Outcomes of Percutaneous Coronary Interventions for Long Diffuse Coronary Artery Disease with Extremely Small Diameter
Abstract
:1. Introduction
2. Methods
2.1. Study Population and Covariates
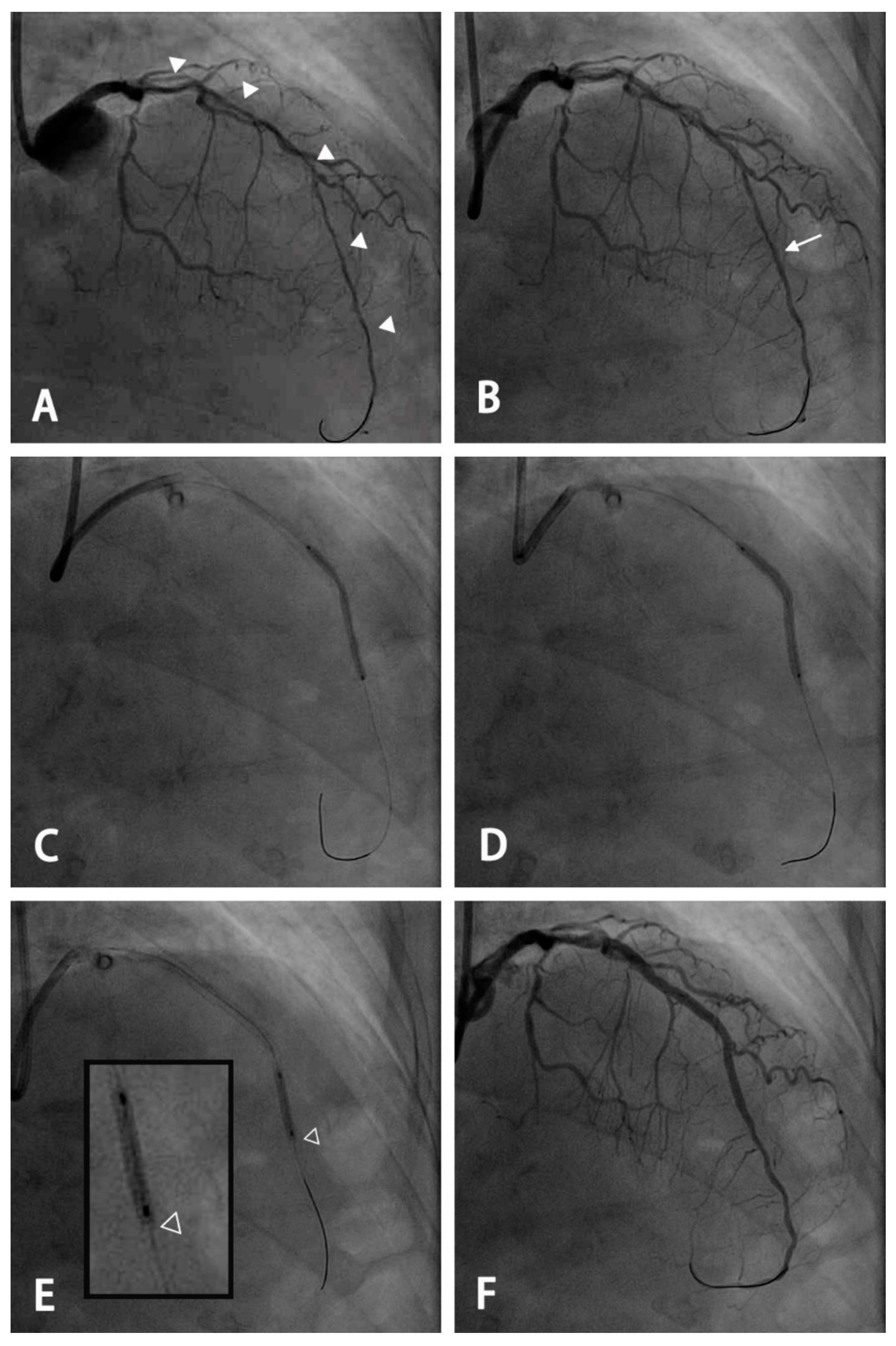
2.2. PCI Procedure
2.3. Definitions and Clinical Follow-Up
2.4. Statistical Analysis
3. Results
3.1. Clinical and Angiographic Characteristics
3.2. Procedural Details and Outcomes
3.3. Long-Term Clinical Outcomes
3.4. Predictors of TVF
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CABG | coronary artery bypass graft |
| CTO | chronic total occlusion |
| DCB | drug-coated balloon |
| DES | drug-eluting stent |
| dsDMax | maximal luminal diameter of the distal segment |
| ESDV | extremely small distal vessel |
| PCI | percutaneous coronary intervention |
| TVF | target vessel failure |
| TVMI | target vessel myocardial infarction |
| TVR | target vessel revascularization |
References
- Lee, C.W.; Park, K.-H.; Kim, Y.-H.; Hong, M.-K.; Kim, J.-J.; Park, S.-W.; Park, S.-J. Clinical and angiographic outcomes after placement of multiple overlapping drug-eluting stents in diffuse coronary lesions. J. Am. Coll. Cardiol. 2006, 98, 918–922. [Google Scholar] [CrossRef] [PubMed]
- Sharp, A.; Latib, A.; Ielasi, A.; Larosa, C.; Godino, C.; Saolini, M.; Magni, V.; Gerber, R.T.; Montorfano, M.; Carlino, M.; et al. Long-term follow-up on a large cohort of “full-metal jacket” percutaneous coronary intervention procedures. Circ. Cardiovasc. Interv. 2009, 2, 416–422. [Google Scholar] [CrossRef]
- Lee, C.W.; Ahn, J.-M.; Lee, J.-Y.; Kim, W.-J.; Park, D.-W.; Kang, S.-J.; Lee, S.-W.; Kim, Y.-H.; Park, S.-W.; Park, S.-J. Long-term (8 year) outcomes and predictors of major adverse cardiac events after full metal jacket drug-eluting stent implantation. Catheter. Cardiovasc. Interv. 2014, 84, 361–365. [Google Scholar] [CrossRef]
- Honda, Y.; Muramatsu, T.; Ito, Y.; Sakai, T.; Hirano, K.; Yamawaki, M.; Araki, M.; Kobayashi, N.; Takimura, H.; Sakamoto, Y.; et al. Impact of ultra-long second-generation drug-eluting stent implantation. Catheter. Cardiovasc. Interv. 2016, 87, E44–E53. [Google Scholar] [CrossRef] [PubMed]
- Durante, A.; Manzillo, G.F.; Burzotta, F.; Trani, C.; Aurigemma, C.; Summaria, F.; Patrizi, R.; Talarico, G.P.; Latib, A.; Figini, F.; et al. Long term follow-up of “full metal jacket” of de novo coronary lesions with new generation zotarolimus-eluting stents. Int. J. Cardiol. 2016, 221, 1008–1012. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, R.; Ako, J.; Morino, Y.; Sonoda, S.; Kaneda, H.; Terashima, M.; Hassan, A.H.M.; Leon, M.B.; Moses, J.W.; Popma, J.J.; et al. Predictors of edge stenosis following sirolimus-eluting stent deployment (a quantitative intravascular ultrasound analysis from the sirius trial). J. Am. Coll. Cardiol. 2005, 96, 1251–1253. [Google Scholar] [CrossRef]
