Three Visual–Diagnostic Methods for the Detection of Enamel Cracks: An In Vitro Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Diagnostic Models
2.2. Documentation of Enamel Cracks
- Score 1: no crack (intact surface),
- Score 2: superficial enamel crack (enamel crack < 50% enamel thickness),
- Score 3: deep enamel crack (crack runs through the first 2/3 of the enamel),
- Score 4: whole thickness enamel crack (crack extends to the dentino-enamel junction),
- Score 5: dentin crack (crack extending beyond the dentino-enamel junction).
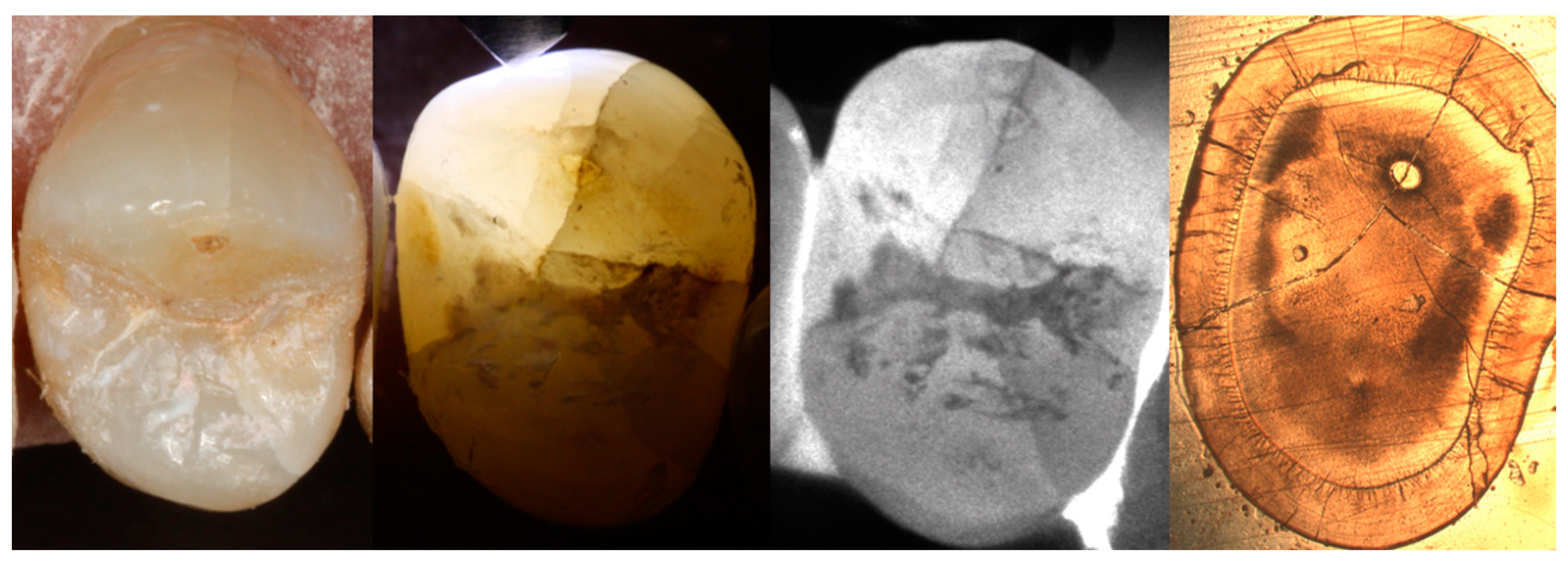
2.3. Visualization of Enamel Cracks
2.4. Preparation and Evaluation of Horizontal Crown Sections
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Veltmaat, A.; Günay, H.; Geurtsen, W. In-vivo-Studie zur Epidemiologie der Kroneninfraktion bei gefüllten Seitenzähnen [In vivo study of the epidemiology of the cracked-tooth syndrome of restored posterior teeth]. Dtsch. Zahnärztl. Z. 1997, 52, 137–140. [Google Scholar]
- Cracking the Cracked Tooth Code: Detection and Treatment of Various Longitudinal Tooth Fractures. Available online: https://www.aae.org/specialty/wp-content/uploads/sites/2/2017/07/ecfesum08.pdf (accessed on 11 October 2022).
