Asymmetry of Gray- and White-Matter Volume and Metabolites in the Central-Vestibular System in Healthy Individuals
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Otological Assessment
2.3. Image Acquisition
2.4. Magnetic-Resonance-Spectroscopy Acquisition
2.5. Spectral Quantification of MRS Data
2.6. Image Processing and Analysis
2.7. Statistical Analysis
3. Results
3.1. Brain-Tissue Volume
3.2. Brain Metabolites of MRS
3.3. Partial Correlation between GMV and Brain Metabolites
4. Discussion
4.1. Comparison with Previous Studies
4.2. Significance of Vestibular GMV and Brain Metabolites in PIVC Corresponding to Vestibular Cortex
4.3. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dieterich, M.; Bense, S.; Lutz, S.; Drzezga, A.; Stephan, T.; Bartenstein, P.; Brandt, T. Dominance for vestibular cortical function in the non-dominant hemisphere. Cereb. Cortex 2003, 13, 994–1007. [Google Scholar] [CrossRef] [PubMed]
- Lopez, C.; Blanke, O. The thalamocortical vestibular system in animals and humans. Brain Res. Rev. 2011, 67, 119–146. [Google Scholar] [CrossRef]
- Bense, S.; Bartenstein, P.; Lochmann, M.; Schlindwein, P.; Brandt, T.; Dieterich, M. Metabolic changes in vestibular and visual cortices in acute vestibular neuritis. Ann. Neurol. 2004, 56, 624–630. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, E.; Roediger, D.; Kucukboyaci, N.E.; Carlson, C.; Devinsky, O.; Kuzniecky, R.; Halgren, E.; Thesen, T. Hemispheric asymmetries of cortical volume in the human brain. Cortex 2013, 49, 200–210. