Abstract
Coronavirus disease 2019 (COVID-19) is associated with pulmonary involvement and cardiac arrhythmias, including supraventricular tachycardia (SVT). Adenosine is commonly used to treat SVT and is generally safe, but is rarely associated with bronchospasm. There are no data regarding the safety of adenosine use in patients with COVID-19 pneumonia and physicians may hesitate to use it in such patients. We surveyed resident physicians and cardiology attendings regarding their level of comfort in administering adenosine to hospitalized COVID-19 patients. We compared a study group of 42 COVID-19 hospitalized patients who received adenosine for SVT to a matched (for age, sex, and co-morbidities) control group of 42 non-COVID-19 hospitalized patients during the same period, all of whom received IV adenosine for SVT. Escalation of care following intravenous adenosine administration was defined as increased/new pressor requirement, need for higher O2 flow rates, need for endotracheal intubation, new nebulizer therapy, or transfer to intensive care unit within 2 h of adenosine administration. Survey results showed that 82% (59/72) of residents and 62% (16/26) of cardiologists expressed hesitation/significant concerns regarding administering adenosine in hospitalized COVID-19 patients. Adenosine use was associated with escalation of care in 47.6% (20/42) COVID-19 as compared to 50% (21/42) non-COVID-19 patients (odds ratio 0.95, 95% CI 0.45–2.01, p = NS). Escalation of care was more likely in patients who were on higher FiO2, on prior nebulizer therapy, required supplemental oxygen, or were already on a ventilator. In conclusion, we identified significant hesitation among physicians regarding the use of adenosine for SVT in hospitalized COVID-19 patients. In this study, there was no evidence of increased harm from administering adenosine to patients with SVT and COVID-19. This finding needs to be confirmed in larger studies. Based on the current evidence, adenosine for treatment of SVT in this setting should not be avoided. Key Points: Question: Given the known bronchospastic effects of adenosine, is the use of adenosine safe for treatment of supraventricular tachycardia in hospitalized patients with COVID-19? Findings: A survey of residents and cardiology attending identified that a majority expressed some level of apprehension in using adenosine for SVT in COVID-19 patients. In our matched cohort study, we found adenosine use to be comparably safe in COVID-19 and non-COVID-19 hospitalized patients. Meaning: Based on current evidence, adenosine for treatment of SVT in this setting should not be avoided.
1. Introduction
The COVID-19 pandemic plunged medicine into chaos and uncertainty in many ways. This disease is associated with a well-described inflammatory course resulting in multi-organ consequences, including cardiopulmonary complications. Notably, one of the many cardiac complications associated with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is arrhythmia, with early data from Wuhan, China, showing 44% (n = 36) of ICU level patients exhibiting some form of arrhythmia, other larger reviews quoting a 6–17% prevalence [1], and an Italian multicenter study specifically quoting a supraventricular tachycardia (SVT) rate of 1.2% (n = 414) [2]. Arrhythmias are believed to be a manifestation of multiple factors including systemic inflammation, hypoxia, adrenergic state, viral myocardial damage, downregulation of angiotensin converting enzyme 2 (ACE2) pathways, volume shifts, metabolic derangements, and potential aggravation of chronic conduction disturbances [3,4].
Adenosine is a naturally occurring organic nucleoside molecule with a well-known and widely used role for SVT termination via cascade modulation of adenylate cyclase, cAMP, and potassium/calcium currents causing transient atrioventricular nodal block. However, adenosine also carries a relative contraindication in patients with asthma [5] and cautionary use in patients with COPD secondary to mast cell-mediated pulmonary bronchospasm [6], although the risk for bronchospasm is reported to be <1% [7]. Given the association of adenosine with potential pulmonary compromise and the relative novelty of SARS-CoV-2, many clinicians may avoid or hesitate to use this potent anti-arrhythmic for the treatment of SVT in such patients.
