Impact and Modification of the New PJI-TNM Classification for Periprosthetic Joint Infections
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Treatment Regimen
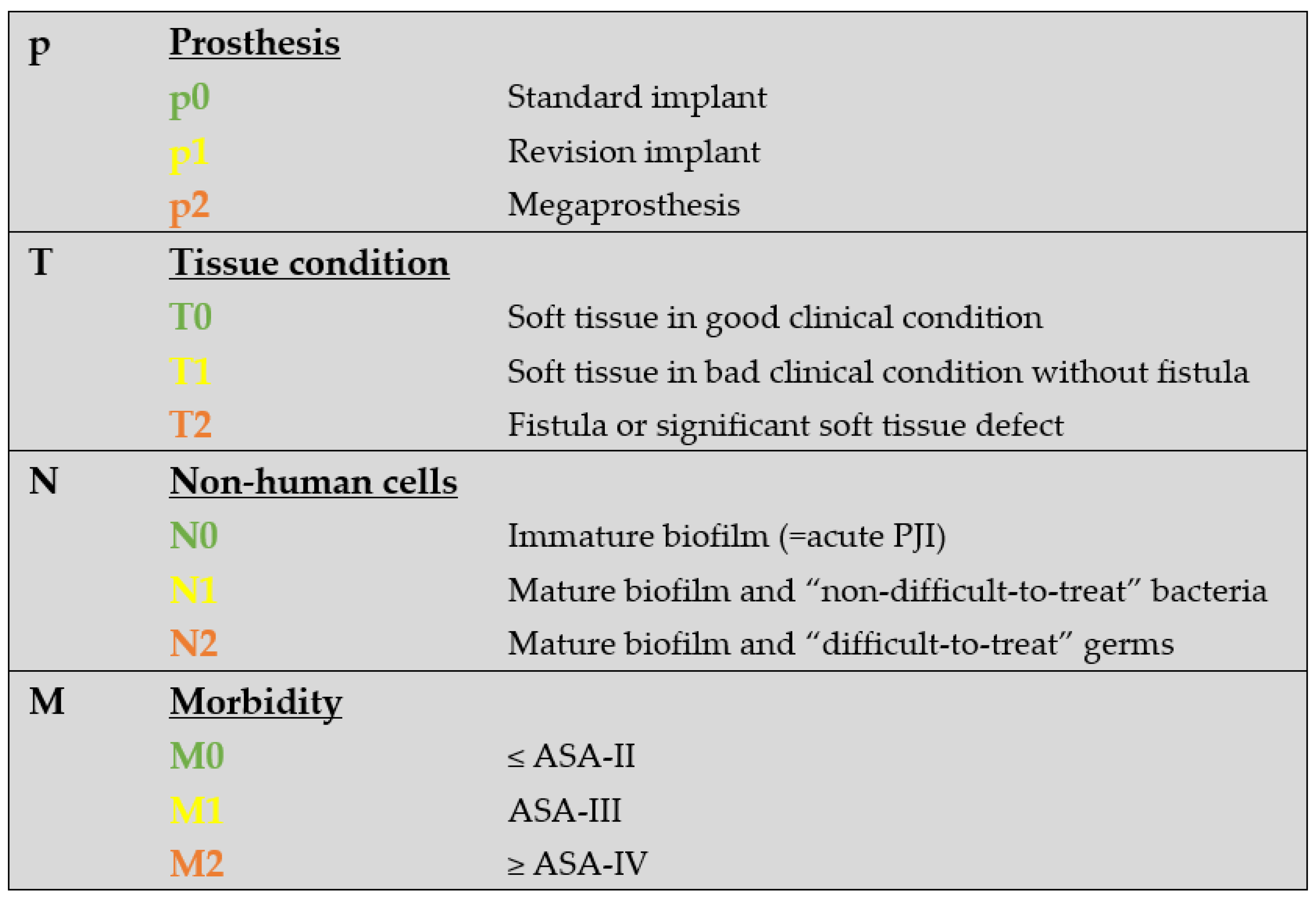
2.2. The PJI-TNM Classification
2.3. Therapy and Outcome Parameters
2.4. Statistical Analysis
3. Results
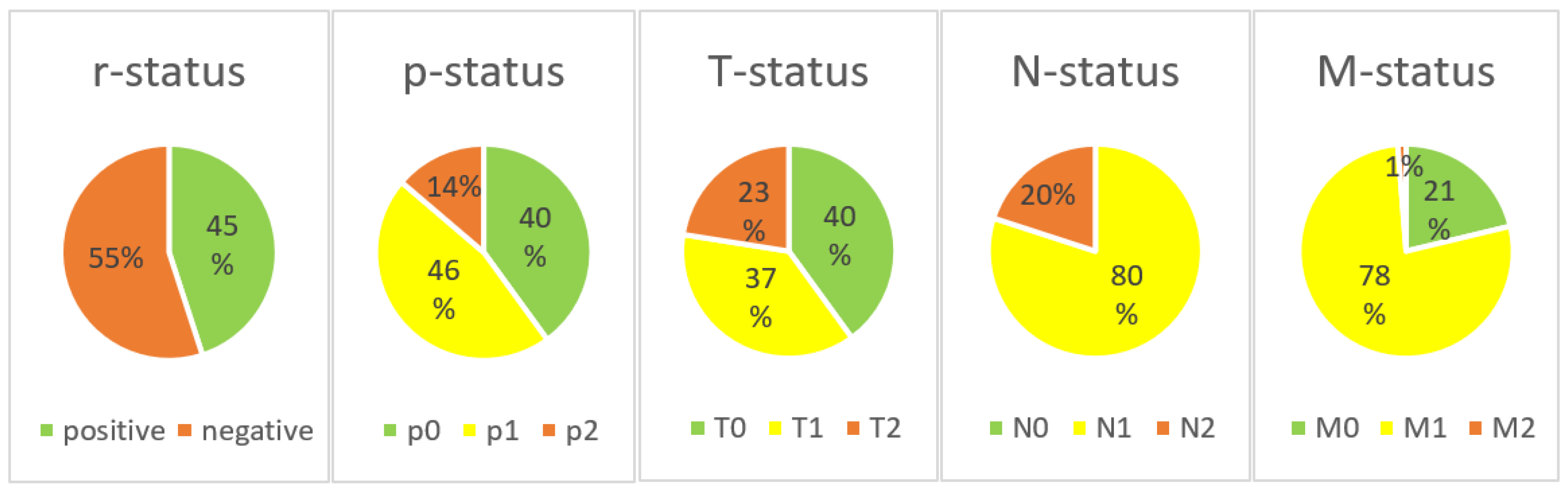
3.1. The PJI-TNM Classification
3.2. Therapy and Outcome Parameters
3.3. Correlation between “PJI-TNM” and Therapy/Outcome Parameters
3.4. Modification of the Original Classification Resulting in “PJI-pTNM”
3.5. Correlation between Modified PJI-pTNM and Therapy/Outcome Parameters
4. Discussion
4.1. The Modified PJI-pTNM Classification
4.2. Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rupp, M.; Lau, E.; Kurtz, S.M.; Alt, V. Projections of Primary TKA and THA in Germany From 2016 Through 2040. Clin. Orthop. Relat. Res. 2020, 478, 1622. [Google Scholar] [CrossRef]
- Sukeik, M.; Haddad, F.S. Periprosthetic joint infections after total hip replacement: An algorithmic approach. SICOT J. 2019, 5, 5. [Google Scholar] [CrossRef]
- Phillips, J.E.; Crane, T.P.; Noy, M.; Elliott, T.S.; Grimer, R.J. The incidence of deep prosthetic infections in a specialist orthopaedic hospital: A 15-year prospective survey. J. Bone Jt. Surg. Br. 2006, 88, 943–948. [Google Scholar] [CrossRef]
- Dale, H.; Fenstad, A.M.; Hallan, G.; Havelin, L.I.; Furnes, O.; Overgaard, S.; Pedersen, A.B.; Kärrholm, J.; Garellick, G.; Pulkkinen, P.; et al. Increasing risk of prosthetic joint infection after total hip arthroplasty. Acta Orthop. 2012, 83, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Sukeik, M.; Haddad, F.S. Two-stage procedure in the treatment of late chronic hip infections--spacer implantation. Int. J. Med. Sci. 2009, 6, 253–257. [Google Scholar] [CrossRef] [PubMed]
- Dombrowski, M.E.; Wilson, A.E.; Wawrose, R.A.; O’Malley, M.J.; Urish, K.L.; Klatt, B.A. A Low Percentage of Patients Satisfy Typical Indications for Single-stage Exchange Arthroplasty for Chronic Periprosthetic Joint Infection. Clin. Orthop. Relat. Res. 2020, 478, 1780–1786. [Google Scholar] [CrossRef]