- Ellis, S.G. Incomplete tooth fracture—Proposal for a new definition. Br. Dent. J. 2001, 190, 424–428. [Google Scholar] [CrossRef] [PubMed]
- Imai, K.; Shimada, Y.; Sadr, A.; Sumi, Y.; Tagami, J. Noninvasive cross-sectional visualization of enamel cracks by optical coherence tomography in vitro. J. Endodont. 2012, 38, 1269–1274. [Google Scholar] [CrossRef] [PubMed]
- Love, R.M. Bacterial penetration of the root canal of intact incisor teeth after a simulated traumatic injury. Endodont. Dent. Traumatol. 1996, 12, 289–293. [Google Scholar] [CrossRef] [PubMed]
- Ravn, J.J. Follow-up study of permanent incisors with enamel-dentin fractures after acute trauma. Scand. J. Dent. Res. 1981, 89, 355–365. [Google Scholar] [CrossRef]
- Walker, B.N.; Makinson, O.F.; Peters, M.C. Enamel cracks. The role of enamel lamellae in caries initiation. Aust. Dent. J. 1998, 43, 110–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, W.S.; Jacobs, H.R.; Thompson, R.E. Thermal fatigue in teeth. J. Dent. Res. 1972, 51, 461–467. [Google Scholar] [CrossRef]
- Lynch, C.D.; McConnell, R.J. The cracked tooth syndrome. J. Can. Dent. Assoc. 2002, 68, 470–475. [Google Scholar]
- Mariona, R.P.; Antony, S.D.P. Diagnostic methods for cracked tooth by two endodontic tools. Drug Invent. Today 2018, 10, 2999–3002. [Google Scholar]
- Nguyen, V.; Palmer, G. A review of the diagnosis and management of the cracked tooth. Dent. Update 2009, 36, 338–340. [Google Scholar] [CrossRef]
- Ghorbanzadeh, A.; Aminifar, S.; Shadan, L.; Ghanati, H. Evaluation of three methods in the diagnosis of dentin cracks caused by apical resection. J. Dent. 2013, 10, 175–185. [Google Scholar]
- Clark, D.J.; Sheets, C.G.; Paquette, J.M. Definitive diagnosis of early enamel and dentin cracks based on microscopic evaluation. J. Esthet. Restor. Dent. 2003, 15, 391–401, discussion 401. [Google Scholar] [CrossRef]
- Kim, J.H.; Eo, S.H.; Shrestha, R.; Ihm, J.J.; Seo, D.G. Association between longitudinal tooth fractures and visual detection methods in diagnosis. J. Dent. 2020, 101, 103466. [Google Scholar] [CrossRef] [PubMed]