- Werner, G.S.; Schwarz, G.; Prochnau, D.; Fritzenwanger, M.; Krack, A.; Betge, S.; Figulla, H.R. Paclitaxel-eluting stents for the treatment of chronic total coronary occlusions: A strategy of extensive lesion coverage with drug-eluting stents. Catheter. Cardiovasc. Interv. 2006, 67, 1–9. [Google Scholar] [CrossRef]
- Mintz, G.S.; Weissman, N.J. Intravascular ultrasound in the drug-eluting stent era. J. Am. Coll. Cardiol. 2006, 48, 421–429. [Google Scholar] [CrossRef]
- Garcia-Garcia, H.M.; McFadden, E.P.; Farb, A.; Mehran, R.; Stone, G.W.; Spertus, J.; Onuma, Y.; Morel, M.-A.; Es, G.-A.v.; Zuckerman, B.; et al. Standardized end point definitions for coronary intervention trials. The academic research consortium-2 consensus document. Circulation 2018, 137, 2635–2650. [Google Scholar] [CrossRef]
- Galassi, A.R.; Tomasello, S.D.; Crea, F.; Costanzo, L.; Campisano, M.B.; Marza, F.; Tamburino, C. Transient impairment of vasomotion function after successful chronic total occlusion recanalization. J. Am. Coll. Cardiol. 2012, 59, 711–718. [Google Scholar] [CrossRef]
- Branden, B.J.L.V.d.; Teeuwen, K.; Koolen, J.J.; Schaaf, R.J.v.d.; Henriques, J.P.S.; Tijssen, J.G.P.; Kelder, J.C.; Vermeersch, P.H.M.J.; Rensing, B.J.W.M.; Suttorp, M.J. Primary stenting of totally occluded native coronary arteries iii (prison iii): A randomized comparison of sirolimus-eluting stent implantation with zotarolimuseluting stent implantation for the treatment of total coronary occlusions. EuroIntervention 2013, 9, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Zocca, P.; Kok, M.M.; Tandjung, K.; Danse, P.W.; Jessurun, G.A.J.; Hautvast, R.W.M.; Houwelingen, K.G.v.; Stoel, M.G.; Schramm, A.R.; Gin, R.M.Y.J.; et al. 5-year outcome following randomized treatment of all-comers with zotarolimus-eluting resolute integrity and everolimus-eluting promus element coronary stents. Final report of the dutch peers (twente ii) trial. J. Am. Coll. Cardiol. Intv 2018, 11, 462–469. [Google Scholar] [CrossRef]