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.K.; Kim, J.H.; Kim, H.J.; Lee, H.J. Changes in the gray matter volume during compensation after vestibular neuritis: A longitudinal VBM study. Restor Neurol. Neurosci. 2014, 32, 663–673. [Google Scholar] [CrossRef]
- Helmchen, C.; Klinkenstein, J.; Machner, B.; Rambold, H.; Mohr, C.; Sander, T. Structural changes in the human brain following vestibular neuritis indicate central vestibular compensation. Ann. N. Y. Acad. Sci. 2009, 1164, 104–115. [Google Scholar] [CrossRef]
- Devantier, L.; Hansen, A.K.; Molby-Henriksen, J.J.; Christensen, C.B.; Pedersen, M.; Hansen, K.V.; Magnusson, M.; Ovesen, T.; Borghammer, P. Positron emission tomography visualized stimulation of the vestibular organ is localized in Heschl’s gyrus. Hum. Brain Mapp. 2020, 41, 185–193. [Google Scholar] [CrossRef] [PubMed]
- zu Eulenburg, P.; Stoeter, P.; Dieterich, M. Voxel-based morphometry depicts central compensation after vestibular neuritis. Ann. Neurol. 2010, 68, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Frank, S.M.; Forster, L.; Pawellek, M.; Malloni, W.M.; Ahn, S.; Tse, P.U.; Greenlee, M.W. Visual Attention Modulates Glutamate-Glutamine Levels in Vestibular Cortex: Evidence from Magnetic Resonance Spectroscopy. J. Neurosci. 2021, 41, 1970–1981. [Google Scholar] [CrossRef]
- Burke, R.E.; Fahn, S. Choline acetyltransferase activity of the principal vestibular nuclei of rat, studied by micropunch technique. Brain Res. 1985, 328, 196–199. [Google Scholar] [CrossRef]
