GLI1 Deficiency Impairs the Tendon–Bone Healing after Anterior Cruciate Ligament Reconstruction: In Vivo Study Using Gli1-Transgenic Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
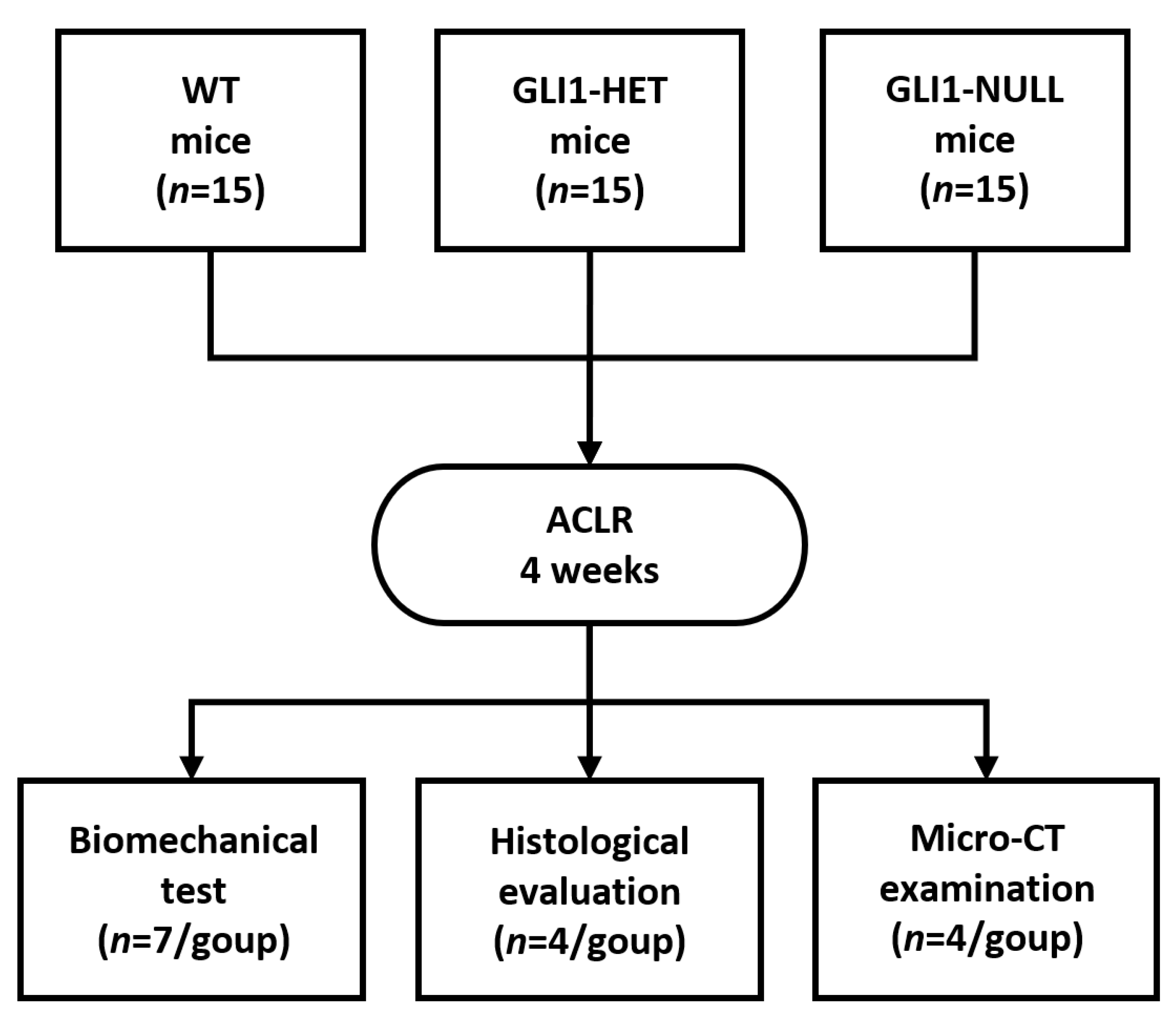
2.2. Study Design
2.3. Biomechanical Testing
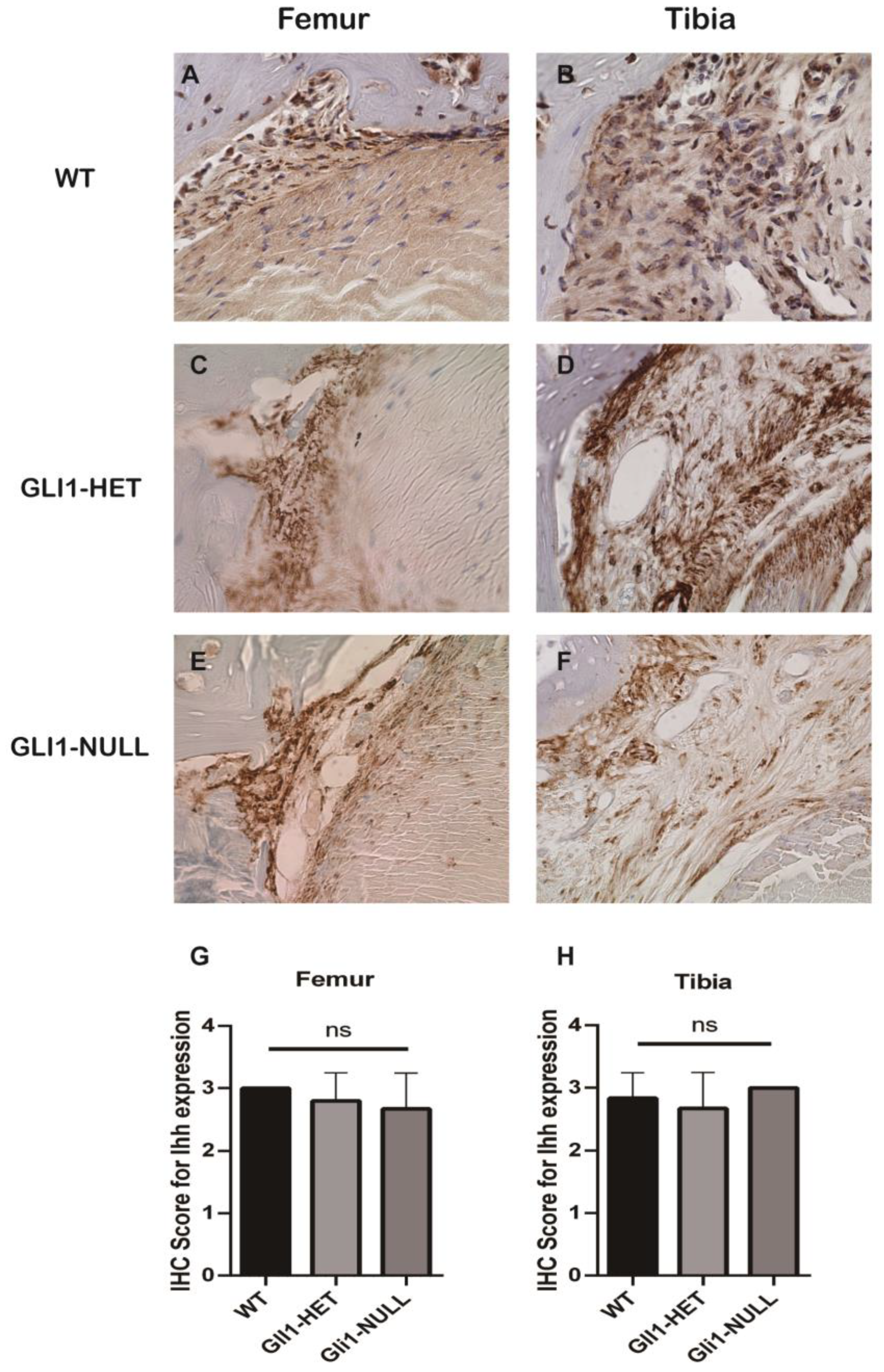
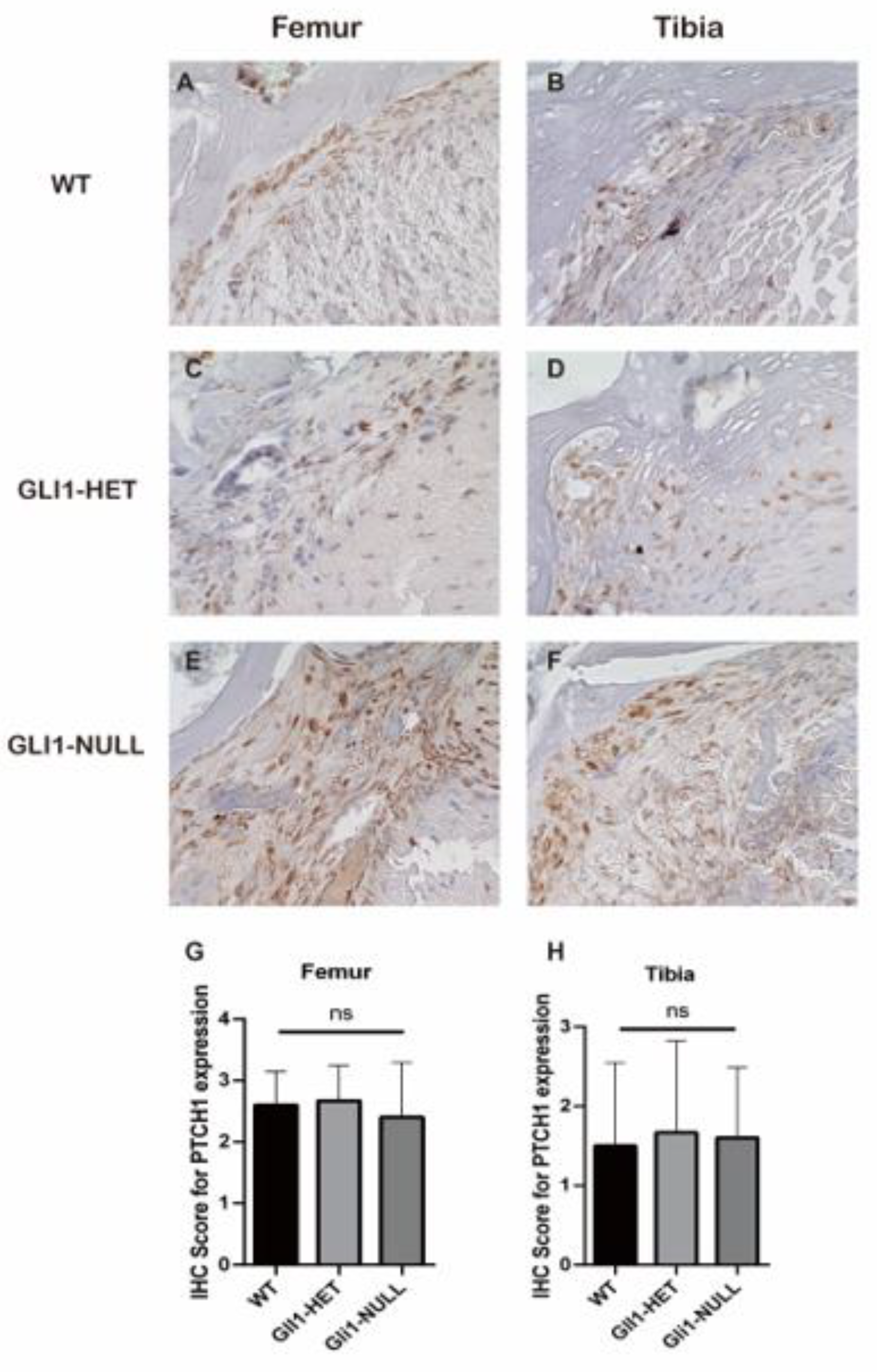
2.4. Histologic Evaluation and Immunohistochemical (IHC) Staining
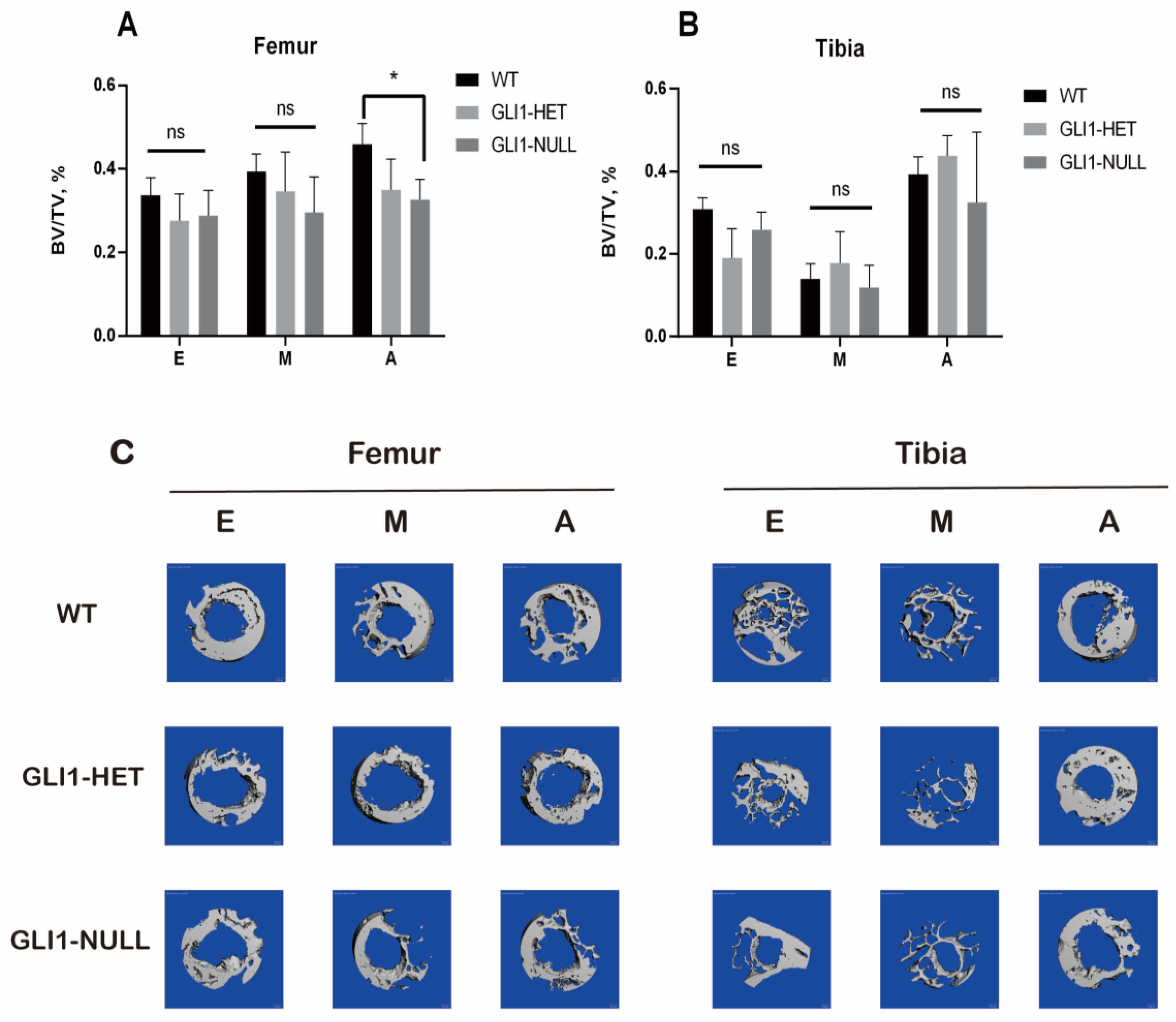
2.5. Micro-CT Examination
2.6. Statistical Analysis
3. Results
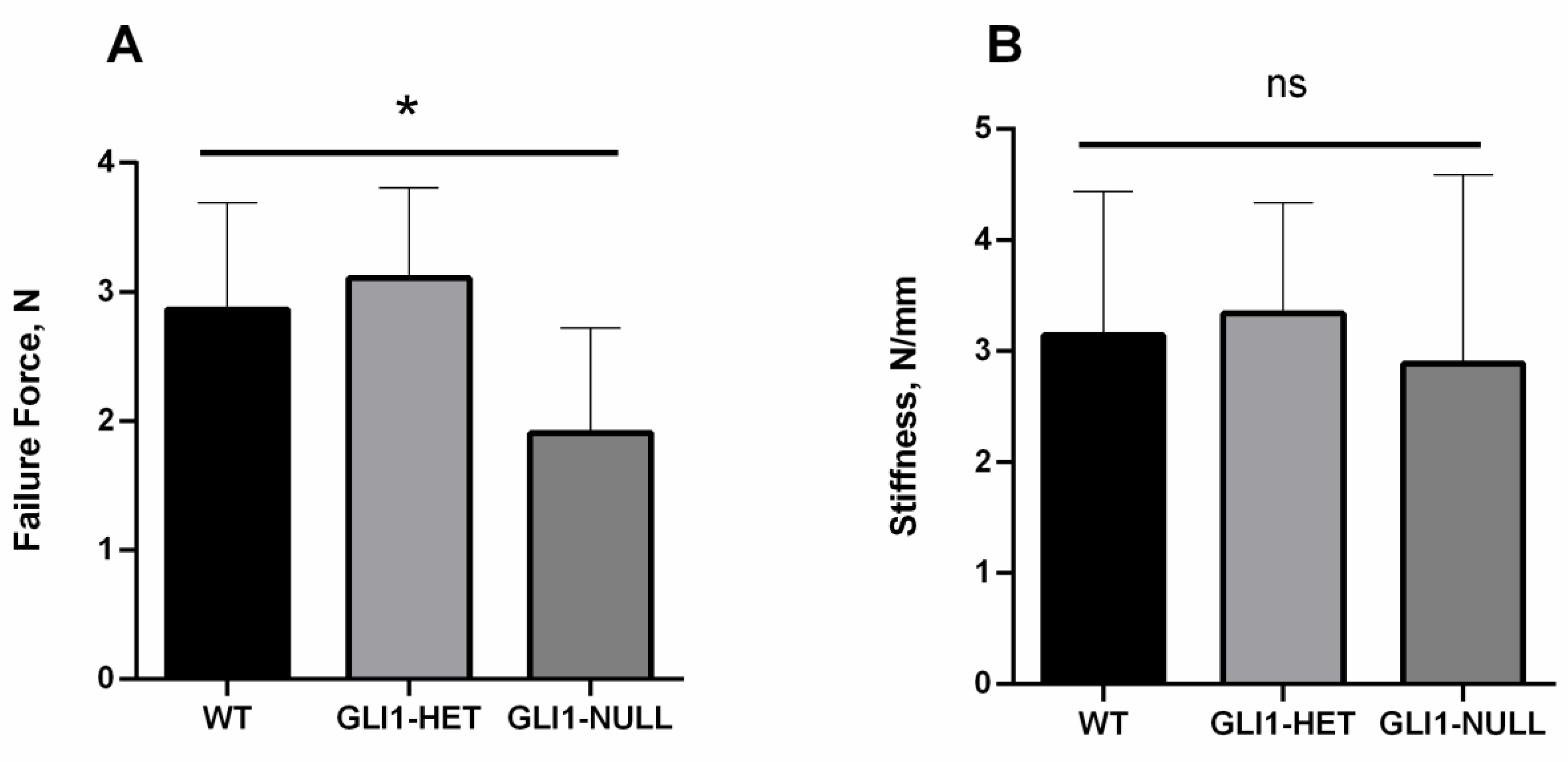
3.1. Biomechanical Evaluation
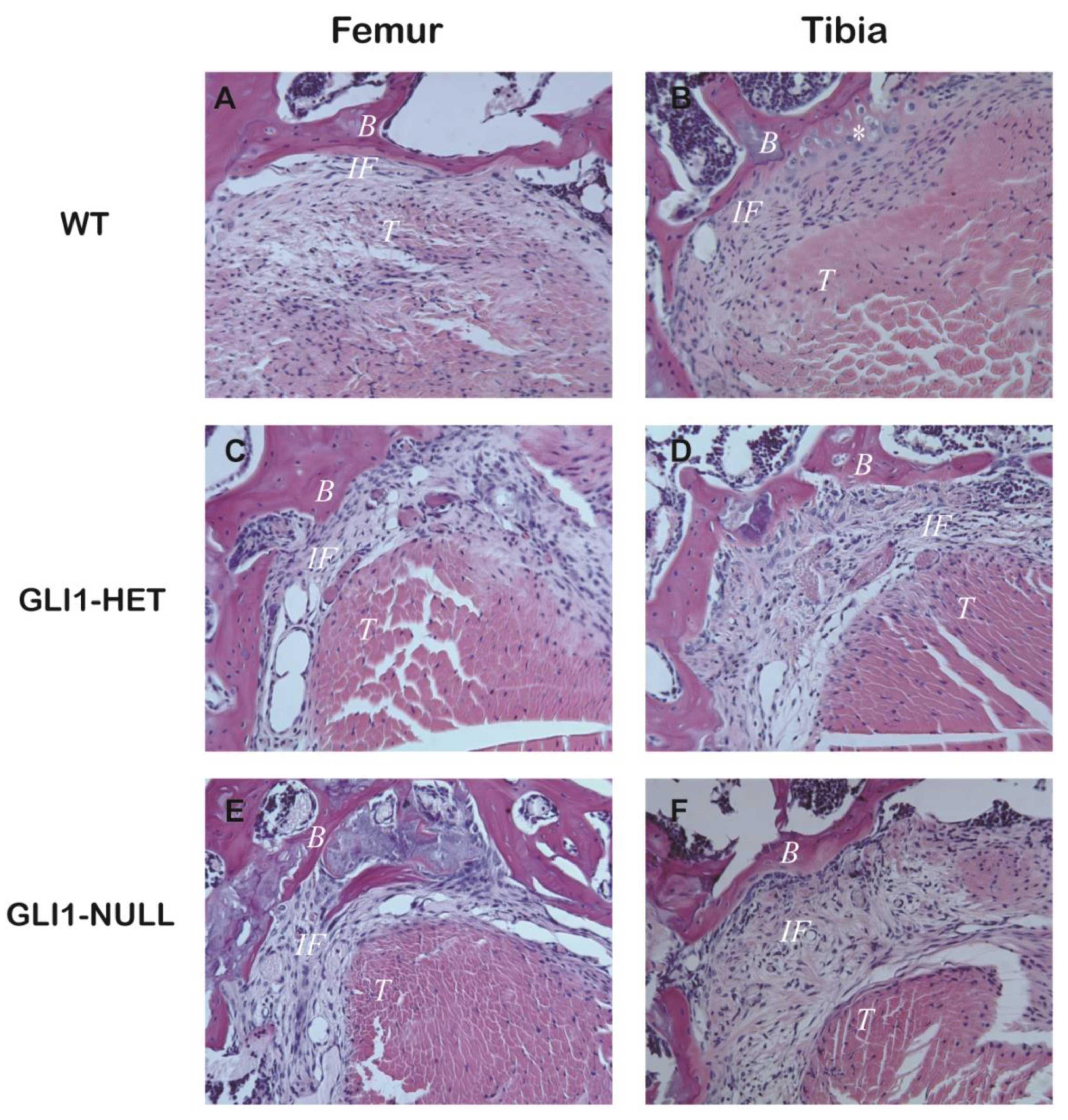
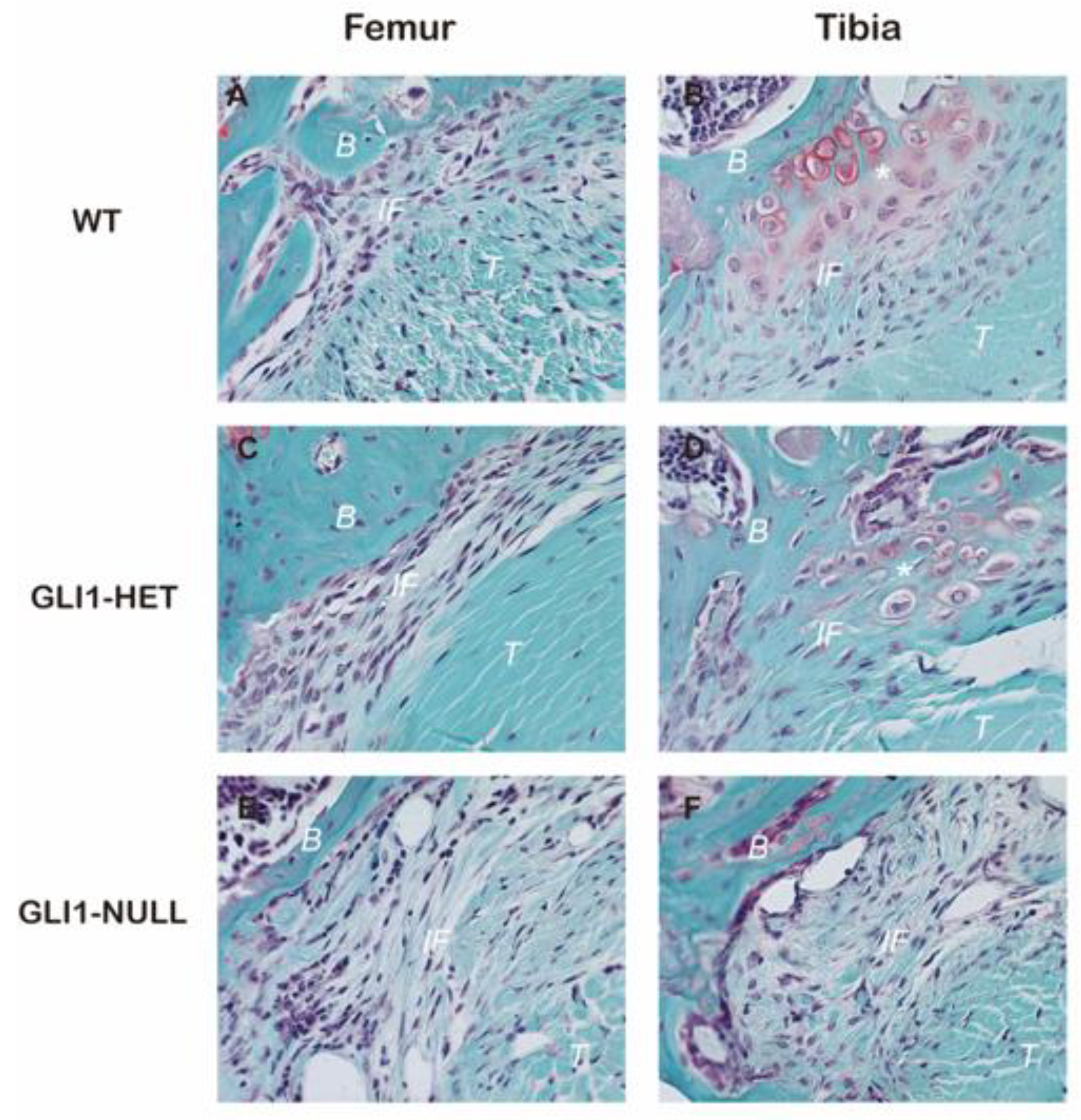
3.2. Histological Evaluation
3.3. Micro-CT
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Grassi, A.; Carulli, C.; Innocenti, M.; Mosca, M.; Zaffagnini, S.; Bait, C.; SIGASCOT Arthroscopy Committee. New Trends in Anterior Cruciate Ligament Reconstruction: A Systematic Review of National Surveys of the Last 5 Years. Joints 2018, 6, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Webster, K.E.; Feller, J.A.; Hameister, K.A. Bone tunnel enlargement following anterior cruciate ligament reconstruction: A randomised comparison of hamstring and patellar tendon grafts with 2-year follow-up. Knee Surg. Sports Traumatol. Arthrosc. 