliaccio, R.; Canu, E.; Magnani, G.; Marcone, A.; Copetti, M.; Falautano, M.; Comi, G.; et al. White Matter Degeneration in Atypical Alzheimer Disease. Radiology 2015, 277, 162–172. [Google Scholar] [CrossRef]
- Henneman, W.J.; Sluimer, J.D.; Barnes, J.; van der Flier, W.M.; Sluimer, I.C.; Fox, N.C.; Scheltens, P.; Vrenken, H.; Barkhof, F. Hippocampal atrophy rates in Alzheimer disease: Added value over whole brain volume measures. Neurology 2009, 72, 999–1007. [Google Scholar] [CrossRef] [Green Version]
- Vemuri, P.; Jack, C.R., Jr. Role of structural MRI in Alzheimer’s disease. Alzheimers Res. Ther. 2010, 2, 23. [Google Scholar] [CrossRef] [Green Version]
- Bell-McGinty, S.; Lopez, O.L.; Meltzer, C.C.; Scanlon, J.M.; Whyte, E.M.; Dekosky, S.T.; Becker, J.T. Differential cortical atrophy in subgroups of mild cognitive impairment. Arch. Neurol. 2005, 62, 1393–1397. [Google Scholar] [CrossRef] [Green Version]
- Tabatabaei-Jafari, H.; Shaw, M.E.; Walsh, E.; Cherbuin, N.; Alzheimer’s Disease Neuroimaging, I. Regional brain atrophy predicts time to conversion to Alzheimer’s disease, dependent on baseline volume. Neurobiol. Aging 2019, 83, 86–94. [Google Scholar] [CrossRef]
- Iglesias, J.E.; Augustinack, J.C.; Nguyen, K.; Player, C.M.; Player, A.; Wright, M.; Roy, N.; Frosch, M.P.; McKee, A.C.; Wald, L.L.; et al. A computational atlas of the hippocampal formation using ex vivo, ultra-high resolution MRI: Application to adaptive segmentation of in vivo MRI. Neuroimage 2015, 115, 117–137. [Google Scholar] [CrossRef]
- Palesi, F.; Vitali, P.; Chiarati, P.; Castellazzi, G.; Caverzasi, E.; Pichiecchio, A.; Colli-Tibaldi, E.; D’Amore, F.; D’Errico, I.; Sinforiani, E.; et al. DTI and MR Volumetry of Hippocampus-PC/PCC Circuit: In Search of Early Micro- and Macrostructural Signs of Alzheimers’s Disease. Neurol. Res. Int. 2012, 2012, 517876. [Google Scholar] [CrossRef]
- Yushkevich, P.A.; Pluta, J.B.; Wang, H.; Xie, L.; Ding, S.L.; Gertje, E.C.; Mancuso, L.; Kliot, D.; Das, S.R.; Wolk, D.A. Automated volumetry and regional thickness analysis of hippocampal subfields and medial temporal cortical structures in mild cognitive impairment. Hum. Brain Mapp. 2015, 36, 258–287. [Google Scholar] [CrossRef] [Green Version]
- de Flores, R.; La Joie, R.; Landeau, B.; Perrotin, A.; Mezenge, F.; de La Sayette, V.; Eustache, F.; Desgranges, B.; Chetelat, G. Effects of age and Alzheimer’s disease on hippocampal subfields: Comparison between manual and FreeSurfer volumetry. Hum. Brain Mapp. 2015, 36, 463–474. [Google Scholar] [CrossRef]
- Kim, G.W.; Kim, B.C.; Park, K.S.; Jeong, G.W. A pilot study of brain morphometry following donepezil treatment in mild cognitive impairment: Volume changes of cortical/subcortical regions and hippocampal subfields. Sci. Rep. 2020, 10, 10912. [Google Scholar] [CrossRef]