- Williamson, J.B.; Lamb, D.G.; Porges, E.C.; Bottari, S.; Woods, A.J.; Datta, S.; Langer, K.; Cohen, R.A. Cerebral Metabolite Concentrations Are Associated With Cortical and Subcortical Volumes and Cognition in Older Adults. Front Aging Neurosci. 2020, 12, 587104. [Google Scholar] [CrossRef]
- Hafkemeijer, A.; Altmann-Schneider, I.; de Craen, A.J.; Slagboom, P.E.; van der Grond, J.; Rombouts, S.A. Associations between age and gray matter volume in anatomical brain networks in middle-aged to older adults. Aging Cell 2014, 13, 1068–1074. [Google Scholar] [CrossRef] [PubMed]
- Yeo, S.S.; Kwon, J.W.; Cho, I.H. Associations between Age-Related Changes in the Core Vestibular Projection Pathway and Balance Ability: A Diffusion Tensor Imaging Study. Behav. Neurol. 2020, 2020, 2825108. [Google Scholar] [CrossRef]
- Ruggieri, M.; Tortorella, C.; Ceci, E.; Paolicelli, D.; Solfrizzi, V.; Di Bitonto, G.; Pica, C.; Mastrapasqua, M.; Livrea, P.; Trojano, M. Age-related changes of serum N-acetyl-aspartate in healthy controls. Age Ageing 2011, 40, 391–395. [Google Scholar] [CrossRef] [PubMed]
- Kirov, I.I.; Sollberger, M.; Davitz, M.S.; Glodzik, L.; Soher, B.J.; Babb, J.S.; Monsch, A.U.; Gass, A.; Gonen, O. Global brain volume and N-acetyl-aspartate decline over seven decades of normal aging. Neurobiol Aging 2021, 98, 42–51. [Google Scholar] [CrossRef]
- Haga, K.K.; Khor, Y.P.; Farrall, A.; Wardlaw, J.M. A systematic review of brain metabolite changes, measured with 1H magnetic resonance spectroscopy, in healthy aging. Neurobiol. Aging 2009, 30, 353–363. [Google Scholar] [CrossRef]
