Factors Associated with Potentially Inappropriate Screening for Vitamin D Deficiency among Women in Medically Underserved Regions of West Texas
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Definition of Variables
2.3. Statistical Analysis
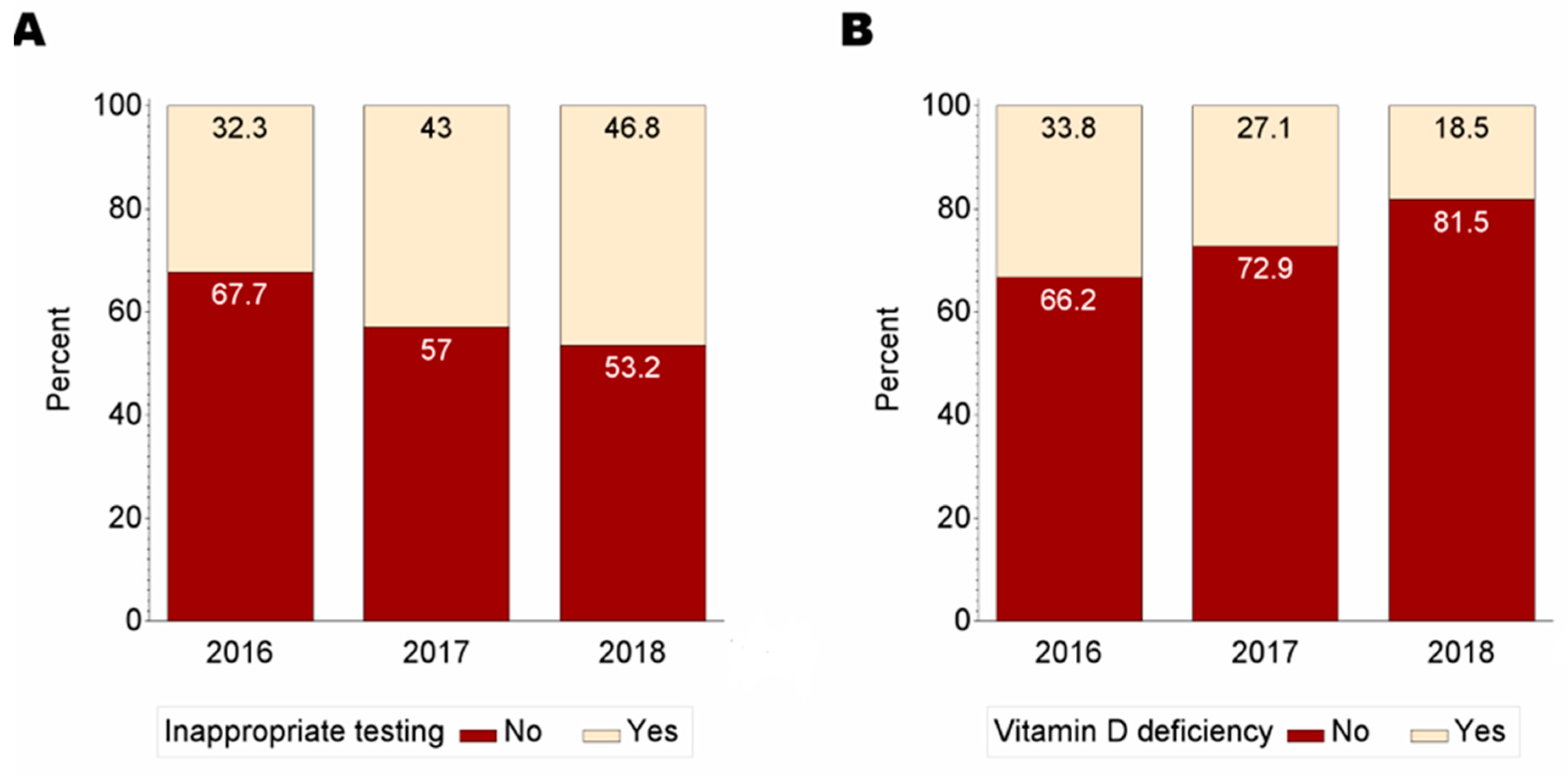
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pilz, S.; Zittermann, A.; Trummer, C.; Theiler-Schwetz, V.; Lerchbaum, E.; Keppel, M.H.; Grübler, M.R.; März, W.; Pandis, M. Vitamin D testing and treatment: A narrative review of current evidence. Endocr. Connect. 2019, 8, R27–R43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barrett-Connor, E.; Siris, E.S.; Wehren, L.E.; Miller, P.D.; Abbott, T.A.; Berger, M.L.; Santora, A.C.; Sherwood, L.M. Osteoporosis and fracture risk in women of different ethnic groups. J. Bone Miner. Res. 2005, 20, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Wactawski-Wende, J.; Kotchen, J.M.; Anderson, G.L.; Assaf, A.R.; Brunner, R.L.; O’Sullivan, M.J.; Margolis, K.L.; Ockene, J.K.; Phillips, L.; Pottern, L.; et al. Calcium plus vitamin D supplementation and the risk of colorectal cancer. New Engl. J. Med. 2006, 354, 684–696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shao, T.; Klein, P.; Grossbard, M.L. Vitamin D and breast cancer. Oncologist 2012, 17, 36–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papandreou, D.; Hamid, Z.T. The Role of Vitamin D in Diabetes and Cardiovascular Disease: An Updated Review of the Literature. Dis. Markers 2015, 2015, 580474. [Google Scholar] [CrossRef] [Green Version]
- Holick, M.F. Vitamin D deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef] [PubMed]
- Barbarawi, M.; Kheiri, B.; Zayed, Y.; Barbarawi, O.; Dhillon, H.; Swaid, B.; Yelangi, A.; Sundus, S.; Bachuwa, G.; Alkotob, M.L.; et al. Vitamin D Supplementation and Cardiovascular Disease Risks in More Than 83 000 Individuals in 21 Randomized Clinical Trials: A Meta-analysis. JAMA Cardiol. 2019, 4, 765–776. [Google Scholar] [CrossRef] [PubMed]
- Chakhtoura, M.; Bacha, D.S.; Gharios, C.; Ajjour, S.; Assaad, M.; Jabbour, Y.; Kahale, F.; Bassatne, A.; Antoun, S.; Akl, E.A.; et al. Vitamin D Supplementation and Fractures in Adults: A Systematic Umbrella Review of Meta-Analyses of Controlled Trials. J. Clin. Endocrinol. Metab. 2021, 107, 882–898. [Google Scholar] [CrossRef]
- Avenell, A.; Mak, J.C.; O’Connell, D. Vitamin D and vitamin D analogues for preventing fractures in post-menopausal women and older men. Cochrane Database Syst. Rev. 2014, 14, CD000227. [Google Scholar]