2001, 9, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Nebelung, W.; Becker, R.; Urbach, D.; Röpke, M.; Roessner, A. Histological findings of tendon-bone healing following anterior cruciate ligament reconstruction with hamstring grafts. Arch. Orthop. Trauma Surg. 2003, 123, 158–163. [Google Scholar] [CrossRef]
- Rodeo, S.A.; Arnoczky, S.P.; Torzilli, P.A.; Hidaka, C.; Warren, R.F. Tendon-healing in a bone tunnel. A biomechanical and histological study in the dog. J. Bone Joint Surg. Am. 1993, 75, 1795–1803. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, M.; McGonagle, D. Entheses: Tendon and ligament attachment sites. Scand. J. Med. Sci. Sports 2009, 19, 520–527. [Google Scholar] [CrossRef]
- Mohler, J. Requirements for hedgehog, a segmental polarity gene, in patterning larval and adult cuticle of Drosophila. Genetics 1988, 120, 1061–1072. [Google Scholar] [CrossRef]
- Schwartz, A.G. Ihh Signaling and Muscle Forces are Required for Enthesis Development. Ph.D. Thesis, Washington University, St. Louis, MO, USA, 2014. [Google Scholar]
- Schwartz, A.G.; Long, F.; Thomopoulos, S. Enthesis fibrocartilage cells originate from a population of Hedgehog-responsive cells modulated by the loading environment. Development 2015, 142, 196–206. [Google Scholar] [CrossRef]
- Liu, C.; Breidenbach, A.; Aschbacher-Smith, L.; Butler, D.; Wylie, C. A role for hedgehog signaling in the differentiation of the insertion site of the patellar tendon in the mouse. PLoS ONE 2013, 8, e65411. [Google Scholar] [CrossRef]
- Breidenbach, A.P.; Aschbacher Smith, L.; Lu, Y.; Dyment, N.A.; Liu, C.; Liu, H.; Wylie, C.; Rao, M.; Shearn, J.T.; Rowe, D.W.; et al. Ablating hedgehog signaling in tenocytes during development impairs biomechanics and matrix organization of the adult murine patellar tendon enthesis. J Orthop Res. 2015, 33, 1142–1151. [Google Scholar] [CrossRef]
- Carbone, A.; Carballo, C.; Ma, R.; Wang, H.; Deng, X.; Dahia, C.; Rodeo, S. Indian hedgehog signaling and the role of graft tension in tendon-to-bone healing: Evaluation in a rat ACL reconstruction model. J. Orthop. Res. 2016, 34, 641–649. [Google Scholar] [CrossRef]
- Zong, J.; Mosca, M.J.; Degen, R.M.; Lebaschi, A.; Carballo, C.; Carbone, A.; Cong, G.-T.; Ying, L.; Deng, X.-H.; Rodeo, S.A. Involvement of Indian hedgehog signaling in mesenchymal stem cell–augmented rotator cuff tendon repair in an athymic rat model. J. Shoulder Elb. Surg. 2017, 26, 580–588. [Google Scholar] [CrossRef] [PubMed]