- Mathew, S.; Thangavel, B.; Mathew, C.A.; Kailasam, S.; Kumaravadivel, K.; Das, A. Diagnosis of cracked tooth syndrome. J. Pharm. Bioallied Sci. 2012, 4, S242–S244. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Holamoge, Y.V.; Li, Z.; Zaid, W.; Osborn, M.L.; Ramos, A.; Miller, J.T.; Li, Y.; Yao, S.; Xu, J. Detection and analysis of enamel cracks by ICG-NIR fluorescence dental imaging. Ann. N. Y. Acad. Sci. 2020, 1475, 52–63. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.M.; Kang, S.R.; Yi, W.J. Automatic detection of tooth cracks in optical coherence tomography images. J. Periodontal. Implant Sci. 2017, 47, 41–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.H.; Lee, J.J.; Chung, H.J.; Park, J.T.; Kim, H.J. Dental optical coherence tomography: New potential diagnostic system for cracked-tooth syndrome. Surg. Radiol. Anat. 2016, 38, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Machoy, M.; Seeliger, J.; Szyszka-Sommerfeld, L.; Koprowski, R.; Gedrange, T.; Woźniak, K. The use of optical coherence tomography in dental diagnostics: A state-of-the-art review. J. Healthcare Eng. 2017, 2017, 7560645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abogazalah, N.; Eckert, G.J.; Ando, M. In vitro performance of near infrared light transillumination at 780-nm and digital radiography for detection of non-cavitated approximal caries. J. Dent. 2017, 63, 44–50. [Google Scholar] [CrossRef] [Green Version]
- Ortiz, M.I.G.; de Melo Alencar, C.; De Paula, B.L.F.; Magno, M.B.; Maia, L.C.; Silva, C.M. Accuracy of near-infrared light transillumination (NILT) compared to bitewing radiograph for detection of interproximal caries in the permanent dentition: A systematic review and meta-analysis. J. Dent. 2020, 98, 103351. [Google Scholar] [CrossRef] [PubMed]
- Reda, R.; Zanza, A.; Cicconetti, A.; Bhandi, S.; Miccoli, G.; Gambarini, G.; Di Nardo, D. Ultrasound Imaging in Dentistry: A Literature Overview. J. Imaging 2021, 7, 238. [Google Scholar] [CrossRef] [PubMed]
- Cicchetti, D.V. Guidelines, criteria, and rules of thumb for evaluating normed and standardized assessment instruments in Psychology. Psychol. Assess. 1994, 6, 284–290. [Google Scholar] [CrossRef]
- Shah, H.; Hernandez, P.; Budin, F.; Chittajallu, D.; Vimort, J.B.; Walters, R.; Mol, A.; Khan, A.; Paniagua, B. Automatic quantification framework to detect cracks in teeth. Proc. SPIE Int. Soc. Opt. Eng. 2018, 10578, 105781K. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharmila, S.; Lavanya, A.; Kumar, R.; Rao, D.H. A detailed review on ergonomics and parts of dental operating microscope. J Adv. Clin. Res. Insights 2021, 8, 87–90. [Google Scholar] [CrossRef]