- Palmer, J.R.; Pannu, T.S.; Villa, J.M.; Manrique, J.; Riesgo, A.M.; Higuera, C.A. The treatment of periprosthetic joint infection: Safety and efficacy of two stage versus one stage exchange arthroplasty. Expert Rev. Med. Devices 2020, 17, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Kildow, B.J.; Della-Valle, C.J.; Springer, B.D. Single vs. 2-Stage Revision for the Treatment of Periprosthetic Joint Infection. J. Arthroplasty 2020, 35, S24–S30. [Google Scholar] [CrossRef] [PubMed]
- Anagnostakos, K.; Fink, B. Antibiotic-loaded cement spacers—Lessons learned from the past 20 years. Expert Rev. Med. Devices 2018, 15, 231–245. [Google Scholar] [CrossRef]
- Zmistowski, B.; Karam, J.A.; Durinka, J.B.; Casper, D.S.; Parvizi, J. Periprosthetic joint infection increases the risk of one-year mortality. J. Bone Jt. Surg. Am. 2013, 95, 2177–2184. [Google Scholar] [CrossRef]
- Alt, V.; Rupp, M.; Langer, M.; Baumann, F.; Trampuz, A. Can the oncology classification system be used for prosthetic joint infection? The PJI-TNM system. Bone Jt. Res. 2020, 9, 79–81. [Google Scholar] [CrossRef] [PubMed]
- Rupp, M.; Kerschbaum, M.; Freigang, V.; Bärtl, S.; Baumann, F.; Trampuz, A.; Alt, V. PJI-TNM as new classification system for periprosthetic joint infections: An evaluation of 20 cases. Orthopade 2021, 50, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Parvizi, J.; Zmistowski, B.; Berbari, E.F.W.; Bauer, T.; Springer, B.D.; Della Valle, C.J.; Garvin, K.L.; Wongworawat, M.D.; Fehring, T.K.; Osmon, D.R.; et al. New definition for periprosthetic joint infection. J. Arthroplasty 2011, 26, 1136–1138. [Google Scholar]
- Parvizi, J.; Gehrke, T.; Chen, A.F. Proceedings of the International Consensus on Periprosthetic Joint Infection. Bone Jt. J. 2013, 95-B, 1450–1452. [Google Scholar] [CrossRef] [PubMed]
- Muhlhofer, H.M.L.; Knebel, C.; Pohlig, F.; Feihl, S.; Harrasser, N.; Schauwecker, J.; von Eisenhart-Rothe, R. Synovial aspiration and serological testing in two-stage revision arthroplasty for prosthetic joint infection: Evaluation before reconstruction with a mean follow-up of twenty seven months. Int. Orthop. 2018, 42, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Preininger, B.; Janz, V.; von Roth, P.; Trampuz, A.; Perka, C.F.; Pfitzner, T. Inadequacy of Joint Aspiration for Detection of Persistent Periprosthetic Infection During Two-Stage Septic Revision Knee Surgery. Orthopedics 2017, 40, 231–234. [Google Scholar] [CrossRef]