- Cavanna, A.E.; Trimble, M.R. The precuneus: A review of its functional anatomy and behavioural correlates. Brain 2006, 129, 564–583. [Google Scholar] [CrossRef]
- Wurthmann, S.; Naegel, S.; Schulte Steinberg, B.; Theysohn, N.; Diener, H.C.; Kleinschnitz, C.; Obermann, M.; Holle, D. Cerebral gray matter changes in persistent postural perceptual dizziness. J. Psychosom. Res. 2017, 103, 95–101. [Google Scholar] [CrossRef]
- Kirsch, V.; Keeser, D.; Hergenroeder, T.; Erat, O.; Ertl-Wagner, B.; Brandt, T.; Dieterich, M. Structural and functional connectivity mapping of the vestibular circuitry from human brainstem to cortex. Brain Struct. Funct. 2016, 221, 1291–1308. [Google Scholar] [CrossRef]
- Gruetter, R.; Novotny, E.J.; Boulware, S.D.; Mason, G.F.; Rothman, D.L.; Shulman, G.I.; Prichard, J.W.; Shulman, R.G. Localized 13C NMR Spectroscopy in the Hum. an Brain of Amino Acid Labeling from d-[1-13C]Glucose. J. Neurochem. 1994, 63, 1377–1385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bekdash, R.A. Choline and the Brain: An Epigenetic Perspective. Adv. Neurobiol. 2016, 12, 381–399. [Google Scholar] [CrossRef] [PubMed]
- Babb, S.M.; Ke, Y.; Lange, N.; Kaufman, M.J.; Renshaw, P.F.; Cohen, B.M. Oral choline increases choline metabolites in human brain. Psychiatry Res. 2004, 130, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Gessler, D.J.; Li, D.; Xu, H.; Su, Q.; Sanmiguel, J.; Tuncer, S.; Moore, C.; King, J.; Matalon, R.; Gao, G. Redirecting N-acetylaspartate metabolism in the central nervous system normalizes myelination and rescues Canavan disease. JCI Insight. 2017, 2, e90807. [Google Scholar] [CrossRef]