- Wada, S.; Lebaschi, A.H.; Nakagawa, Y.; Caballo, C.; Uppstrom, T.; Cong, G.-T.; Album, Z.; Hall, A.; Ying, L.; Deng, X.-H.; et al. Postoperative tendon loading with treadmill running delays tendon-to-bone healing: Immunohistochemical evaluation in a murine rotator cuff repair model. J. Orthop. Res. 2019, 37, 1628–1637. [Google Scholar] [CrossRef]
- Kimura, H.; Stephen, D.; Joyner, A.; Curran, T. Gli1 is important for medulloblastoma formation in Ptc1+/− mice. Oncogene 2005, 24, 4026–4036. [Google Scholar] [CrossRef] [PubMed]
- Camp, C.L.; Lebaschi, A.; Cong, G.; Album, Z.; Carballo, C.; Deng, X.-H.; Rodeo, S.A. Timing of postoperative mechanical loading affects healing following anterior cruciate ligament reconstruction: Analysis in a murine model. J. Bone Joint Surg. 2017, 99, 1382–1391. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Lebaschi, A.; Camp, C.L.; Carballo, C.; Coleman, N.; Zong, J.; Grawe, B.; Rodeo, S. Expression of signaling molecules involved in embryonic development of the insertion site is inadequate for reformation of the native enthesis: Evaluation in a novel murine ACL reconstruction model. J. Bone Joint Surg. Am. Vol. 2018, 100, e102. [Google Scholar] [CrossRef] [PubMed]