- Chow, N.; Hwang, K.S.; Hurtz, S.; Green, A.E.; Somme, J.H.; Thompson, P.M.; Elashoff, D.A.; Jack, C.R.; Weiner, M.; Apostolova, L.G.; et al. Comparing 3T and 1.5T MRI for mapping hippocampal atrophy in the Alzheimer’s Disease Neuroimaging Initiative. AJNR Am. J. Neuroradiol. 2015, 36, 653–660. [Google Scholar] [CrossRef]
- Khan, W.; Westman, E.; Jones, N.; Wahlund, L.O.; Mecocci, P.; Vellas, B.; Tsolaki, M.; Kloszewska, I.; Soininen, H.; Spenger, C.; et al. Automated Hippocampal Subfield Measures as Predictors of Conversion from Mild Cognitive Impairment to Alzheimer’s Disease in Two Independent Cohorts. Brain Topogr. 2015, 28, 746–759. [Google Scholar] [CrossRef] [Green Version]
- Teipel, S.; Grothe, M.J.; Neuroimaging, A.s.D. Does posterior cingulate hypometabolism result from disconnection or local pathology across preclinical and clinical stages of Alzheimer’s disease? Eur. J. Nucl. Med. Mol. I 2016, 43, 526–536. [Google Scholar] [CrossRef]
- Beversdorf, D.Q.; Nagaraja, H.N.; Bornstein, R.A.; Scharre, D.W. The Effect of Donepezil on Problem-solving Ability in Individuals with Amnestic Mild Cognitive Impairment: A Pilot Study. Cogn. Behav. Neurol. 2021, 34, 182–187. [Google Scholar] [CrossRef]
- Cavedo, E.; Grothe, M.J.; Colliot, O.; Lista, S.; Chupin, M.; Dormont, D.; Houot, M.; Lehericy, S.; Teipel, S.; Dubois, B.; et al. Reduced basal forebrain atrophy progression in a randomized Donepezil trial in prodromal Alzheimer’s disease. Sci. Rep. 2017, 7, 11706. [Google Scholar] [CrossRef] [Green Version]
- Kasa, P.; Papp, H.; Kasa, P., Jr.; Torok, I. Donepezil dose-dependently inhibits acetylcholinesterase activity in various areas and in the presynaptic cholinergic and the postsynaptic cholinoceptive enzyme-positive structures in the human and rat brain. Neuroscience 2000, 101, 89–100. [Google Scholar] [CrossRef]
- Scali, C.; Casamenti, F.; Bellucci, A.; Costagli, C.; Schmidt, B.; Pepeu, G. Effect of subchronic administration of metrifonate, rivastigmine and donepezil on brain acetylcholine in aged F344 rats. J. Neural. Transm. 2002, 109, 1067–1080. [Google Scholar] [CrossRef] [PubMed]
- Risacher, S.L.; Wang, Y.; Wishart, H.A.; Rabin, L.A.; Flashman, L.A.; McDonald, B.C.; West, J.D.; Santulli, R.B.; Saykin, A.J. Cholinergic Enhancement of Brain Activation in Mild Cognitive Impairment during Episodic Memory Encoding. Front. Psychiatry 2013, 4, 105. [Google Scholar] [CrossRef] [Green Version]
- Petrella, J.R.; Prince, S.E.; Krishnan, S.; Husn, H.; Kelley, L.; Doraiswamy, P.M. Effects of donepezil on cortical activation in mild cognitive impairment: A pilot double-blind placebo-controlled trial using functional MR imaging. AJNR Am. J. Neuroradiol. 2009, 30, 411–416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- La Rocca, M.; Amoroso, N.; Monaco, A.; Bellotti, R.; Tangaro, S. A novel approach to brain connectivity reveals early structural changes in Alzheimer’s disease. Physiol. Meas. 2018, 39, 074005. [Google Scholar] [CrossRef] [PubMed]
