Is It All about Surface Topography? An Intra-Individual Clinical Outcome Analysis of Two Different Implant Surfaces in Breast Reconstruction
Abstract
1. Introduction
2. Materials and Methods
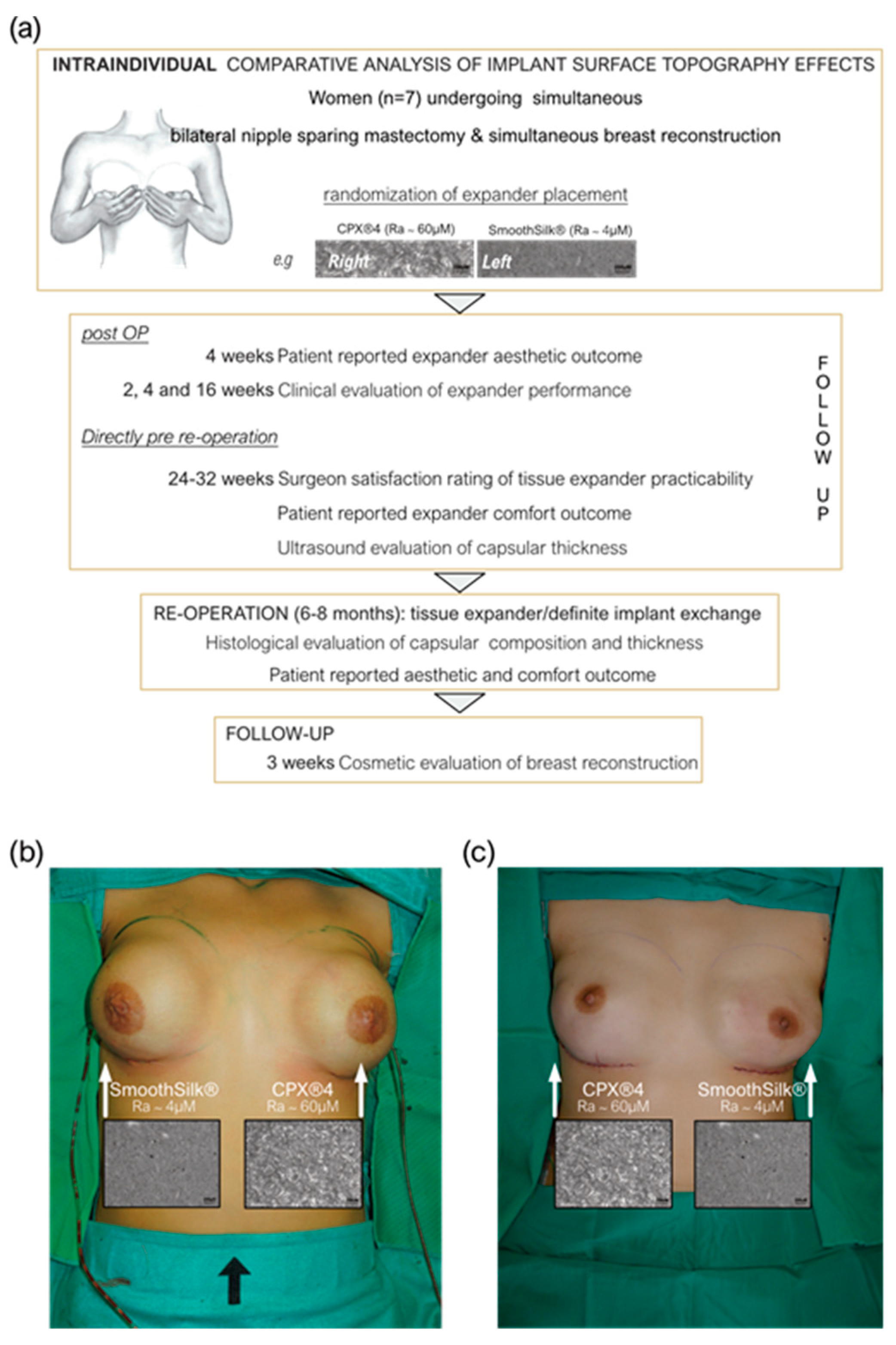
2.1. Study Population
2.2. Study Design
2.3. Statistical Analysis
3. Results
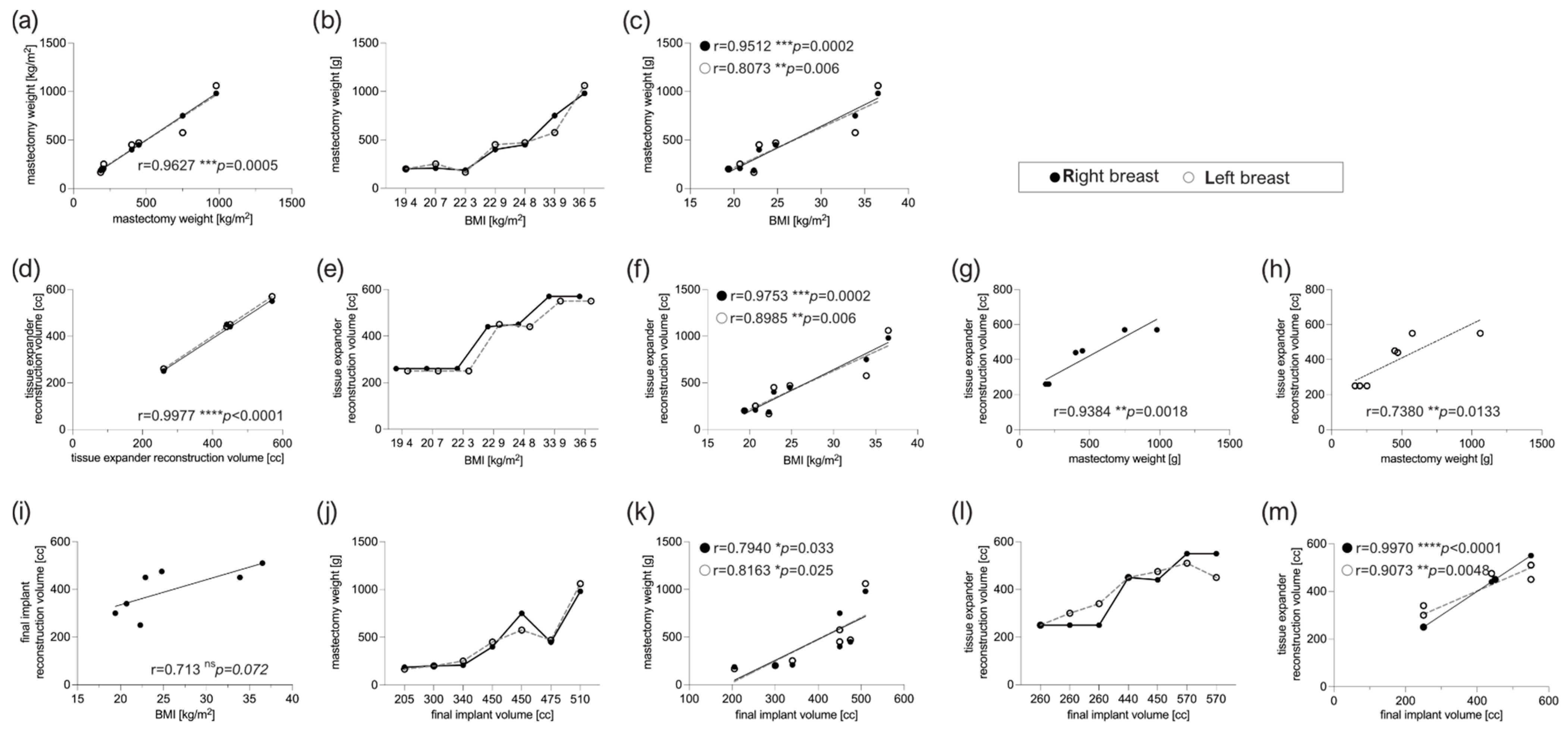
3.1. Patient Characteristics