- Kilicarslan, R.; Alkan, A.; Aralasmak, A.; Aksoy, F.; Toprak, H.; Yetis, H.; Ozturan, O. Magnetic resonance spectroscopy features of Heschl’s gyri in patients with unilateral acoustic neuroma: Preliminary study. Acad. Radiol. 2014, 21, 1501–1505. [Google Scholar] [CrossRef]
- Hassanien, O.A.; Younes, R.L.; Dawoud, R.M.; Kotait, M.A. Vestibular cortex assessment using DTI & MRS complemented by rotatory chair technique in vestibulopathy patients with normal appearing brain MRI. Egypt. J. Radiol. Nuc. Med. 2017, 48, 611–619. [Google Scholar] [CrossRef]
- Haris, M.; Cai, K.; Singh, A.; Hariharan, H.; Reddy, R. In vivo mapping of brain myo-inositol. Neuroimage 2011, 54, 2079–2085. [Google Scholar] [CrossRef]
- Iwasaki, S.; Yamasoba, T. Dizziness and Imbalance in the Elderly: Age-related Decline in the Vestibular System. Aging Dis. 2015, 6, 38–47. [Google Scholar] [CrossRef]
- McGarvie, L.A.; MacDougall, H.G.; Halmagyi, G.M.; Burgess, A.M.; Weber, K.P.; Curthoys, I.S. The Video Head Impulse Test (vHIT) of Semicircular Canal Function—Age-Dependent Normative Values of VOR Gain in Healthy Subjects. Front. Neurol. 2015, 6, 154. [Google Scholar] [CrossRef]
- Cyran, C.A.; Boegle, R.; Stephan, T.; Dieterich, M.; Glasauer, S. Age-related decline in functional connectivity of the vestibular cortical network. Brain Struct. Funct. 2016, 221, 1443–1463. [Google Scholar] [CrossRef]