- Jaimes, C.; Cheng, H.H.; Soul, J.; Ferradal, S.; Rathi, Y.; Gagoski, B.; Newburger, J.W.; Grant, P.E.; Zollei, L. Probabilistic tractography-based thalamic parcellation in healthy newborns and newborns with congenital heart disease. J. Magn. Reason. Imaging 2018, 47, 1626–1637. [Google Scholar] [CrossRef]
- Kim, G.W.; Park, S.E.; Park, K.; Jeong, G.W. White Matter Connectivity and Gray Matter Volume Changes Following Donepezil Treatment in Patients with Mild Cognitive Impairment: A Preliminary Study Using Probabilistic Tractography. Front. Aging Neurosci. 2021, 12, 604940. [Google Scholar] [CrossRef]
- Leung, K.K.; Bartlett, J.W.; Barnes, J.; Manning, E.N.; Ourselin, S.; Fox, N.C.; Alzheimer’s Disease Neuroimaging, I. Cerebral atrophy in mild cognitive impairment and Alzheimer disease: Rates and acceleration. Neurology 2013, 80, 648–654. [Google Scholar] [CrossRef] [Green Version]
- King-Robson, J.; Wilson, H.; Politis, M.; Alzheimer’s Disease Neuroimaging, I. Associations Between Amyloid and Tau Pathology, and Connectome Alterations, in Alzheimer’s Disease and Mild Cognitive Impairment. J. Alzheimers Dis. 2021, 82, 541–560. [Google Scholar] [CrossRef]
- Morris, J.C. Revised criteria for mild cognitive impairment may compromise the diagnosis of Alzheimer disease dementia. Arch. Neurol. 2012, 69, 700–708. [Google Scholar] [CrossRef] [Green Version]
- Gaynes, B.N.; Asher, G.; Gartlehner, G.; Hoffman, V.; Green, J. AHRQ Technology Assessments. In Definition of Treatment-Resistant Depression in the Medicare Population; Agency for Healthcare Research and Quality: Rockville, MD, USA, 2018. [Google Scholar]
- Kim, G.W.; Farabaugh, A.H.; Vetterman, R.; Holmes, A.; Nyer, M.; Nasiriavanaki, Z.; Fava, M.; Holt, D.J. Diminished frontal pole size and functional connectivity in young adults with high suicidality. J. Affect Disord. 2022, 310, 484–492. [Google Scholar] [CrossRef]
- Theisen, F.; Leda, R.; Pozorski, V.; Oh, J.M.; Adluru, N.; Wong, R.; Okonkwo, O.; Dean, D.C., III; Bendlin, B.B.; Johnson, S.C.; et al. Evaluation of striatonigral connectivity using probabilistic tractography in Parkinson’s disease. Neuroimage Clin. 2017, 16, 557–563. [Google Scholar] [CrossRef]
- Cho, K.I.; Shenton, M.E.; Kubicki, M.; Jung, W.H.; Lee, T.Y.; Yun, J.Y.; Kim, S.N.; Kwon, J.S. Altered Thalamo-Cortical White Matter Connectivity: Probabilistic Tractography Study in Clinical-High Risk for Psychosis and First-Episode Psychosis. Schizophr. Bull. 2016, 42, 723–731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Lian, S.; Zhang, Y.; Zhao, Q. Efficacy and safety of donepezil for mild cognitive impairment: A systematic review and meta-analysis. Clin. Neurol. Neurosurg. 2022, 213, 107134. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Yang, H.; Zhang, J. Donepezil’s Effects on Brain Functions of Patients with Alzheimer Disease: A Regional Homogeneity Study Based on Resting-State Functional Magnetic Resonance Imaging. Clin. Neuropharmacol. 2019, 42, 42–48. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, S.; Sun, W. Expression of miR-28-3p in patients with Alzheimer’s disease before and after treatment and its clinical value. Exp. Ther. Med. 2020, 20, 2218–2226. [Google Scholar] [CrossRef] [PubMed]