- Bouillon, R.; Manousaki, D.; Rosen, C.; Trajanoska, K.; Rivadeneira, F.; Richards, J.B. The health effects of vitamin D supplementation: Evidence from human studies. Nat. Rev. Endocrinol. 2022, 18, 96–110. [Google Scholar] [CrossRef]
- Zheng, Y.T.; Cui, Q.Q.; Hong, Y.M.; Yao, W.G. A meta-analysis of high dose, intermittent vitamin D supplementation among older adults. PLoS ONE 2015, 10, e0115850. [Google Scholar] [CrossRef]
- Mateussi, M.V.; Latorraca, C.O.C.; Daou, J.P.; Martimbianco, A.L.C.; Riera, R.; Pacheco, R.L.; Pachito, D.V. What do Cochrane systematic reviews say about interventions for vitamin D supplementation? Sao Paulo Med. J. 2017, 135, 497–507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mithal, A.; Wahl, D.A.; Bonjour, J.P.; Burckhardt, P.; Dawson-Hughes, B.; Eisman, J.A.; El-Hajj Fuleihan, G.; Josse, R.G.; Lips, P.; Morales-Torres, J.; et al. Global vitamin D status and determinants of hypovitaminosis D. Osteoporos. Int. 2009, 20, 1807–1820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wierzbicka, A.; Oczkowicz, M. Sex differences in vitamin D metabolism, serum levels and action. Br. J. Nutr. 2022, 128, 2115–2130. [Google Scholar] [CrossRef] [PubMed]
- Ning, Z.; Song, S.; Miao, L.; Zhang, P.; Wang, X.; Liu, J.; Hu, Y.; Xu, Y.; Zhao, T.; Liang, Y.; et al. High prevalence of vitamin D deficiency in urban health checkup population. Clin. Nutr. 2016, 35, 859–863. [Google Scholar] [CrossRef] [PubMed]
- Kakarala, R.R.; Chandana, S.R.; Harris, S.S.; Kocharla, L.P.; Dvorin, E. Prevalence of vitamin D deficiency in uninsured women. J. Gen. Intern. Med. 2007, 22, 1180–1183. [Google Scholar] [CrossRef] [Green Version]
- Bilinski, K.; Boyages, S. The rise and rise of vitamin D testing. Br. Med. J. 2012, 345, e4743. [Google Scholar] [CrossRef]
- Woodford, H.J.; Barrett, S.; Pattman, S. Vitamin D: Too much testing and treating? Clin. Med. 2018, 18, 196–200. [Google Scholar] [CrossRef] [Green Version]
- Crowe, F.L.; Jolly, K.; MacArthur, C.; Manaseki-Holland, S.; Gittoes, N.; Hewison, M.; Scragg, R.; Nirantharakumar, K. Trends in the incidence of testing for vitamin D deficiency in primary care in the UK: A retrospective analysis of The Health Improvement Network (THIN), 2005–2015. BMJ Open 2019, 9, 028355. [Google Scholar] [CrossRef]
- Rodd, C.; Sokoro, A.; Lix, L.M.; Thorlacius, L.; Moffatt, M.; Slater, J.; Bohm, E. Increased rates of 25-hydroxy vitamin D testing: Dissecting a modern epidemic. Clin. Biochem. 2018, 59, 56–61. [Google Scholar] [CrossRef]