- Huang, W.; Alexander, G.E.; Daly, E.M.; Shetty, H.U.; Krasuski, J.S.; Rapoport, S.I.; Schapiro, M.B. High Brain myo-Inositol Levels in the Predementia Phase of Alzheimer’s Disease in Adults With Down’s Syndrome: A 1H MRS Study. Am. J. Psychiatry 1999, 156, 1879–1886. [Google Scholar] [CrossRef]
- Chen, S.Q.; Wang, P.J.; Ten, G.J.; Zhan, W.; Li, M.H.; Zang, F.C. Role of myo-inositol by magnetic resonance spectroscopy in early diagnosis of Alzheimer’s disease in APP/PS1 transgenic mice. Dement. Geriatr. Cogn. Disord. 2009, 28, 558–566. [Google Scholar] [CrossRef] [PubMed] [Green Version]

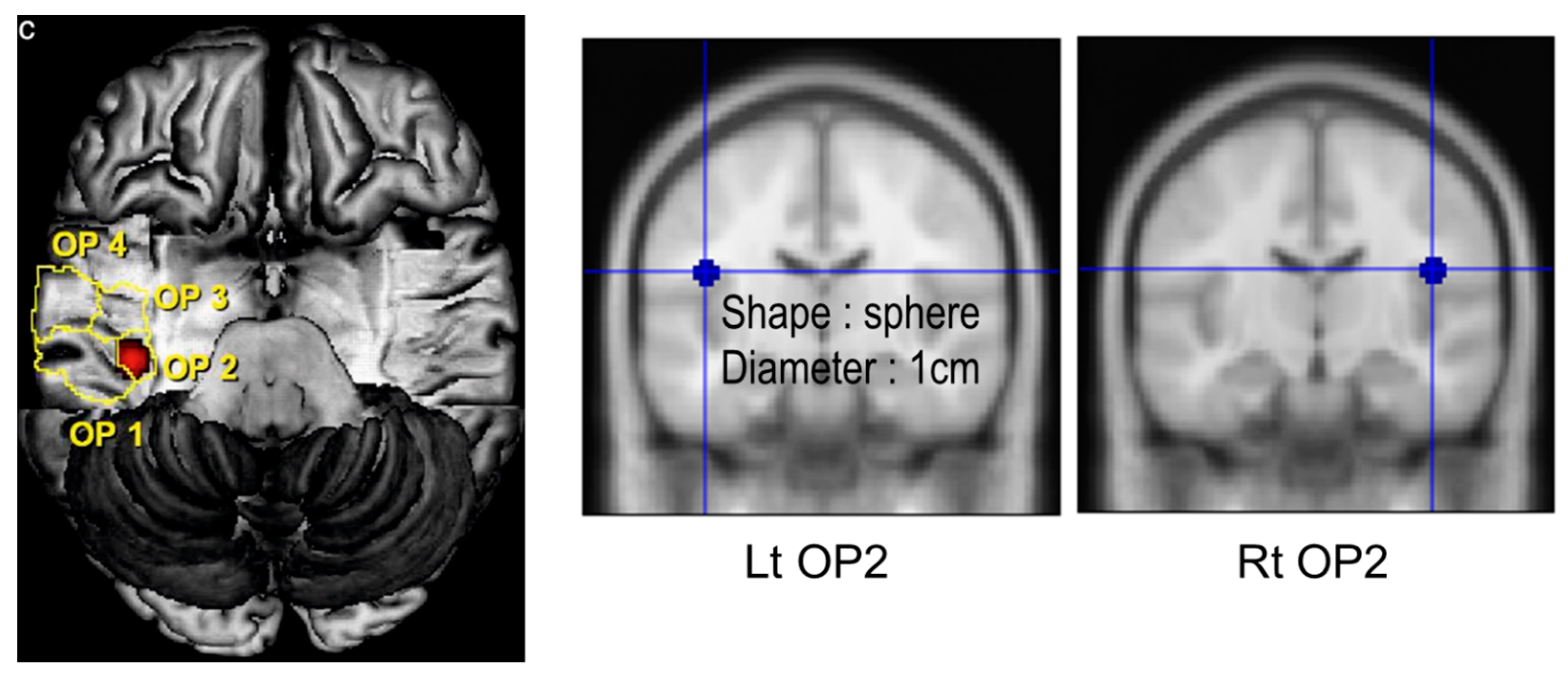
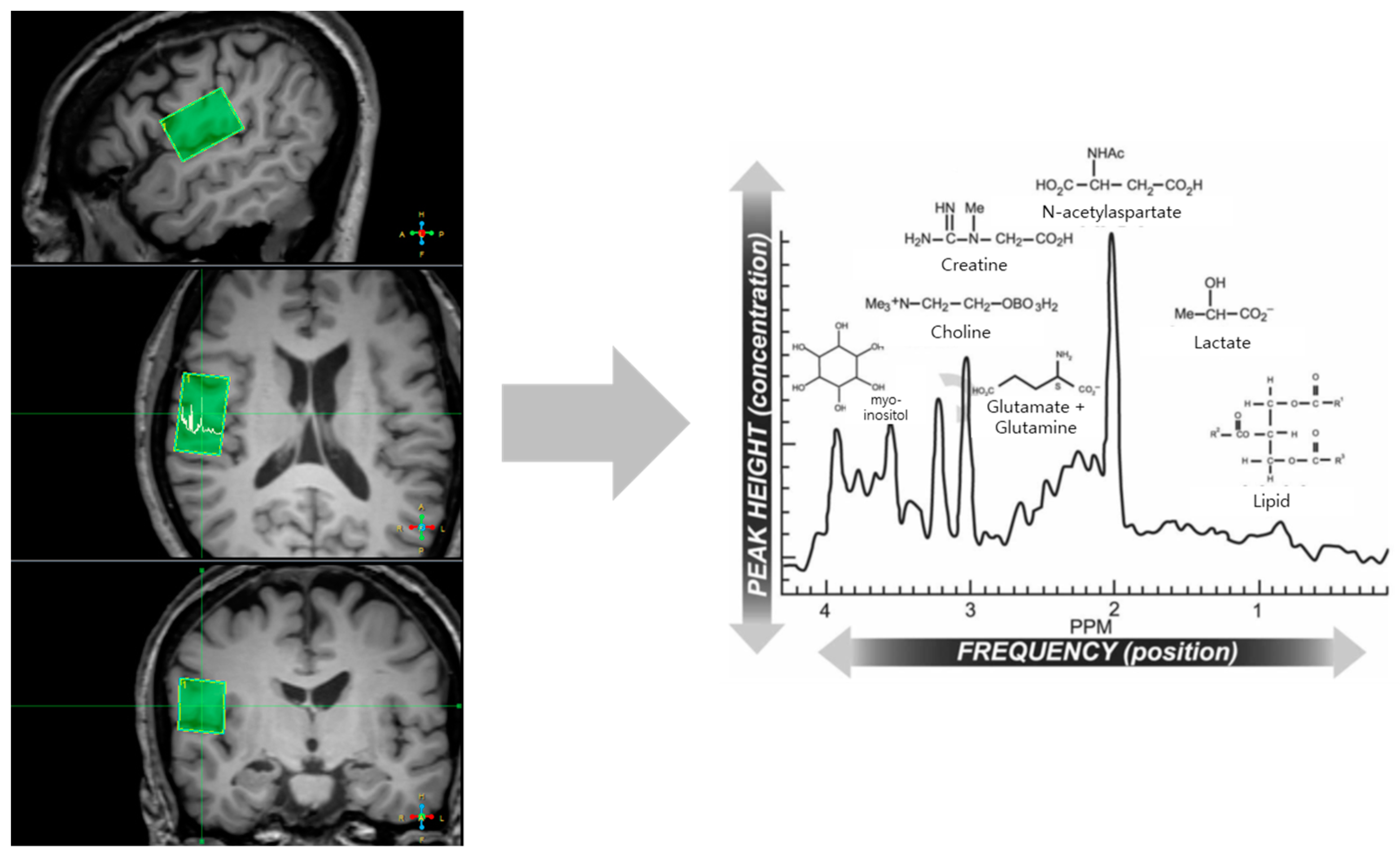
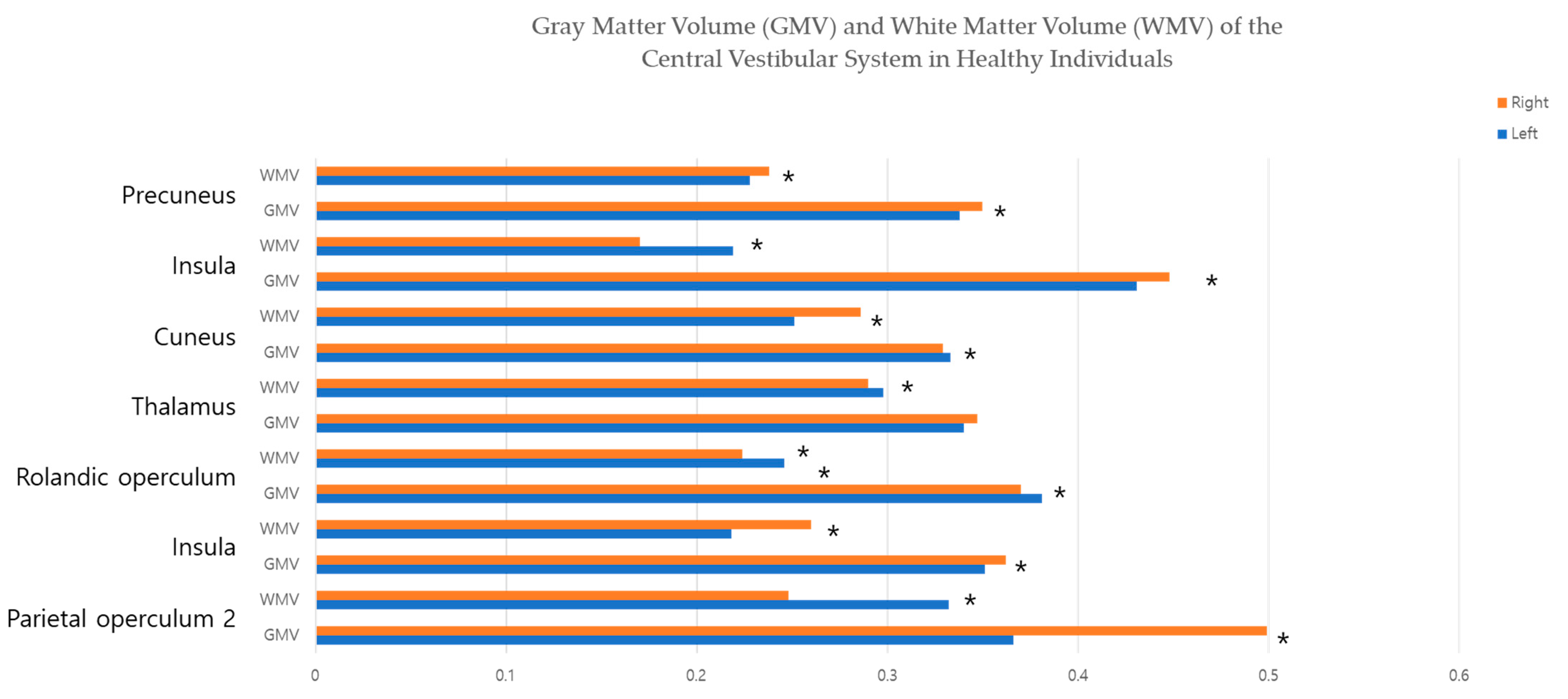

| Region of Interest | Left Side (Mean ± SD) | Right Side (Mean ± SD) | Significance (p-Value) |
|---|---|---|---|
| Parietal operculum 2 | |||
| GMV | 0.366 ± 0.071 | 0.499 ± 0.066 | <0.001 * |
| WMV | 0.332 ± 0.034 | 0.248 ± 0.053 | <0.001 * |
| Caudate | |||
| GMV | 0.351 ± 0.041 | 0.362 ± 0.041 | 0.001 * |
| WMV | 0.218 ± 0.025 | 0.260 ± 0.029 | <0.001 * |
| Rolandic operculum | |||
| GMV | 0.381 ± 0.051 | 0.370 ± 0.046 | 0.038 * |
| WMV | 0.246 ± 0.030 | 0.224 ± 0.025 | <0.001 * |
| Thalamus | |||
| GMV | 0.340 ± 0.048 | 0.347 ± 0.059 | 0.266 |
| WMV | 0.298 ± 0.041 | 0.290 ± 0.045 | 0.020 * |
| Cuneus | |||
| GMV | 0.333 ± 0.048 | 0.329 ± 0.041 | 0.543 |
| WMV | 0.251 ± 0.038 | 0.286 ± 0.042 | <0.001 * |