- Barnes, J.; Bartlett, J.W.; van de Pol, L.A.; Loy, C.T.; Scahill, R.I.; Frost, C.; Thompson, P.; Fox, N.C. A meta-analysis of hippocampal atrophy rates in Alzheimer’s disease. Neurobiol. Aging 2009, 30, 1711–1723. [Google Scholar] [CrossRef] [Green Version]
- Saribudak, A.; Subick, A.A.; Kim, N.H.; Rutta, J.A.; Uyar, M.U. Gene Expressions, Hippocampal Volume Loss, and MMSE Scores in Computation of Progression and Pharmacologic Therapy Effects for Alzheimer’s Disease. IEEE/ACM Trans. Comput. Biol. Bioinform. 2020, 17, 608–622. [Google Scholar] [CrossRef]
- Hashimoto, M.; Kazui, H.; Matsumoto, K.; Nakano, Y.; Yasuda, M.; Mori, E. Does donepezil treatment slow the progression of hippocampal atrophy in patients with Alzheimer’s disease? Am. J. Psychiatry 2005, 162, 676–682. [Google Scholar] [CrossRef] [Green Version]
- He, J.; Farias, S.; Martinez, O.; Reed, B.; Mungas, D.; Decarli, C. Differences in brain volume, hippocampal volume, cerebrovascular risk factors, and apolipoprotein E4 among mild cognitive impairment subtypes. Arch. Neurol. 2009, 66, 1393–1399. [Google Scholar] [CrossRef] [Green Version]
- Laakso, M.P.; Soininen, H.; Partanen, K.; Helkala, E.L.; Hartikainen, P.; Vainio, P.; Hallikainen, M.; Hanninen, T.; Riekkinen, P.J. Volumes of hippocampus, amygdala and frontal lobes in the MRI-based diagnosis of early Alzheimer’s disease: Correlation with memory functions. J. Neural. Transm. Park Dis. Dement. Sect. 1995, 9, 73–86. [Google Scholar] [CrossRef]
- Tang, X.; Qin, Y.; Wu, J.; Zhang, M.; Zhu, W.; Miller, M.I. Shape and diffusion tensor imaging based integrative analysis of the hippocampus and the amygdala in Alzheimer’s disease. Magn. Reason. Imaging 2016, 34, 1087–1099. [Google Scholar] [CrossRef]
- Ceceli, A.O.; Bradberry, C.W.; Goldstein, R.Z. The neurobiology of drug addiction: Cross-species insights into the dysfunction and recovery of the prefrontal cortex. Neuropsychopharmacology 2021, 47, 276–291. [Google Scholar] [CrossRef]
- Pierpaoli, C.; Jezzard, P.; Basser, P.J.; Barnett, A.; Di Chiro, G. Diffusion tensor MR imaging of the human brain. Radiology 1996, 201, 637–648. [Google Scholar] [CrossRef]
- Song, S.K.; Yoshino, J.; Le, T.Q.; Lin, S.J.; Sun, S.W.; Cross, A.H.; Armstrong, R.C. Demyelination increases radial diffusivity in corpus callosum of mouse brain. Neuroimage 2005, 26, 132–140. [Google Scholar] [CrossRef]
- Burianova, H.; McIntosh, A.R.; Grady, C.L. A common functional brain network for autobiographical, episodic, and semantic memory retrieval. Neuroimage 2010, 49, 865–874. [Google Scholar] [CrossRef]