- Carbonell-Abella, C. Why concerns about vitamin D deficiency should not lead to over testing and overtreatment. Eur. J. Gen. Pract. 2020, 26, 163–165. [Google Scholar] [CrossRef] [PubMed]
- LeFevre, M.L.; LeFevre, N.M. Vitamin D Screening and Supplementation in Community-Dwelling Adults: Common Questions and Answers. Am. Fam. Physician 2018, 97, 254–260. [Google Scholar] [PubMed]
- American Society for Clinical Pathology. Don’t Perform Population Based Screening for 25-OH-Vitamin D Deficiency. Available online: https://www.choosingwisely.org/clinician-lists/american-society-clinical-pathology-population-based-screening-for-vitamin-d-deficiency/ (accessed on 23 December 2022).
- Breth-Petersen, M.; Bell, K.; Pickles, K.; McGain, F.; McAlister, S.; Barratt, A. Health, financial and environmental impacts of unnecessary vitamin D testing: A triple bottom line assessment adapted for healthcare. BMJ Open 2022, 12, 056997. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Evaluation, Treatment, and Prevention of Vitamin D Deficiency: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rockwell, M.S.; Wu, Y.; Salamoun, M.; Hulver, M.W.; Epling, J.W. Patterns of Clinical Care Subsequent to Nonindicated Vitamin D Testing in Primary Care. J. Am. Board Fam. Med. 2020, 33, 569–579. [Google Scholar] [CrossRef]
- Aralica, M.; Šupak Smolčić, V.; Turk Wensveen, T.; Hrabrić Vlah, S.; Selar, M.; Bilić Zulle, L. An analysis of the vitamin D overtesting in a tertiary healthcare centre. Biochem. Med. 2022, 32, 020701. [Google Scholar] [CrossRef]
- Essig, S.; Merlo, C.; Reich, O.; Trottmann, M. Potentially inappropriate testing for vitamin D deficiency: A cross-sectional study in Switzerland. BMC Health Serv. Res. 2020, 20, 020–05956. [Google Scholar] [CrossRef]
- Granado-Lorencio, F.; Blanco-Navarro, I.; Pérez-Sacristán, B. Criteria of adequacy for vitamin D testing and prevalence of deficiency in clinical practice. Clin. Chem. Lab. Med. 2016, 54, 791–798. [Google Scholar] [CrossRef]
- Gonzalez-Chica, D.; Stocks, N. Changes to the frequency and appropriateness of vitamin D testing after the introduction of new Medicare criteria for rebates in Australian general practice: Evidence from 1.5 million patients in the NPS MedicineInsight database. BMJ Open 2019, 9, 024797. [Google Scholar] [CrossRef]
- TexasCounties.net. The Regions of Texas. Available online: https://www.texascounties.net/statistics/regions.htm (accessed on 23 December 2022).