There are few data on the safety of adenosine use in patients with COVID-19. A review article analyzing arrhythmia and COVID-19 states that adenosine can be used for treatment of SVT, but recognized that more data are needed for validation [8]. Other review articles have mentioned caution with the use of beta blockers and amiodarone in the setting of COVID-19 but not with the use of adenosine [9]. On the other hand, one review suggested that the use of adenosine and its derivatives may impede SARS-CoV-2 viral entry, ARDS progression and thrombosis in the setting of COVID-19 infection, with one study from Italy showing therapeutic benefit from inhaled adenosine in the setting of COVID-19 infection [10,11]. There are many reports that focus on malignant arrhythmias, atrial fibrillation, and heart block, but there are limited data with specific attention to SVT and the safety of adenosine use [12,13].
2. Materials and Methods
2.1. Survey
We surveyed resident physicians in the fields of internal medicine, family medicine, and general surgery as well as attending cardiology physicians regarding their level of comfort in administering adenosine to patients with COVID-19 pneumonia. The survey was sent to residents from Internal Medicine, Family Medicine and General Surgery via email with an attached web link. The emails were sent out to the residents by the chief residents of each specialty rather than directly by the study investigators. Reminder emails were sent out once each week for a total of 3 weeks. The survey was also sent to all attendings in the division of cardiology as an email sent directly by the senior author (BP), with 2 reminder emails once a week for 2 weeks. The survey questions are shown in Figure 1. The same survey was also sent to the cardiology attendings, but without the last two questions.
Figure 1.
Survey questions sent out to residents.
2.2. Retrospective Review
We searched the electronic health record that included all five hospitals in our health system for all patients admitted with the diagnosis of COVID-19 who received adenosine for the treatment of SVT between the dates of February 01, 2020 and 1 February 2021. We compared a study group which consisted of COVID-19 hospitalized patients (n = 42) who received adenosine for SVT with a control group. The control group consisted of an equal number of non-COVID-19 hospitalized patients (n = 42) during the same period who received IV adenosine for SVT. The groups were matched for age, sex and comorbidities. Both the study group and control group had 42 patients with a mean age of 66 years old, and a gender split of 32 males and 10 females each. Additionally, the groups were matched for comorbidities including history of pulmonary disease, history of arrhythmia and history of conduction system disease. We obtained relevant medical history, including history of pulmonary disease, history of arrhythmia, and prior use of anti-arrhythmic therapy or rate controlling medications. The highest dosage of adenosine given as well as its effects on cardiac rhythm were documented. Each chart was reviewed for possible escalation of care related to adenosine and any resulting changes in management. Escalation of care was defined as any one of the following within two hours of adenosine administration: increased or new pressor requirement, need for higher O2 flow, endotracheal intubation, new bronchodilator nebulizer therapy, or transfer to an intensive care unit. We documented these variables before and after adenosine administration. The same electronic health record was searched (during the same time period) for age-/gender-matched hospitalized patients who received IV adenosine for SVT but who did not have an associated diagnosis of COVID-19. The exact same clinical and outcomes parameters were collected.
2.3. Statistical Analysis
Data were analyzed separately in both groups, and also for the entire population (both study and the control groups) based on whether or not adenosine use was associated with escalation of care as defined in our study. Continuous variables were compared between the two groups using the two-tailed Student’s t test assuming equal variances. Dichotomous variables were compared using the Fisher’s exact test. When groups had <5 data points, the Yates correction was applied. p values < 0.05 were considered to be statistically significant.
3. Results
3.1. Survey
The survey was sent to a total of 181 residents from Internal Medicine (109), Family Medicine (30) and General Surgery (42) via email. The response rate from residents was 39.8% (72/181). The survey was also sent to all 49 attendings in the division of cardiology and the attending response rate was 53.1% (26/49).