- Schwab, S.; Afyouni, S.; Chen, Y.; Han, Z.; Guo, Q.; Dierks, T.; Wahlund, L.O.; Grieder, M. Functional Connectivity Alterations of the Temporal Lobe and Hippocampus in Semantic Dementia and Alzheimer’s Disease. J. Alzheimers Dis. 2020, 76, 1461–1475. [Google Scholar] [CrossRef]
- Wang, L.; Zang, Y.; He, Y.; Liang, M.; Zhang, X.; Tian, L.; Wu, T.; Jiang, T.; Li, K. Changes in hippocampal connectivity in the early stages of Alzheimer’s disease: Evidence from resting state fMRI. Neuroimage 2006, 31, 496–504. [Google Scholar] [CrossRef]
- Li, M.; Long, C.; Yang, L. Hippocampal-prefrontal circuit and disrupted functional connectivity in psychiatric and neurodegenerative disorders. Biomed. Res. Int. 2015, 2015, 810548. [Google Scholar] [CrossRef] [Green Version]
- Berron, D.; van Westen, D.; Ossenkoppele, R.; Strandberg, O.; Hansson, O. Medial temporal lobe connectivity and its associations with cognition in early Alzheimer’s disease. Brain 2020, 143, 1233–1248. [Google Scholar] [CrossRef]
- Kraljevic, N.; Schaare, H.L.; Eickhoff, S.B.; Kochunov, P.; Yeo, B.T.T.; Kharabian Masouleh, S.; Valk, S.L. Behavioral, Anatomical and Heritable Convergence of Affect and Cognition in Superior Frontal Cortex. Neuroimage 2021, 243, 118561. [Google Scholar] [CrossRef]
- Velayudhan, L.; Francis, S.; Dury, R.; Paul, S.; Bestwn, S.; Gowland, P.; Bhattacharyya, S. Hippocampal functional connectivity in Alzheimer’s disease: A resting state 7T fMRI study. Int. Psychogeriatr. 2021, 33, 95–96. [Google Scholar] [CrossRef] [PubMed]
- Zaidel, L.; Allen, G.; Cullum, C.M.; Briggs, R.W.; Hynan, L.S.; Weiner, M.F.; McColl, R.; Gopinath, K.S.; McDonald, E.; Rubin, C.D. Donepezil effects on hippocampal and prefrontal functional connectivity in Alzheimer’s disease: Preliminary report. J. Alzheimers Dis. 2012, 31, S221–S226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scheff, S.W.; Price, D.A.; Schmitt, F.A.; Scheff, M.A.; Mufson, E.J. Synaptic loss in the inferior temporal gyrus in mild cognitive impairment and Alzheimer’s disease. J. Alzheimers Dis. 2011, 24, 547–557. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liang, P.; Jia, X.; Qi, Z.; Yu, L.; Yang, Y.; Zhou, W.; Lu, J.; Li, K. Baseline and longitudinal patterns of hippocampal connectivity in mild cognitive impairment: Evidence from resting state fMRI. J. Neurol. Sci. 2011, 309, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Bottini, G.; Berlingeri, M.; Basilico, S.; Passoni, S.; Danelli, L.; Colombo, N.; Sberna, M.; Franceschi, M.; Sterzi, R.; Paulesu, E. GOOD or BAD responder? Behavioural and neuroanatomical markers of clinical response to donepezil in dementia. Behav. Neurol. 2012, 25, 61–72. [Google Scholar] [CrossRef]
- Kaasinen, V.; Nagren, K.; Jarvenpaa, T.; Roivainen, A.; Yu, M.; Oikonen, V.; Kurki, T.; Rinne, J.O. Regional effects of donepezil and rivastigmine on cortical acetylcholinesterase activity in Alzheimer’s disease. J. Clin. Psychopharmacol. 2002, 22, 615–620. [Google Scholar] [CrossRef]