- Health Resources and Services Administration. Medically Underserved Area and Medically Underserved Population Designations Throughout the U.S. Available online: https://data.hrsa.gov/tools/shortage-area/mua-find (accessed on 23 December 2022).
- Institute of Medicine. Dietary Reference Intakes for Calcium and Vitamin D; The National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- Herrick, K.A.; Storandt, R.J.; Afful, J.; Pfeiffer, C.M.; Schleicher, R.L.; Gahche, J.J.; Potischman, N. Vitamin D status in the United States, 2011–2014. Am. J. Clin. Nutr. 2019, 110, 150–157. [Google Scholar] [CrossRef] [Green Version]
- National Institute of Health. Vitamin D: Fact Sheet for Health Professionals. Available online: https://ods.od.nih.gov/factsheets/VitaminD-HealthProfessional/ (accessed on 6 January 2023).
- Ross, A.C.; Manson, J.E.; Abrams, S.A.; Aloia, J.F.; Brannon, P.M.; Clinton, S.K.; Durazo-Arvizu, R.A.; Gallagher, J.C.; Gallo, R.L.; Jones, G.; et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: What clinicians need to know. J. Clin. Endocrinol. Metab. 2011, 96, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Choosing Wisely. Vitamin D Tests: When You Need Them—And When You Don’t. Available online: https://www.choosingwisely.org/patient-resources/vitamin-d-tests/ (accessed on 28 December 2022).
- Bilinski, K.; Boyages, S. Evidence of overtesting for vitamin D in Australia: An analysis of 4.5 years of Medicare Benefits Schedule (MBS) data. BMJ Open 2013, 3, 002955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amrein, K.; Scherkl, M.; Hoffmann, M.; Neuwersch-Sommeregger, S.; Köstenberger, M.; Tmava Berisha, A.; Martucci, G.; Pilz, S.; Malle, O. Vitamin D deficiency 2.0: An update on the current status worldwide. Eur. J. Clin. Nutr. 2020, 74, 1498–1513. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Baylin, A.; Levy, P.D. Vitamin D deficiency and insufficiency among US adults: Prevalence, predictors and clinical implications. Br. J. Nutr. 2018, 119, 928–936. [Google Scholar] [CrossRef] [Green Version]
- Cui, A.; Xiao, P.; Ma, Y.; Fan, Z.; Zhou, F.; Zheng, J.; Zhang, L. Prevalence, trend, and predictor analyses of vitamin D deficiency in the US population, 2001–2018. Front. Nutr. 2022, 9, 965376. [Google Scholar] [CrossRef]
- Mishra, S.; Stierman, B.; Gahche, J.J.; Potischman, N. Dietary Supplement Use Among Adults: United States, 2017–2018. NCHS Data Brief 2021, 399, 1–8. [Google Scholar]
- Rockwell, M.; Kraak, V.; Hulver, M.; Epling, J. Clinical Management of Low Vitamin D: A Scoping Review of Physicians’ Practices. Nutrients 2018, 10, 493. [Google Scholar] [CrossRef] [Green Version]
- Washington Health Alliance. First, Do No Harm: Calculating Health Care Waste in Washington State. Available online: https://www.wacommunitycheckup.org/highlights/calculating-health-care-waste-in-washington-state-dec-2018/ (accessed on 28 December 2022).