- Heijden, L.C.v.d.; Kok, M.M.; Danse, P.W.; Schramm, A.R.; Hartmann, M.; Löwik, M.M.; Linssen, G.C.M.; Stoel, M.G.; Doggen, C.J.M.; Birgelen, C.v. Small-vessel treatment with contemporary newer-generation drug-eluting coronary stents in all-comers: Insights from 2-year dutch peers (twente ii) randomized trial. Am. Heart J. 2016, 176, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Buiten, R.A.; Ploumen, E.H.; Zocca, P.; Doggen, C.J.M.; Heijden, L.C.v.d.; Kok, M.M.; Danse, P.W.; Schotborgh, C.E.; Scholte, M.; Frits, H.A.F.d.M.; et al. Outcomes in patients treated with thin-strut, very thin-strut, or ultrathin-strut drug-eluting stents in small coronary vessels. A prespecified analysis of the randomized bio-resort trial. JAMA Cardiol. 2019, 4, 659–669. [Google Scholar] [CrossRef] [PubMed]
- Price, M.J.; Saito, S.; Shlofmitz, R.A.; Spriggs, D.J.; Attubato, M.; McLaurin, B.; Almonacid, A.P.; Brar, S.; Liu, M.; Moe, E.; et al. First report of the resolute onyx 2.0-mm zotarolimus-eluting stent for the treatment of coronary lesions with very small reference vessel diameter. J. Am. Coll. Cardiol. Intv. 2017, 10, 1381–1388. [Google Scholar] [CrossRef]
- Kang, S.-J.; Cho, Y.-R.; Park, G.-M.; Ahn, J.-M.; Kim, W.-J.; Lee, J.-Y.; Park, D.-W.; Lee, S.-W.; Kim, Y.-H.; Lee, C.W.; et al. Intravascular ultrasound predictors for edge restenosis after newer generation drug-eluting stent implantation. Am. J. Cardiol. 2013, 111, 1408–1414. [Google Scholar] [CrossRef]
- Ngaage, D.L.; Hashmi, I.; Griffin, S.; Cowen, M.E.; Cale, A.R.; Guvendik, L. To graft or not to graft? Do coronary artery characteristics influence early outcomes of coronary artery bypass surgery? Analysis of coronary anastomoses of 5171 patients. J. Thorac. Cardiovasc. Surg. 2010, 140, 66–72. [Google Scholar] [CrossRef]
- Ramström, J.; Lund, O.; Cadavid, E.; Thuren, J.; Oxelbark, S.; Henze, A. Multiarterial coronary artery bypass grafting with special reference to small vessel disease and results in women. Eur. Heart J. 1993, 14, 634–639. [Google Scholar] [CrossRef]
- O’Connor, N.J.; Morton, J.R.; Birkmeyer, J.D.; Olmstead, E.M.; O’Connor, G.T. Effect of coronary artery diameter in patients undergoing coronary bypass surgery. Northern new england cardiovascular disease study group. Circulation 1996, 93, 652–655. [Google Scholar] [CrossRef]
- Desai, N.D.; Naylor, C.D.; Kiss, A.; Cohen, E.A.; Feder-Elituv, R.; Miwa, S.; Radhakrishnan, S.; Dubbin, J.; Schwartz, L.; Fremes, S.E. Impact of patient and target-vessel characteristics on arterial and venous bypass graft patency. Insight from a randomized trial. Circulation 2007, 115, 684–691. [Google Scholar] [CrossRef]