- Tan, T.L.; Kheir, M.M.; Rondon, A.J.; Parvizi, J.; George, J.; Higuera, C.A.; Shohat, N.; Chen, A.F. Determining the Role and Duration of the “Antibiotic Holiday” Period in Periprosthetic Joint Infection. J. Arthroplasty 2018, 33, 2976–2980. [Google Scholar] [CrossRef]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Charlson, M.; Szatrowski, T.P.; Peterson, J.; Gold, J. Validation of a combined comorbidity index. J. Clin. Epidemiol. 1994, 47, 1245–1251. [Google Scholar] [CrossRef]
- Engh, G.A.; Parks, N.L. The management of bone defects in revision total knee arthroplasty. Instr. Course Lect. 1997, 46, 227–236. [Google Scholar]
- Keats, A.S. The ASA classification of physical status—A recapitulation. Anesthesiology 1978, 49, 233–236. [Google Scholar] [CrossRef] [PubMed]
- Tsukayama, D.T.; Estrada, R.; Gustilo, R.B. Infection after total hip arthroplasty. A study of the treatment of one hundred and six infections. J. Bone Jt. Surg. Am. 1996, 78, 512–523. [Google Scholar] [CrossRef] [PubMed]
- Izakovicova, P.; Borens, O.; Trampuz, A. Periprosthetic joint infection: Current concepts and outlook. EFORT Open Rev. 2019, 4, 482–494. [Google Scholar] [CrossRef] [PubMed]



| R | If the Infection Involves a Previously Infected Implant, the Situation Is Considered as “Reinfection” and an “r” Is Put in Front of the Classification | ||
|---|---|---|---|
| T | T0 | a | Stable standard implant without important soft tissue defect |
| b | Stable revision implant without important soft tissue defect | ||
| T1 | a | Loosened standard implant without important soft tissue defect | |
| b | Loosened revision implant without important soft tissue defect | ||
| T2 | a | Severe soft tissue defect with standard implant | |
| b | Severe soft tissue defect with revision implant | ||
| N | N0 | a | No mature biofilm formation (former: acute), directly postoperatively |
| b | No mature biofilm formation (former: acute), late hematogenous | ||
| N1 | a | Mature biofilm formation (former: chronic) w/o “difficult-to-treat bacteria” | |
| b | Mature biofilm formation (former: chronic) with culture-negative infection | ||
| N2 | a | Mature biofilm formation (former: chronic) with “difficult-to-treat bacteria” | |
| b | Mature biofilm formation (former: chronic) with polymicrobial infection | ||
| c | Mature biofilm formation (former: chronic) with fungi | ||
| M | M0 | Not or only mildly compromised (Charlson Comorbidity Index: 0–1) | |
| M1 | Moderately compromised patient (Charlson Comorbidity Index: 2–3) | ||