- Mafi, J.N.; Russell, K.; Bortz, B.A.; Dachary, M.; Hazel, W.A., Jr.; Fendrick, A.M. Low-Cost, High-Volume Health Services Contribute The Most To Unnecessary Health Spending. Health Aff. 2017, 36, 1701–1704. [Google Scholar] [CrossRef]
- Bilinski, K.; Boyages, S. The Vitamin D paradox: Bone density testing in females aged 45 to 74 did not increase over a ten-year period despite a marked increase in testing for vitamin D. J. Endocrinol. Investig. 2013, 36, 914–922. [Google Scholar]
- Koch, C.; Roberts, K.; Petruccelli, C.; Morgan, D.J. The Frequency of Unnecessary Testing in Hospitalized Patients. Am. J. Med. 2018, 131, 500–503. [Google Scholar] [CrossRef]
- Felcher, A.H.; Gold, R.; Mosen, D.M.; Stoneburner, A.B. Decrease in unnecessary vitamin D testing using clinical decision support tools: Making it harder to do the wrong thing. J. Am. Med. Inform. Assoc. 2017, 24, 776–780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naugler, C.; Hemmelgarn, B.; Quan, H.; Clement, F.; Sajobi, T.; Thomas, R.; Turin, T.C.; Hnydyk, W.; Chin, A.; Wesenberg, J. Implementation of an intervention to reduce population-based screening for vitamin D deficiency: A cross-sectional study. CMAJ Open 2017, 5, E36–E39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson, L.F.; Xu, Z.; Mishra, G.D.; Dobson, A.J.; Doust, J. Did changes to recommended testing criteria affect the rate of vitamin D testing among Australian women. Arch. Osteoporos. 2020, 15, 020–00840. [Google Scholar] [CrossRef] [PubMed]
- Thommasen, A.; Clement, F.; Kinniburgh, D.W.; Lau, C.K.; Guo, M.; Viczko, J.; Guggisberg, K.; Thomas, R.E.; Turin, T.C.; Wesenberg, J.C.; et al. Canadian family physician knowledge and attitudes toward laboratory utilization management. Clin. Biochem. 2016, 49, 4–7. [Google Scholar] [CrossRef]
| Vitamin D Test Performed | |||
|---|---|---|---|
| Characteristics, % | No (n = 19,654) | Yes (n = 1753) | p Value |
| Age, years | <0.001 | ||
| 18–34 | 45.1 | 7.9 | |
| 35–64 | 32.9 | 41.2 | |
| ≥65 | 22.0 | 50.9 | |
| Year of admission | 0.014 | ||
| 2016 | 38.4 | 41.8 | |
| 2017 | 32.8 | 31.7 | |
| 2018 | 28.8 | 26.5 | |
| Race | 0.003 | ||
| White | 83.0 | 84.9 | |
| Black | 6.3 | 7.0 | |
| Other | 4.7 | 3.0 | |
| Unknown | 6.0 | 5.0 | |
| Type of insurance | <0.001 | ||
| Public | 57.6 | 75.6 | |
| Private | 31.6 | 15.2 | |
| Uninsured | 10.8 | 9.2 | |
| Marital status | <0.001 | ||
| Single | 38.8 | 27.2 | |
| Married | 40.7 | 38.8 | |
| Divorced/separated/widowed | 16.6 | 31.0 | |
| Other/unknown | 3.9 | 3.1 | |
| Vitamin D supplement intake | 4.5 | 35.7 | |
| Prevalent medical conditions | |||
| Cardiovascular disease | 24.9 | 48.7 | <0.001 |
| Diabetes | 6.5 | 13.7 | <0.001 |
| Cancer | 4.0 | 5.8 | 0.001 |
| Chronic obstructive pulmonary disease | 3.2 | 5.2 | <0.001 |
| Clinical indications for vitamin D testing | 75.7 | 60.5 | <0.001 |
| Lymphoma | 0.2 | 0.5 | 0.138 |
| Osteoporosis | 0.1 | 0.3 | 0.006 |
| Chronic kidney disease | 1.4 | 16.5 | <0.001 |
| Liver disease | 2.2 | 4.1 | <0.001 |
| Pancreatic insufficiency | 0.4 | 0.6 | 0.260 |