- Jang, K.; Lim, H.C.; Jung, W.Y.; Moon, S.W.; Wang, J.H. Efficacy and safety of human umbilical cord blood–derived mesenchymal stem cells in anterior cruciate ligament reconstruction of a rabbit model: New strategy to enhance tendon graft healing. Arthrosc. J. Arthrosc. Relat. Surg. 2015, 31, 1530–1539. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Sun, Y.; Wan, F.; Ding, Z.; Chen, S.; Chen, J. Advantages of an Attached Semitendinosus Tendon Graft in Anterior Cruciate Ligament Reconstruction in a Rabbit Model. Am. J. Sports Med. 2018, 46, 3227–3236. [Google Scholar] [CrossRef]
- Varghese, F.; Bukhari, A.B.; Malhotra, R.; De, A. IHC Profiler: An open source plugin for the quantitative evaluation and automated scoring of immunohistochemistry images of human tissue samples. PLoS ONE 2014, 9, e96801. [Google Scholar] [CrossRef]
- Sun, Y.; Guo, W.; Ren, T.; Liang, W.; Zhou, W.; Lu, Q.; Jiao, G.; Yan, T. Gli1 inhibition suppressed cell growth and cell cycle progression and induced apoptosis as well as autophagy depending on ERK1/2 activity in human chondrosarcoma cells. Cell Death Dis. 2014, 5, e979. [Google Scholar] [CrossRef]