- Cortese, B.; Micheli, A.; Picchi, A.; Coppolaro, A.; Bandinelli, L.; Severi, A.; Limbruno, U. Paclitaxel-coated balloon versus drug-eluting stent during PCI of small coronary vessels, a prospective randomised clinical trial. The PICCOLETO Study. Heart 2010, 96, 1291–1296. [Google Scholar] [CrossRef] [PubMed]
- Latib, A.; Colombo, A.; Castriota, F.; Micari, A.; Cremonesi, A.; Felice, F.d.; Marchese, A.; Tespili, M.; Presbitero, P.; Sgueglia, G.A.; et al. A Randomized Multicenter Study Comparing a Paclitaxel Drug-Eluting Balloon With a Paclitaxel-Eluting Stent in Small Coronary Vessels: The BELLO (Balloon Elution and Late Loss Optimization) Study. J. Am. Coll. Cardiol. 2012, 60, 2473–2480. [Google Scholar] [CrossRef] [PubMed]
- Jeger, R.V.; Farah, A.; Ohlow, M.A.; Mangner, N.; Möbius-Winkler, S.; Leibundgut, G.; Weilenmann, D.; Wöhrle, J.; Richter, S.; Schreiber, M.; et al. Drug-coated balloons for small coronary artery disease (BASKET-SMALL 2): An open-label randomised non-inferiority trial. Lancet 2018, 392, 849–856. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Qiao, S.; Su, X.; Chen, Y.; Jin, Z.; Chen, H.; Xu, B.; Kong, X.; Pang, W.; Liu, Y.; et al. Drug-Coated Balloon Versus Drug-Eluting Stent for Small-Vessel Disease. The RESTORE SVD China Randomized Trial. JACC Cardiovasc. Interv. 2018, 11, 2381–2392. [Google Scholar] [CrossRef]


| ESDV Group N = 307 | Non-ESDV Group N = 429 | p Value | |
|---|---|---|---|
| Age (years) | 65.1 ± 11.8 | 64.5 ± 10.8 | 0.52 |
| Male | 232(75.6) | 358(83.0) | 0.013 |
| Presentation of myocardial infarction | 79(25.7) | 120(28.0) | 0.50 |
| Diabetes mellitus | 163(53.1) | 209(48.7) | 0.24 |
| Hypertension | 235(76.5) | 332(77.4) | 0.79 |
| Hyperlipidemia | 268(87.3) | 355(82.8) | 0.09 |
| Smoking | 81(26.4) | 144(33.6) | 0.037 |
| Chronic kidney disease | 104(33.9) | 111(25.9) | 0.019 |
| left ventricular ejection fraction < 40% | 31(10.1) | 40(9.3) | 0.73 |
| History of myocardial infarction | 25(8.1) | 52(12.1) | 0.08 |
| History of stroke | 12(3.9) | 24(5.6) | 0.30 |
| Peripheral artery disease | 27(8.8) | 45(10.5) | 0.45 |
| Number of vessel disease | 0.56 | ||
| 1-vessel disease | 48(15.6) | 78(18.2) | |
| 2-vessel disease | 109(35.5) | 156(36.4) | |
| 3-vessel disease | 150(48.9) | 195(45.5) | |
| Culprit vessel | 0.24 | ||
| Left anterior descending artery | 187(60.9) | 241(56.2) | |
| Left circumflex artery | 39(12.7) | 50(11.7) | |
| Right coronary artery | 81(26.4) | 138(32.2) | |
| Left main coronary disease | 45(14.7) | 69(16.1) | 0.60 |
| Chronic total occlusion | 142(46.3) | 84(19.6) | <0.001 |