| Pregnancy-related hospitalization | 41.4 | 2.2 | <0.001 |
| Obesity | 52.9 | 45.8 | <0.001 |
| Malabsorption syndromes | 0.6 | 0.9 | 0.109 |
| Potentially Inappropriate Testing | |||
|---|---|---|---|
| Characteristics, % | No (n = 1059) | Yes (n = 693) | p Value |
| Age, years | <0.001 | ||
| 18–34 | 9.5 | 5.5 | |
| 35–64 | 49.5 | 28.6 | |
| ≥ 65 | 41.0 | 65.9 | |
| Year of admission | <0.001 | ||
| 2016 | 46.8 | 34.2 | |
| 2017 | 29.8 | 34.4 | |
| 2018 | 23.3 | 31.4 | |
| Race | <0.001 | ||
| Non-White | 20.9 | 6.2 | |
| White | 79.1 | 93.8 | |
| Type of insurance | 0.002 | ||
| Public | 72.7 | 80.1 | |
| Private | 17.3 | 12.0 | |
| Uninsured | 10.0 | 7.9 | |
| Marital status | 0.007 | ||
| Single | 29.7 | 23.4 | |
| Married | 39.0 | 38.5 | |
| Divorced/separated/widowed | 28.7 | 34.3 | |
| Other/unknown | 2.6 | 3.8 | |
| Prevalent medical conditions | |||
| Cardiovascular disease | 55.8 | 37.8 | <0.001 |
| Diabetes | 16.9 | 8.9 | <0.001 |
| Cancer | 5.9 | 5.5 | 0.753 |
| Vitamin D supplement intake | 0.022 | ||
| No | 66.5 | 61.0 | |
| Yes | 33.5 | 39.0 | |
| Vitamin D deficiency | <0.001 | ||
| No | 66.6 | 81.2 | |
| Yes | 33.4 | 18.8 | |
| Died during inpatient stay | <0.001 | ||
| No | 84.4 | 92.1 | |
| Yes | 15.6 | 7.9 | |
| Variables | OR (95% CI) | p Value |
|---|---|---|
| Age group | <0.001 | |
| 18–34 years | 1 | |
| 35–64 years | 2.10 (1.45–3.06) | |
| ≥65 years | 3.07 (2.05–4.59) | |
| Race | <0.001 | |
| Non-White | 1 | |
| White | 2.71 (1.95–3.78) | |
| Marital status | 0.758 | |
| Single | 1 | |
| Married | 0.94 (0.74–1.19) | |
| Divorced/separated/widowed | 0.88 (0.68–1.12) | |
| Other/unknown | 0.89 (0.56–1.44) | |
| Type of insurance | 0.004 | |
| Private | 1 | |
| Public | 1.62 (1.20–2.17) | |
| Uninsured | 1.14 (0.79–1.65) | |
| Vitamin D supplement intake | 7.05 (5.82–8.54) | <0.001 |
| Cardiovascular disease | 0.75 (0.63–0.90) | 0.002 |
| Diabetes | 1.06 (0.78–1.43) | 0.723 |
| Cancer | 0.71 (0.50–1.03) | 0.068 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Appiah, D.; Kamrudin, S.; de Riese, C. Factors Associated with Potentially Inappropriate Screening for Vitamin D Deficiency among Women in Medically Underserved Regions of West Texas. J. Clin. Med. 2023, 12, 993. https://doi.org/10.3390/jcm12030993
Appiah D, Kamrudin S, de Riese C. Factors Associated with Potentially Inappropriate Screening for Vitamin D Deficiency among Women in Medically Underserved Regions of West Texas. Journal of Clinical Medicine. 2023; 12(3):993. https://doi.org/10.3390/jcm12030993
Chicago/Turabian StyleAppiah, Duke, Samira Kamrudin, and Cornelia de Riese. 2023. "Factors Associated with Potentially Inappropriate Screening for Vitamin D Deficiency among Women in Medically Underserved Regions of West Texas" Journal of Clinical Medicine 12, no. 3: 993. https://doi.org/10.3390/jcm12030993
APA StyleAppiah, D., Kamrudin, S., & de Riese, C. (2023). Factors Associated with Potentially Inappropriate Screening for Vitamin D Deficiency among Women in Medically Underserved Regions of West Texas. Journal of Clinical Medicine, 12(3), 993. https://doi.org/10.3390/jcm12030993