| Insula | |||
| GMV | 0.431 ± 0.044 | 0.448 ± 0.044 | 0.001 * |
| WMV | 0.219 ± 0.022 | 0.170 ± 0.016 | <0.001 * |
| Precuneus | |||
| GMV | 0.338 ± 0.033 | 0.350 ± 0.036 | 0.004 * |
| WMV | 0.228 ± 0.026 | 0.238 ± 0.028 | 0.004 * |
| Metabolite of Parietal Operculum 2 | Left Side (Mean ± SD) | Right Side (Mean ± SD) | Significance (p-Value) |
|---|---|---|---|
| mIns/tCr ratio | 0.591 ± 0.064 | 0.578 ± 0.049 | 0.333 |
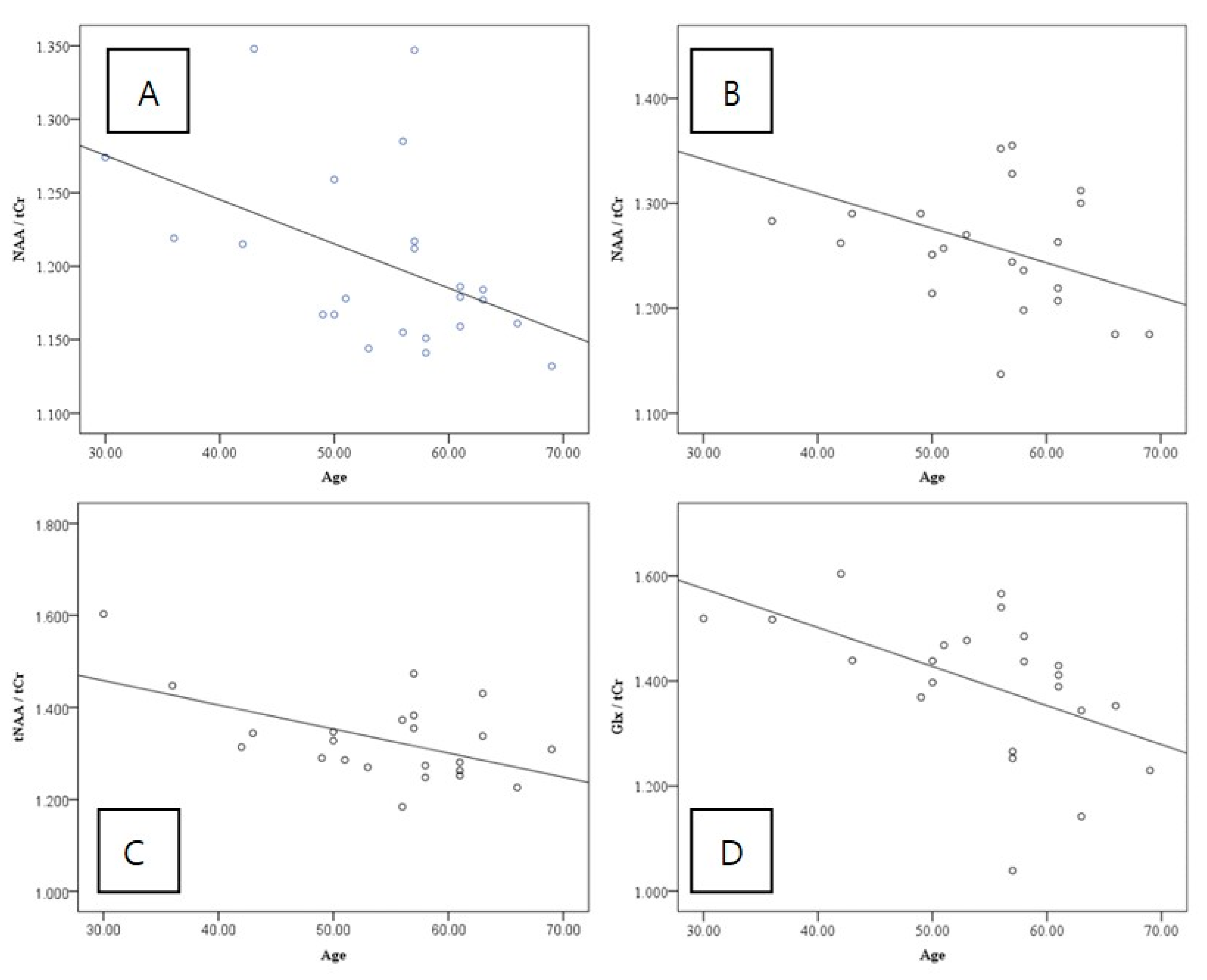
| NAA/tCr ratio | 1.202 ± 0.062 | 1.262 ± 0.065 | <0.001 * |
| tNAA/tCr ratio | 1.263 ± 0.078 | 1.331 ± 0.092 | 0.002 * |
| GPC/tCr ratio | 0.242 ± 0.016 | 0.223 ± 0.039 | 0.034 * |
| Glx/tCr ratio | 1.545 ± 0.111 | 1.444 ± 0.026 | <0.001 * |
| Metabolite of Parietal Operculum 2 | Left Side Pearson Correlation | Left Side p-Value | Right Side Pearson Correlation | Right Side p-Value |
|---|---|---|---|---|
| mIns/tCr ratio | 0.112 | 0.610 | −0.209 | 0.339 |
| NAA/tCr ratio | −0.463 | 0.026 * | −0.478 | 0.021 * |
| tNAA/tCr ratio | −0.332 | 0.121 | −0.537 | 0.008 * |
| GPC/tCr ratio | 0.134 | 0.542 | −0.082 | 0.723 |
| Glx/tCr ratio | 0.134 | 0.542 | −0.514 | 0.012 * |
| Region of Interest | Left Side Pearson Correlation | Left Side p-Value | Right Side Pearson Correlation | Right Side p-Value |
|---|---|---|---|---|
| Parietal operculum 2 | 0.006 | 0.980 | −0.035 | 0.884 |
| Caudate | 0.064 | 0.790 | 0.016 | 0.947 |
| Rolandic operculum | −0.117 | 0.623 | −0.156 | 0.511 |
| Thalamus | 0.082 | 0.731 | 0.049 | 0.838 |