- Yu, H.; Fu, F.; Yao, S.; Luo, H.; Xu, T.; Jin, H.; Tong, P.; Chen, D.; Wu, C.; Ruan, H. Biomechanical, histologic, and molecular characteristics of graft-tunnel healing in a murine modified ACL reconstruction model. J. Orthop. Transl. 2020, 24, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Berg, E.E.; Pollard, M.E.; Kang, Q. Interarticular bone tunnel healing. Arthrosc. J. Arthrosc. Relat. Surg. 2001, 17, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Nakase, J.; Kitaoka, K.; Toratani, T.; Kosaka, M.; Ohashi, Y.; Tsuchiya, H. Grafted tendon healing in femoral and tibial tunnels after anterior cruciate ligament reconstruction. J. Orthop. Surg. 2014, 22, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Rodeo, S.A.; Kawamura, S.; Kim, H.; Dynybil, C.; Ying, L. Tendon healing in a bone tunnel differs at the tunnel entrance versus the tunnel exit: An effect of graft-tunnel motion? Am. J. Sports Med. 2006, 34, 1790–1800. [Google Scholar] [CrossRef] [PubMed]
- Hettrich, C.M.; Gasinu, S.; Beamer, B.S.; Stasiak, M.; Fox, A.; Birmingham, P.; Ying, O.; Deng, X.-H.; Rodeo, S.A. The effect of mechanical load on tendon-to-bone healing in a rat model. Am. J. Sports Med. 2014, 42, 1233–1241. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, E.; Fukuda, Y.; Loh, J.C.; Debski, R.E.; Fu, F.H.; Woo, S.L. The effect of soft-tissue graft fixation in anterior cruciate ligament reconstruction on graft-tunnel motion under anterior tibial loading. Arthroscopy 2002, 18, 960–967. [Google Scholar] [CrossRef]
- Weiler, A.; Unterhauser, F.N.; Faensen, B.; Hunt, P.; Bail, H.J.; Haas, N.P. Comparison of tendon-to-bone healing using extracortical and anatomic interference fit fixation of soft tissue grafts in a sheep model of ACL reconstruction. In Proceedings of the Transactions of the Annual Meeting-Orthopaedic Research Society, Dallas, TX, USA, 10–13 February 2002. [Google Scholar]
- Yamakado, K.; Kitaoka, K.; Yamada, H.; Hashiba, K.; Nakamura, R.; Tomita, K. The influence of mechanical stress on graft healing in a bone tunnel. Arthrosc. J. Arthrosc. Relat. Surg. 2002, 18, 82–90. [Google Scholar] [CrossRef] [PubMed]



| Failure Mode | WT | GLI1-HET | GLI1-NULL |
|---|---|---|---|
| Pull out from femur | 5 | 6 | 6 |
| Pull out from tibia | 1 | 0 | 1 |