| M2 | Severely compromised patient (Charlson Comorbidity Index 4–5) | ||
| M3 | a | Patient refuses surgical treatment | |
| b | Patient does not benefit from surgical treatment | ||
| c | Patient does not survive surgical treatment |
| Therapy and Outcome Parameters |
|---|
| Mortality (survived or died) |
| Type of spacer used (articulating or static) |
| Reimplantation at second-stage surgery (yes or no) |
| Unplanned revision surgery during interim period (yes or no) |
| First-stage surgery: duration of surgery (min), blood loss (ml), bone loss (AORI) |
| Duration of interim period (days) |
| r-Status | T-Status | N-Status | M-Status | |
|---|---|---|---|---|
| Mortality (yes, no) | p = 0.706 | p = 0.61 | p = 0.77 | p < 0.01 *; V = 0.37 |
| Spacer (articulating, static) | p < 0.01 *; φ = 0.29 | p = 0.11 | p = 0.18 | p = 0.74 |
| Reimplantation (yes, no) | p = 0.20 | p = 0.04 *; V = 0.28 | p = 0.67 | p = 0.63 |
| Duration of 1st stage (min) | p < 0.01 *; r = 0.43 | p = 0.18 | p = 0.08 | p = 0.42 |
| Blood loss at 1st stage (mL) | p = 0.09 | p = 0.04 *; r = 0.24 | p = 0.15 | p = 0.67 |
| Bone loss at 1st stage | p < 0.01 *; r = 0.32 | p = 0.02 *; r = 0.26 | p = 0.65 | p = 0.92 |
| Spacer revision (yes, no) | p = 0.25 | p = 0.95 | p = 0.12 | p = 0.90 |
| Interim period (days) | p = 0.24 | p = 0.29 | p = 0.98 | p = 0.55 |
| p-Status | T-Status | N-Status | M-Status | |
|---|---|---|---|---|
| Mortality (yes, no) | p = 0.09 | p = 0.33 | p = 0.60 | p = 0.04 *; φ = 0.23 |
| Spacer (art., static) | p < 0.01 *; φ = 0.62 | p = 0.04 *; φ = 0.23 | p = 0.04 *; φ = 0.23 | p = 0.03 *; φ = 0.25 |
| Reimplantation (yes, no) | p = 0.74 | p = 0.01 *; φ = 0.28 | p = 0.35 | p = 0.10 |
| Duration of 1st stage (min) | p < 0.01 *; r = 0.37 | p = 0.10 | p = 0.01 *; r = 0.31 | p = 0.72 |
| Blood loss at 1st stage (mL) | p < 0.01 *; r = 0.36 | p < 0.01 *; r = 0.32 | p = 0.58 | p = 0.16 |
| Bone loss at 1st stage (AORI) | p < 0.01 *; r = 0.69 | p = 0.04 *; r = 0.23 | p = 0.84 | p = 0.37 |
| Revision of spacer (yes, no) | p = 0.47 | p = 0.76 | p = 0.02 *; φ = 0.28 | p = 0.50 |
| Interim period (days) | p = 0.37 | p = 0.045 *; r = 0.25 | p = 0.02 *; r = 0.29 | p = 0.16 |
| No. | Mod. PJI-pTNM | Original PJI-TNM |
|---|---|---|
| 1 | p0T0N1M0 (4×) | T0aN1aM0, T0aN2bM0, T1aN1bM0 (2×) |