- Bud, M.; Jitaru, S.; Lucaciu, O.; Korkut, B.; Dumitrascu-Timis, L.; Ionescu, C.; Cimpean, S.; Delean, A. The advantages of the dental operative microscope in restorative dentistry. Med. Pharm. Rep. 2021, 94, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Davies, G.M.; Worthington, H.V.; Clarkson, J.E.; Thomas, P.; Davies, R.M. The use of fibre-optic transillumination in general dental practice. Br. Dent. J. 2001, 191, 145–147. [Google Scholar] [CrossRef] [Green Version]
- Peers, A.; Hill, F.J.; Mitropoulos, C.M.; Holloway, P.J. Validity and reproducibility of clinical examination, fibre-optic transillumination, and bite-wing radiology for the diagnosis of small approximal carious lesions: An in vitro study. Caries Res. 1993, 27, 307–311. [Google Scholar] [CrossRef] [PubMed]
- Kühnisch, J.; Söchtig, F.; Pitchika, V.; Laubender, R.; Neuhaus, K.W.; Lussi, A.; Hickel, R. In vivo validation of near-infrared light transillumination for interproximal dentin caries detection. Clin. Oral Investig. 2016, 20, 821–829. [Google Scholar] [CrossRef] [PubMed]
- Casalegno, F.; Newton, T.; Daher, R.; Abdelaziz, M.; Lodi-Rizzini, A.; Schürmann, F.; Krejci, I.; Markram, H. Caries detection with near-infrared transillumination using deep learning. J. Dent. Res. 2019, 98, 1227–1233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohammad-Rahimi, H.; Motamedian, S.R.; Rohban, M.H.; Krois, J.; Uribe, S.E.; Mahmoudinia, E.; Rokhshad, R.; Nadimi, M.; Schwendicke, F. Deep learning for caries detection: A systematic review. J. Dent. 2022, 122, 104115. [Google Scholar] [CrossRef] [PubMed]
- Ei, T.Z.; Shimada, Y.; Abdou, A.; Sadr, A.; Yoshiyama, M.; Sumi, Y.; Tagami, J. Three-dimensional assessment of proximal contact enamel using optical coherence tomography. Dent. Mater. 2019, 35, e74–e82. [Google Scholar] [CrossRef] [PubMed]
- Litzenburger, F.; Heck, K.; Kaisarly, D.; Kunzelmann, K.H. Diagnostic validity of early proximal caries detection using near-infrared imaging technology on 3D range data of posterior teeth. Clin. Oral Investig. 2022, 26, 543–553. [Google Scholar] [CrossRef]