| Distal vessel maximal diameter (mm) | 1.7 ± 0.3 | 2.7 ± 0.5 | <0.005 |
| Moderate to severe calcification | 113(36.8) | 165(38.5) | 0.65 |
| Duration of DAPT (months) | 10.3 ± 3.0 | 10.4 ± 3.0 | 0.70 |
| ESDV Group N = 307 | Non-ESDV Group N = 429 | p Value | |
|---|---|---|---|
| Stent length (mm) | 62.6 ± 18.1 | 59.1 ± 16.0 | 0.007 |
| Number of stents | 1.9 ± 0.5 | 1.9 ± 1.1 | 0.46 |
| Mean stent diameter (mm) | 2.8 ± 0.3 | 3.0 ± 0.4 | <0.001 |
| Stent deployment pressure (atm) | 6.6 ± 1.8 | 8.9 ± 3.1 | <0.0001 |
| Post-dilatation | 305(99.3) | 426(99.3) | 1.00 |
| Post-dilatation pressure (atm) | 18.6 ± 4.0 | 19.6 ± 4.4 | 0.10 |
| Image guidance | 59(19.2) | 70(16.3) | 0.33 |
| Residual diameter stenosis (%) | 14.4 ± 9.6 | 14.9 ± 9.5 | 0.97 |
| Patients with residual diameter stenosis >30% | 10(3.0) | 11(2.7) | 1.00 |
| Post-PCI TIMI flow grade < 3 | 2(0.7) | 2(0.5) | 1.00 |
| Distal edge dissection | 1(0.3) | 2(0.5) | 1.00 |
| Acute stent thrombosis | 0(0) | 0(0) | 1.00 |
| Procedural success | 294(95.8) | 414(96.5) | 0.70 |
| Overall Population N = 736 | Propensity Score Matching-Matched Patients N = 474 | |||||||
|---|---|---|---|---|---|---|---|---|
| ESDV Group N = 307 | Non-ESDV Group N = 429 | Hazard Ratio (95% CI) | p Value | ESDV Group N =237 | Non-ESDV Group N =237 | Hazard Ratio (95% CI) | p Value | |
| Target vessel failure | 50(16.3) | 52(12.1) | 1.38(0.93–2.04) | 0.10 | 32(13.5) | 31(13.1) | 1.09(0.67–1.79) | 0.73 |
| Cardiac death | 12(3.9) | 15(3.5) | 1.12(0.52–2.39) | 0.77 | 7(3.0) | 10(4.2) | 0.72(0.27–1.89) | 0.50 |
| TVMI | 15(4.9) | 13(3.0) | 1.59(0.76–3.35) | 0.22 | 7(3.0) | 9(3.8) | 0.79(0.30–2.13) | 0.64 |
| Clinical driven TVR | 39(12.7) | 39(9.1) | 1.45(0.93–2.26) | 0.10 | 24(10.1) | 22(9.3) | 1.16(0.65–2.07) | 0.61 |
| death | 49(16.0) | 66(15.4) | 0.83 | 35(14.8) | 47(19.8) | 0.15 | ||
| stroke | 5(1.6) | 8(1.9) | 0.81 | 2(0.8) | 4(1.7) | 0.69 | ||
| Stent thrombosis | 3(1.0) | 1(0.2) | 0.31 | 1(0.4) | 0(0) | 1.00 | ||
| Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|
| Hazard Ratio (95% CI) | p Value | Hazard Ratio (95% CI) | p Value | |
| Age | 1.005(0.987–1.023) | 0.59 | ||
| Male | 0.546(0.355–0.840) | 0.006 | 0.732(0.467–1.145) | 0.17 |
| Presentation of myocardial infarction | 1.056(0.680–1.639) | 0.81 | ||
| Diabetes mellitus | 2.410(1.586–3.664) | <0.001 | 1.679(1.077–2.620) | 0.022 |
| Hypertension | 1.997(1.135–3.511) | 0.016 | 1.321(0.730–2.392) | 0.36 |
| Hyperlipidemia | 1.243(0.680–2.270) | 0.48 | ||
| Smoking | 0.859(0.559–1.321) | 0.49 | ||