The survey results showed that 82% (59/72) of responding residents and 62% (16/26) of responding cardiology attendings had either some hesitation or significant concerns about administering adenosine in hospitalized COVID-19 patients. The level of hesitation was significantly greater among residents as compared to attending (p = 0.0353). Only 27.3% of the responding residents and 38.5% of responding cardiology attendings had no concerns about administering adenosine in COVID-19 pneumonia patients for the treatment of SVT. Survey details are shown in Figure 2.
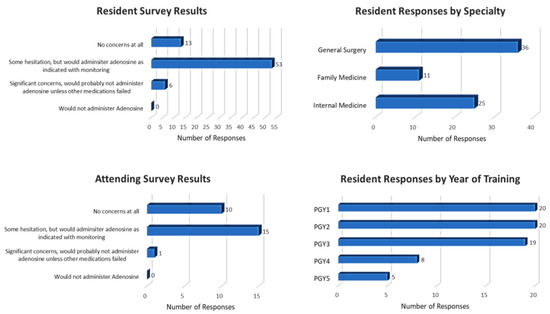
Figure 2.
Survey results.
3.2. Baseline Characteristics of the Study and Control Groups
Table 1 shows the clinical variables of the total study population and the two groups. There were no statistically significant differences in the study and control cohorts (except for a higher incidence of prior arrhythmia and greater need for supplemental O2 in the control group).

Table 1.
Baseline characteristics of total population in this study.
3.3. Study Group
Table 2 shows the baseline clinical characteristics in the 42 patients in the entire study group, and separately in patients who did not and did require escalation of care. Adenosine use for SVT was associated with escalation of care within 2 h in 47.6% (20/42) of patients. Escalation of care was significantly more likely to occur in COVID-19 patients who required prior use of nebulizers as compared to those who did not (11/20 vs. 8/22, p = 0.0045).

Table 2.
Study group. Clinical variables are shown for all study group patients, and also separately for patients who did not and did require escalation of care.
3.4. Control Group
Table 3 shows the baseline clinical characteristics in the 42 matched patients in the entire control group, and separately in patients who did not and did require escalation of care. Adenosine use for SVT was associated with escalation of care within 2 h in 50% (21/42) of patients. Escalation of care was significantly more likely to occur in non-COVID-19 patients who required higher pre-adenosine FIO2 (66.9 vs. 41.7, p = 0.0284), who were on supplemental oxygen at the time of adenosine administration (20/21 vs. 10/21, p = 0.0006), and had received previous nebulizer therapy (13/21 vs. 6/21, p = 0.0495) as compared to patients who did not require escalation of care.

Table 3.
Control group. Clinical variables are shown for all control group patients, and also separately for patients who did not and did require escalation of care.
The odds ratio of risk of escalation for COVID-19 vs. non-COVID-19 patients was 0.95, 95% CI 0.45–2.01, p = NS).
Among the 41 patients (from both groups) who were defined as needing escalation of care, requiring more nebulizer therapy after adenosine administration was significantly more common in the COVID-19 group as compared to the non-COVID-19 group (12/20 vs. 5/21, p = 0.0187); all other parameters of escalation of care (escalating pressor requirement, escalating O2 flow, requiring intubation, requiring nebulizers, or requiring higher transfer to an intensive care unit within two hours of adenosine administration) were comparable in the study group vs. the control group (all p = NS), as shown in Table 4.

Table 4.
Comparison of individual escalation parameters between the study and control groups.
4. Discussion
Our survey results indicate that there is apprehension/concern regarding adenosine use in COVID-19 patients among 82% of residents and 62% of cardiology attendings. According to the survey, these concerns were significantly more common amongst house staff compared to attendings. This is important since the clinical decision to administer adenosine for arrhythmia often calls for quick action. Similarly, the higher incidence of supplemental O2 in the control group was not associated with any differences in pre-adenosine FiO2 or O2 flow, need for nebulizers, or being on a ventilator. Overall, our data shows that there is no significant difference in the risk of requiring escalation of care after adenosine use for SVT in COVID-19 patients as compared to a matched cohort of hospitalized non-COVID-19 patients. Among patients who met our definition of escalation of care, the need for nebulizer therapy was significantly more common in the COVID-19 group as compared to the control group, suggesting that clinicians should be especially alert to the bronchospastic effects of adenosine in hospitalized COVID-19 patients. These findings are biologically plausible given what is known about the pathophysiology of COVID-19 and the pharmacology of adenosine, and our study represents the first such report.