- Goveas, J.S.; Xie, C.; Ward, B.D.; Wu, Z.; Li, W.; Franczak, M.; Jones, J.L.; Antuono, P.G.; Li, S.J. Recovery of hippocampal network connectivity correlates with cognitive improvement in mild Alzheimer’s disease patients treated with donepezil assessed by resting-state fMRI. J. Magn. Reason. Imaging 2011, 34, 764–773. [Google Scholar] [CrossRef] [Green Version]
- Kotani, S.; Yamauchi, T.; Teramoto, T.; Ogura, H. Donepezil, an acetylcholinesterase inhibitor, enhances adult hippocampal neurogenesis. Chem. Biol. Interact 2008, 175, 227–230. [Google Scholar] [CrossRef]
- Dong, H.; Yuede, C.M.; Coughlan, C.A.; Murphy, K.M.; Csernansky, J.G. Effects of donepezil on amyloid-beta and synapse density in the Tg2576 mouse model of Alzheimer’s disease. Brain Res. 2009, 1303, 169–178. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.J.; Gasperini, R.; Foa, L.; Small, D.H. Amyloid-beta decreases cell-surface AMPA receptors by increasing intracellular calcium and phosphorylation of GluR2. J. Alzheimers Dis. 2010, 21, 655–666. [Google Scholar] [CrossRef] [Green Version]
- Leyhe, T.; Stransky, E.; Eschweiler, G.W.; Buchkremer, G.; Laske, C. Increase of BDNF serum concentration during donepezil treatment of patients with early Alzheimer’s disease. Eur. Arch. Psychiatry Clin. Neurosci. 2008, 258, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Y.; Fu, Q.; Pei, J.; Fan, M.; Yu, Q.; Guo, M.; Zhou, H.; Wang, T.; Wang, L.; Chen, Y. Modulation of Brain Activity and Functional Connectivity by Acupuncture Combined with Donepezil on Mild-to-Moderate Alzheimer’s Disease: A Neuroimaging Pilot Study. Front. Neurol. 2022, 13, 912923. [Google Scholar] [CrossRef] [PubMed]


| ROIs | Patients with AD | Healthy Controls (HC) | Statistical Analysis | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | 6-Month Follow-Up | Baseline vs. Follow-Up | Baseline vs. HC | Follow-Up vs. HC | |||||
| p-Value | Cohen’s d | p-Value | Cohen’s d | p-Value | Cohen’s d | ||||
| Amygdala | 2.5 (0.4) | 2.5 (0.4) | 3.1 (0.4) | p = 0.59 | 0.31 | p < 0.05 * | 1.70 | p < 0.05 * | 1.70 |
| Caudate nucleus | 7.6 (0.4) | 7.8 (0.9) | 7.2 (1.1) | p = 0.31 | 0.41 | p = 0.63 | 0.33 | p = 0.17 | 0.68 |
| Hippocampus | 6.2 (0.9) | 6.1 (0.9) | 7.7 (0.5) | p = 0.68 | 0.34 | p < 0.05 * | 2.27 | p < 0.05 * | 2.34 |
| Putamen | 8.3 (0.8) | 8.5 (1.0) | 8.7 (1.0) | p = 0.11 | 0.61 | p = 0.35 | 0.45 | p = 0.45 | 0.18 |
| Thalamus | 13.0 (1.0) | 13.1 (1.1) | 13.6 (1.5) | p = 0.86 | 0.11 | p = 0.69 | 0.43 | p = 0.45 | 0.39 |
| SFG | 36.4 (2.4) | 37.7 (2.7) | 39.3 (5.8) | p = 0.21 | 0.72 | p = 0.20 | 0.69 | p = 0.69 | 0.37 |
| MFG | 37.9 (3.0) | 39.2 (4.9) | 38.7 (5.6) | p = 0.52 | 0.63 | p = 0.90 | 0.20 | p = 0.76 | 0.11 |
| IFG | 17.9 (2.2) | 18.8 (1.7) | 20.0 (2.6) | p = 0.11 | 0.67 | p = 0.09 | 0.91 | p = 0.31 | 0.58 |
| STG | 21.3 (1.9) | 21.8 (2.1) | 22.5 (2.9) | p = 0.52 | 0.49 | p = 0.23 | 0.53 | p = 0.51 | 0.28 |