| Midsubstance failure | 1 | 1 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Liu, S.; Song, Z.; Chen, D.; Album, Z.; Green, S.; Deng, X.; Rodeo, S.A. GLI1 Deficiency Impairs the Tendon–Bone Healing after Anterior Cruciate Ligament Reconstruction: In Vivo Study Using Gli1-Transgenic Mice. J. Clin. Med. 2023, 12, 999. https://doi.org/10.3390/jcm12030999
Liu Y, Liu S, Song Z, Chen D, Album Z, Green S, Deng X, Rodeo SA. GLI1 Deficiency Impairs the Tendon–Bone Healing after Anterior Cruciate Ligament Reconstruction: In Vivo Study Using Gli1-Transgenic Mice. Journal of Clinical Medicine. 2023; 12(3):999. https://doi.org/10.3390/jcm12030999
Chicago/Turabian StyleLiu, Yake, Shaohua Liu, Zhe Song, Daoyun Chen, Zoe Album, Samuel Green, Xianghua Deng, and Scott A. Rodeo. 2023. "GLI1 Deficiency Impairs the Tendon–Bone Healing after Anterior Cruciate Ligament Reconstruction: In Vivo Study Using Gli1-Transgenic Mice" Journal of Clinical Medicine 12, no. 3: 999. https://doi.org/10.3390/jcm12030999
APA StyleLiu, Y., Liu, S., Song, Z., Chen, D., Album, Z., Green, S., Deng, X., & Rodeo, S. A. (2023). GLI1 Deficiency Impairs the Tendon–Bone Healing after Anterior Cruciate Ligament Reconstruction: In Vivo Study Using Gli1-Transgenic Mice. Journal of Clinical Medicine, 12(3), 999. https://doi.org/10.3390/jcm12030999