- Göstemeyer, G.; Preus, M.; Elhennawy, K.; Schwendicke, F.; Paris, S.; Askar, H. Accuracy of different approaches for detecting proximal root caries lesions in vitro. Clin. Oral Investig. 2022. epub ahead of print. [Google Scholar] [CrossRef]
- Versiani, M.A.; Souza, E.; De-Deus, G. Critical appraisal of studies on dentinal radicular microcracks in endodontics: Methodological issues, contemporary concepts, and future perspectives. Endodont. Top. 2015, 33, 87–156. [Google Scholar] [CrossRef]
- De-Deus, G.; Cavalcante, D.M.; Belladonna, F.G.; Carvalhal, J.; Souza, E.M.; Lopes, R.T.; Versiani, M.A.; Silva, E.J.N.L.; Dummer, P.M.H. Root dentinal microcracks: A post-extraction experimental phenomenon? Int. Endodont. J. 2019, 52, 857–865. [Google Scholar] [CrossRef]
- Ozuna, J.; Barborka, B.; Abubakr, N.H. A Retrospective Evaluation of the Prevalence of Cracked Teeth Among an Adult Population in Nevada. Eur. Endodont. J. 2021, 6, 160–163. [Google Scholar] [CrossRef]


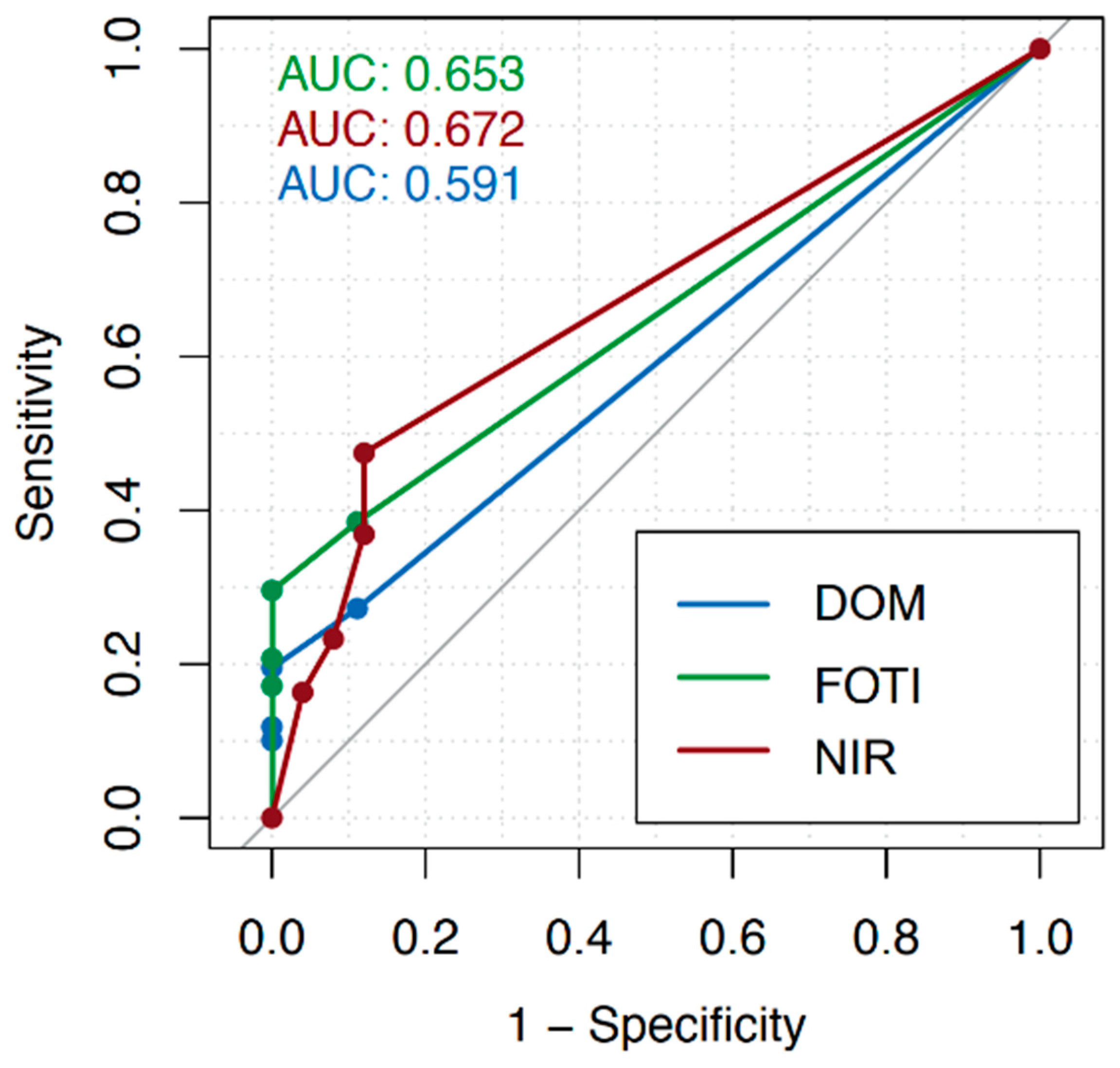
| Cutoff Score | DOM | FOTI | NIR | |||
|---|---|---|---|---|---|---|
| Sensitivity | Specificity | Sensitivity | Specificity | Sensitivity | Specificity | |
| 2 | 0.50 | 0.60 | 0.59 | 0.72 | 0.47 | 0.88 |
| 3 | 0.35 | 0.72 | 0.46 | 0.88 | 0.37 | 0.88 |
| 4 | 0.20 | 0.92 | 0.26 | 0.96 | 0.23 | 0.92 |
| 5 | 0.12 | 1.00 | 0.18 | 1.00 | 0.16 | 0.96 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hausdörfer, T.; Harms, L.; Kanzow, P.; Hülsmann, M. Three Visual–Diagnostic Methods for the Detection of Enamel Cracks: An In Vitro Study. J. Clin. Med. 2023, 12, 973. https://doi.org/10.3390/jcm12030973
Hausdörfer T, Harms L, Kanzow P, Hülsmann M. Three Visual–Diagnostic Methods for the Detection of Enamel Cracks: An In Vitro Study. Journal of Clinical Medicine. 2023; 12(3):973. https://doi.org/10.3390/jcm12030973
Chicago/Turabian StyleHausdörfer, Tim, Lisa Harms, Philipp Kanzow, and Michael Hülsmann. 2023. "Three Visual–Diagnostic Methods for the Detection of Enamel Cracks: An In Vitro Study" Journal of Clinical Medicine 12, no. 3: 973. https://doi.org/10.3390/jcm12030973
APA StyleHausdörfer, T., Harms, L., Kanzow, P., & Hülsmann, M. (2023). Three Visual–Diagnostic Methods for the Detection of Enamel Cracks: An In Vitro Study. Journal of Clinical Medicine, 12(3), 973. https://doi.org/10.3390/jcm12030973