| MTG | 19.0 (2.8) | 20.1 (2.8) | 21.2 (3.1) | p = 0.77 | 0.51 | p = 0.15 | 0.71 | p = 0.40 | 0.37 |
| ITG | 19.4 (4.2) | 20.3 (4.2) | 20.6 (3.8) | p = 0.21 | 0.61 | p = 0.45 | 0.31 | p = 0.63 | 0.07 |
| ROIs | Patients with AD | Healthy Controls (HC) | Statistical Analysis | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | 6-Month Follow-Up | Baseline vs. Follow-Up | Baseline vs. HC | Follow-Up vs. HC | |||||
| p-Value | Cohen’s d | p-Value | Cohen’s d | p-Value | Cohen’s d | ||||
| Amygdala | 13.9 (8.6) | 15.3 (14.2) | 22.6 (20.2) | p = 0.67 | 0.13 | p = 0.41 | 0.62 | p = 0.33 | 0.46 |
| Caudate nucleus | 1.2 (2.5) | 1.5 (1.4) | 3.9 (2.5) | p = 0.28 | 0.18 | p = 0.01 | 1.16 | p = 0.03 | 1.32 |
| Putamen | 0.7 (0.6) | 0.9 (0.6) | 2.0 (1.4) | p = 0.33 | 0.47 | p = 0.01 | 1.30 | p = 0.01 | 1.16 |
| Thalamus | 6.6 (8.2) | 6.8 (10.1) | 8.7 (7.3) | p = 0.86 | 0.07 | p = 0.09 | 0.28 | p = 0.03 | 0.23 |
| SFG | 0.2 (0.2) | 0.3 (0.3) | 1.3 (1.2) | p = 0.43 | 0.45 | p < 0.05 * | 1.33 | p = 0.01 | 1.20 |
| MFG | 0.3 (0.3) | 0.3 (0.3) | 1.5 (1.6) | p = 0.48 | 0.21 | p = 0.01 | 1.19 | p = 0.02 | 1.16 |
| IFG | 0.5 (0.7) | 0.5 (0.4) | 1.2 (1.3) | p = 0.20 | 0.16 | p = 0.06 | 0.84 | p = 0.18 | 0.86 |
| STG | 0.5 (0.6) | 1.0 (0.8) | 0.9 (0.6) | p = 0.11 | 0.77 | p = 0.08 | 0.68 | p = 0.87 | 0.14 |
| MTG | 0.9 (0.8) | 1.2 (0.9) | 1.4 (1.3) | p = 0.51 | 0.42 | p = 0.29 | 0.52 | p = 0.81 | 0.19 |
| ITG | 3.2 (4.9) | 5.0 (4.6) | 7.5 (6.9) | p < 0.05 * | 2.53 | p = 0.01 | 0.80 | p = 0.57 | 0.47 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, G.-W.; Park, K.; Kim, Y.-H.; Jeong, G.-W. Increased Hippocampal-Inferior Temporal Gyrus White Matter Connectivity following Donepezil Treatment in Patients with Early Alzheimer’s Disease: A Diffusion Tensor Probabilistic Tractography Study. J. Clin. Med. 2023, 12, 967. https://doi.org/10.3390/jcm12030967
Kim G-W, Park K, Kim Y-H, Jeong G-W. Increased Hippocampal-Inferior Temporal Gyrus White Matter Connectivity following Donepezil Treatment in Patients with Early Alzheimer’s Disease: A Diffusion Tensor Probabilistic Tractography Study. Journal of Clinical Medicine. 2023; 12(3):967. https://doi.org/10.3390/jcm12030967
Chicago/Turabian StyleKim, Gwang-Won, Kwangsung Park, Yun-Hyeon Kim, and Gwang-Woo Jeong. 2023. "Increased Hippocampal-Inferior Temporal Gyrus White Matter Connectivity following Donepezil Treatment in Patients with Early Alzheimer’s Disease: A Diffusion Tensor Probabilistic Tractography Study" Journal of Clinical Medicine 12, no. 3: 967. https://doi.org/10.3390/jcm12030967
APA StyleKim, G.-W., Park, K., Kim, Y.-H., & Jeong, G.-W. (2023). Increased Hippocampal-Inferior Temporal Gyrus White Matter Connectivity following Donepezil Treatment in Patients with Early Alzheimer’s Disease: A Diffusion Tensor Probabilistic Tractography Study. Journal of Clinical Medicine, 12(3), 967. https://doi.org/10.3390/jcm12030967