| 2 | p0T0N1M1 (12×) | T0aN1aM1, T0aN1aM2 (3×), T0aN1bM2, T0aN2bM1, T1aN1aM0, T1aN1aM1 (3×), T1aN1aM2, T1aN1bM0 |
| 3 | p0T0N2M1 (3×) | T0aN2aM0, T0aN2aM1, T1aN2bM0 |
| 4 | p0T1N1M0 (3×) | T0aN1bM0, T1aN1aM0, T1aN1bM0 |
| 5 | p0T1N1M1 (3×) | T0aN1aM0, T0aN1bM1, T1bN1aM1 |
| 6 | p0T1N2M1 | T0aN2aM0 |
| 7 | p0T2N1M1 (4×) | T2aN1aM1 (2×), T2aN1bM0, T2aN2bM1 |
| 8 | p0T2N1M2 | T2aN1bM2 |
| 9 | p1T0N1M1 (6×) | T0bN1aM1 (2×), T0bN1aM2, T1bN1aM1, T1bN1bM0, T1bN1bM1 |
| 10 | p1T0N2M1 (2×) | T0bN2aM0, T0bN2aM1 |
| 11 | p1T1N1M0 (5×) | T0bN1bM0 (2×), T1bN1bM0 (2×), T1bN2bM0 |
| 12 | p1T1N1M1 (13×) | T0bN1aM0, T0bN1bM1 (3×), T1bN1aM0, T1bN1bM0 (4×), T1bN1bM1, T1bN2bM0 (2×), T1bN2bM1 |
| 13 | p1T1N2M0 (2×) | T0bN2aM0, T0bN2aM1 |
| 14 | p1T1N2M1 (2×) | T0bN2bM2, T1bN2aM1 |
| 15 | p1T2N1M1 (3×) | T2bN1aM1, T2bN1aM2, T2bN2bM0 |
| 16 | p1T2N2M0 | T2bN2aM0 |
| 17 | p1T2N2M1 (4×) | T2bN2aM0, T2bN2aM1, T2bN2aM1, T2bN2bM0 |
| 18 | p2T0N2M0 | T0bN2aM0 |
| 19 | p2T0N2M1 | T1bN2aM2 |
| 20 | p2T1N1M0 | T1bN1aM1 |
| 21 | p2T1N1M1 (3×) | T0bN1aM2, T1bN1bM0 (2×) |
| 22 | p2T2N1M0 | T2bN1aM0 |
| 23 | p2T2N1M1 (2×) | T2bN1aM1 (2×) |
| 24 | p2T2N1M1 | T2bN1aM0 |
| 25 | p2T2N2M1 | T2bN2bM0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lunz, A.; Lehner, B.; Voss, M.N.; Knappe, K.; Jaeger, S.; Innmann, M.M.; Renkawitz, T.; Omlor, G.W. Impact and Modification of the New PJI-TNM Classification for Periprosthetic Joint Infections. J. Clin. Med. 2023, 12, 1262. https://doi.org/10.3390/jcm12041262
Lunz A, Lehner B, Voss MN, Knappe K, Jaeger S, Innmann MM, Renkawitz T, Omlor GW. Impact and Modification of the New PJI-TNM Classification for Periprosthetic Joint Infections. Journal of Clinical Medicine. 2023; 12(4):1262. https://doi.org/10.3390/jcm12041262
Chicago/Turabian StyleLunz, Andre, Burkhard Lehner, Moritz N. Voss, Kevin Knappe, Sebastian Jaeger, Moritz M. Innmann, Tobias Renkawitz, and Georg W. Omlor. 2023. "Impact and Modification of the New PJI-TNM Classification for Periprosthetic Joint Infections" Journal of Clinical Medicine 12, no. 4: 1262. https://doi.org/10.3390/jcm12041262
APA StyleLunz, A., Lehner, B., Voss, M. N., Knappe, K., Jaeger, S., Innmann, M. M., Renkawitz, T., & Omlor, G. W. (2023). Impact and Modification of the New PJI-TNM Classification for Periprosthetic Joint Infections. Journal of Clinical Medicine, 12(4), 1262. https://doi.org/10.3390/jcm12041262