Our study has several limitations inherent to a relatively small retrospective analysis. We report on a relatively small number of patients. The higher incidence of prior arrhythmia in the control group suggests that they may have been predisposed to requiring adenosine, but does not influence response to adenosine, and would not be expected to influence likelihood of escalation of care. Similarly, the higher incidence of supplemental O2 in the control group was not associated with any differences in pre-adenosine FiO2 or O2 flow, need for nebulizers, or being on a ventilator. There was a potential for misclassification of true COVID status in patients who were asymptomatic and therefore not re-tested for COVID during their hospitalization. Our definition of “escalation of care” was meant to identify any and all possible adverse consequences of adenosine use, though we recognize that some of the qualifiers (such as increased O2 requirements) may not have been clinically important. Another point of discussion is that the two-hour time window following adenosine administration may have been relatively long, since the immediate effects of adenosine last only for a brief duration. However, the adenosine-triggered bronchospastic effects may progress and precipitate clinical deterioration in the ensuing two hours, which explains the reasoning behind the chosen “catchment” window. It is probable that the occurrence of any arrhythmia may be a manifestation of the severity of the underlying illness, and that any escalation of care after adenosine may have been unrelated to the drug. This is consistent with a study from Wuhan, China in which 16.7% of 138 patients admitted for COVID-19 were found to have arrhythmia, with 44.4% of these patients requiring escalation to the intensive care unit [14]. Similarly, it is also possible that any increase in pressor requirement may have been due to the arrhythmia rather than adenosine. Finally, although our matching effort identified a comparable control population, there were some differences, notably a higher incidence of prior arrhythmia, and also greater need for supplemental O2 prior to receiving adenosine.
5. Conclusions
Our study identified that there is a significant level of hesitation among physicians regarding the use of adenosine for treatment of SVT in the setting of COVID-19 pneumonia, and there are no prior data to address this concern. Within the limitations of a small sample size retrospective review, our data demonstrates that adenosine use in COVID-19 pneumonia patients is not associated with adverse outcomes as compared to non-COVID-19 hospitalized patients with comparable co-morbidity. Adenosine for the treatment of SVT in this setting should not be avoided.
Author Contributions
Conceptualization, T.Z. and B.B.P.; methodology, T.Z., R.L.R., A.M. and B.B.P.; formal analysis, T.Z., R.L.R., A.M. and B.B.P.; investigation, T.Z., R.L.R., A.M. and B.B.P.; resources, T.Z. and B.B.P.; data curation, T.Z., R.L.R., A.M. and B.B.P.; writing—original draft preparation, T.Z., R.L.R. and B.B.P.; writing—review and editing, T.Z., R.L.R., A.M. and B.B.P.; visualization, B.B.P.; supervision, T.Z. and B.B.P.; project administration, T.Z., R.L.R. and B.B.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board of Thomas Jefferson University Hospital Office of Human Research, protocol code 21E.130, on 2/15/21.
Informed Consent Statement
Patient consent was waived due to the retrospective nature of the study, per review by the Institutional Review Board.