| Cuneus | 0.260 | 0.269 | 0.091 | 0.702 |
| Insula | −0.114 | 0.632 | −0.234 | 0.322 |
| Precuneus | 0.010 | 0.966 | 0.142 | 0.549 |
| Metabolite | Left Side (Partial Correlation) | Left Side p-Value | Right Side (Partial Correlation) | Right Side p-Value |
|---|---|---|---|---|
| mIns/tCr ratio | 0.138 | 0.574 | −0.059 | 0.810 |
| NAA/tCr ratio | 0.301 | 0.210 | −0.130 | 0.594 |
| tNAA/tCr ratio | 0.329 | 0.169 | 0.129 | 0.598 |
| GPC/tCr ratio | 0.003 | 0.991 | 0.094 | 0.701 |
| Glx/tCr ratio | 0.280 | 0.246 | −0.062 | 0.801 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwon, K.J.; Byun, J.Y. Asymmetry of Gray- and White-Matter Volume and Metabolites in the Central-Vestibular System in Healthy Individuals. J. Clin. Med. 2023, 12, 1272. https://doi.org/10.3390/jcm12041272
Kwon KJ, Byun JY. Asymmetry of Gray- and White-Matter Volume and Metabolites in the Central-Vestibular System in Healthy Individuals. Journal of Clinical Medicine. 2023; 12(4):1272. https://doi.org/10.3390/jcm12041272
Chicago/Turabian StyleKwon, Ki Jin, and Jae Yong Byun. 2023. "Asymmetry of Gray- and White-Matter Volume and Metabolites in the Central-Vestibular System in Healthy Individuals" Journal of Clinical Medicine 12, no. 4: 1272. https://doi.org/10.3390/jcm12041272
APA StyleKwon, K. J., & Byun, J. Y. (2023). Asymmetry of Gray- and White-Matter Volume and Metabolites in the Central-Vestibular System in Healthy Individuals. Journal of Clinical Medicine, 12(4), 1272. https://doi.org/10.3390/jcm12041272