| Chronic kidney disease | 2.753(1.862–4.068) | <0.001 | 1.826(1.175–2.836) | 0.007 |
| Left ventricular ejection fraction < 40% | 2.062(1.191–3.571) | 0.01 | 1.233(0.685–2.218) | 0.49 |
| Old myocardial infarction | 1.772(1.038–3.025) | 0.036 | 1.504(0.864–2.618) | 0.15 |
| Old stroke | 2.216(1.117–4.397) | 0.023 | 1.358(0.665–2.773) | 0.40 |
| peripheral artery disease | 2.986(1.809–4.927) | <0.001 | 1.571(0.889–2.774) | 0.12 |
| Number of diseased vessels | 1.141(0.612–2.127) 1.402(0.779–2.524) | 0.68 0.26 | ||
| Culprit vessel | 1.340(0.974–1.843) 0.671(0.401–1.123) | 0.072 0.13 | ||
| Left main disease | 1.657(1.041–2.636) | 0.033 | 1.336(0.828–2.154) | 0.26 |
| CTO | 1.110(0.736–1.672) | 0.62 | ||
| Duration of DAPT | 0.976(0.912–1.043) | 0.47 | ||
| Moderate to severe calcification | 1.865(1.264–2.751) | 0.002 | 1.295(0.851–1.969) | 0.23 |
| stent length, mm | 1.007(0.996–1.017) | 0.23 | ||
| stent number | 1.051(0.866–1.275) | 0.62 | ||
| stent diameter (mean) | 0.802(0.449–1.435) | 0.46 | ||
| dsDMax ≤ 2 mm | 1.381(0.937–2.037) | 0.10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ho, C.-T.; Hsiao, F.-C.; Tung, Y.-C.; Cordero, S.T.; del Castillo, D.V., III; Lee, H.-F.; Chou, S.-H.; Lin, C.-P.; Yen, K.-C.; Hsu, L.-A.; et al. Outcomes of Percutaneous Coronary Interventions for Long Diffuse Coronary Artery Disease with Extremely Small Diameter. J. Clin. Med. 2023, 12, 1285. https://doi.org/10.3390/jcm12041285
Ho C-T, Hsiao F-C, Tung Y-C, Cordero ST, del Castillo DV III, Lee H-F, Chou S-H, Lin C-P, Yen K-C, Hsu L-A, et al. Outcomes of Percutaneous Coronary Interventions for Long Diffuse Coronary Artery Disease with Extremely Small Diameter. Journal of Clinical Medicine. 2023; 12(4):1285. https://doi.org/10.3390/jcm12041285
Chicago/Turabian StyleHo, Chien-Te, Fu-Chih Hsiao, Ying-Chang Tung, Sharon T. Cordero, Dominador V. del Castillo, III, Hsin-Fu Lee, Shing-Hsien Chou, Chia-Pin Lin, Kun-Chi Yen, Lung-An Hsu, and et al. 2023. "Outcomes of Percutaneous Coronary Interventions for Long Diffuse Coronary Artery Disease with Extremely Small Diameter" Journal of Clinical Medicine 12, no. 4: 1285. https://doi.org/10.3390/jcm12041285
APA StyleHo, C.-T., Hsiao, F.-C., Tung, Y.-C., Cordero, S. T., del Castillo, D. V., III, Lee, H.-F., Chou, S.-H., Lin, C.-P., Yen, K.-C., Hsu, L.-A., & Chang, C.-J. (2023). Outcomes of Percutaneous Coronary Interventions for Long Diffuse Coronary Artery Disease with Extremely Small Diameter. Journal of Clinical Medicine, 12(4), 1285. https://doi.org/10.3390/jcm12041285