Data Availability Statement
Data sharing not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Manolis, A.S.; Manolis, A.A.; Manolis, T.A.; Apostolopoulos, E.J.; Papatheou, D.; Melita, H. COVID-19 infection and cardiac arrhythmias. Trends Cardiovasc. Med. 2020, 30, 451–460. [Google Scholar] [CrossRef] [PubMed]
- Russo, V.; Di Maio, M.; Mottola, F.F.; Pagnano, G.; Attena, E.; Verde, N.; Di Micco, P.; Silverio, A.; Scudiero, F.; Nunziata, L.; et al. Clinical characteristics and prognosis of hospitalized COVID-19 patients with incident sustained tachyarrhythmias: A multicenter observational study. Eur. J. Clin. Investig. 2020, 50, e13387. [Google Scholar] [CrossRef] [PubMed]
- Kochi, A.N.; Tagliari, A.P.; Forleo, G.B.; Fassini, G.M.; Tondo, C. Cardiac and arrhythmic complications in patients with COVID-19. J. Cardiovasc. Electrophysiol. 2020, 31, 1003–1008. [Google Scholar] [CrossRef] [PubMed]
- Dherange, P.; Lang, J.; Qian, P.; Overfeld, B.; Sauer, W.H.; Koplan, B.; Tedrow, U. Arrhythmias and COVID-19: A Review. JACC Clin. Electrophysiol. 2020, 6, 1193–1204. [Google Scholar] [CrossRef] [PubMed]
- Neumar, R.W.; Otto, C.W.; Link, M.S.; Kronick, S.L.; Shuster, M.; Callaway, C.W.; Kudenchuk, P.J.; Ornato, J.P.; McNally, B.; Silvers, S.M.; et al. Part 8: Adult advanced cardiovascular life support: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 2010, 122, S729–S767. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.B. Adenosine and its role in asthma. Indian J. Clin. Biochem. 2001, 16, 140–144. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Lokhandwala, Y.; Rai, N.; Malviya, A. Adenosine—A drug with myriad utility in the diagnosis and treatment of arrhythmias. J. Arrhythmia. 2020, 37, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Falcone, C.; Caracciolo, M.; Correale, P.; Macheda, S.; Vadalà, E.G.; La Scala, S.; Tesciona, M.; Danieli, R.; Ferrarelli, A.; Tarsitano, M.G.; et al. Can Adenosine Fight COVID-19 Acute Respiratory Distress Syndrome? J. Clin. Med. 2020, 9, 3045. [Google Scholar] [CrossRef] [PubMed]
- Desai, A.D.; Boursiquot, B.C.; Melki, L.; Wan, E.Y. Management of Arrhythmias Associated with COVID-19. Curr. Cardiol. Rep. 2021, 23, 2. [Google Scholar] [CrossRef] [PubMed]
- Geiger, J.D.; Khan, N.; Murugan, M.; Boison, D. Possible Role of Adenosine in COVID-19 Pathogenesis and Therapeutic Opportunities. Front. Pharmacol. 2020, 11, 594487. [Google Scholar] [CrossRef] [PubMed]
- Caracciolo, M.; Correale, P.; Mangano, C.; Foti, G.; Falcone, C.; Macheda, S.; Cuzzola, M.; Conte, M.; Falzea, A.C.; Iuliano, E.; et al. Efficacy and Effect of Inhaled Adenosine Treatment in Hospitalized COVID-19 Patients. Front. Immunol. 2021, 12, 613070. [Google Scholar] [CrossRef] [PubMed]
- Rattanawong, P.; Shen, W.; El Masry, H.; Sorajja, D.; Srivathsan, K.; Valverde, A.; Scott, L.R. Guidance on short-term management of atrial fibrillation in Coronavirus disease 2019. J. Am. Heart Assoc. 2020, 9, e017529. [Google Scholar] [CrossRef] [PubMed]
- Kochav, S.M.; Coromilas, E.; Nalbandian, A.; Ranard, L.S.; Gupta, A.; Chung, M.K.; Gopinathannair, R.; Biviano, A.B.; Garan, H.; Wan, E.Y. Cardiac arrhythmias in COVID-19 infection. Circ. Arrhythm. Electrophysiol. 2020, 13, e008719. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Hu, B.; Hu, C. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA 2020, 323, 1061–1069. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).