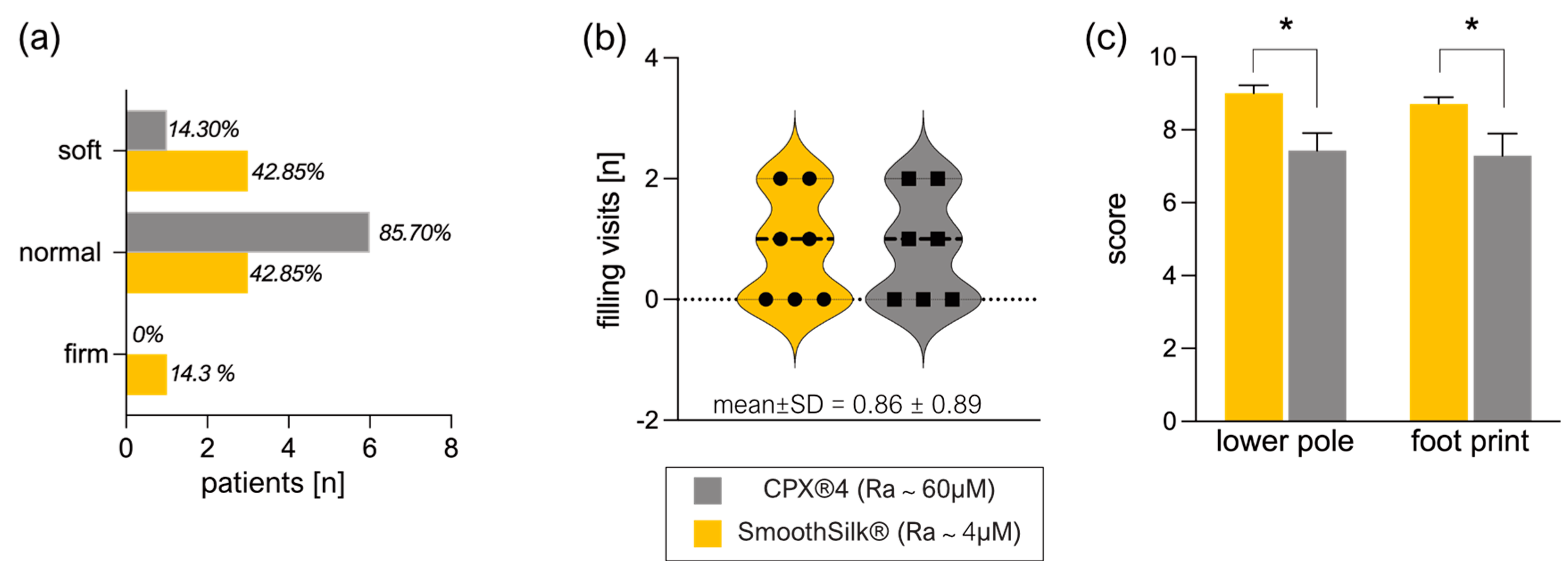
3.2. Clinical Evaluation of Expander Performance
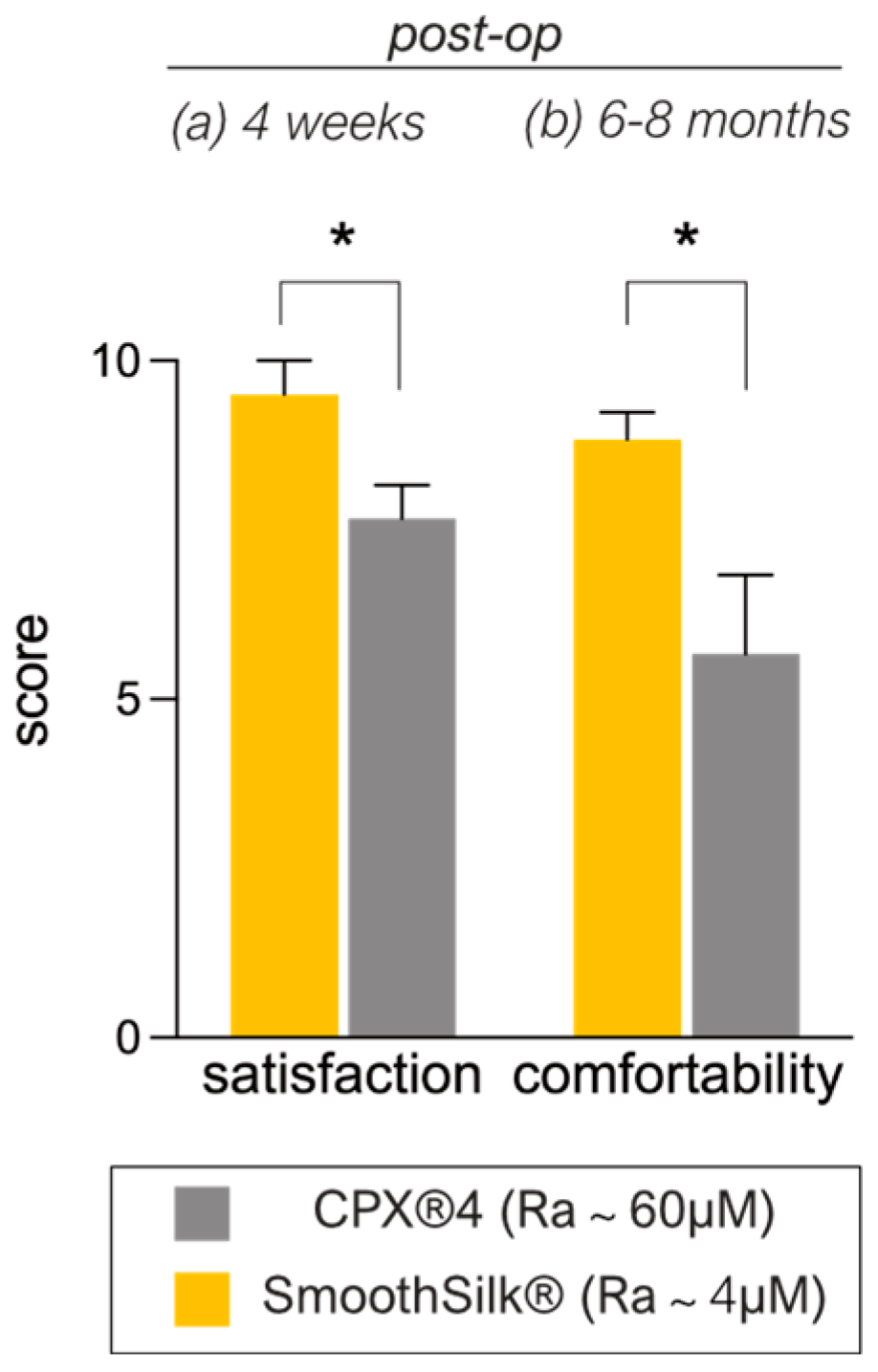
3.3. Patient-Reported Aesthetic and Comfort Outcome after Expander Reconstruction
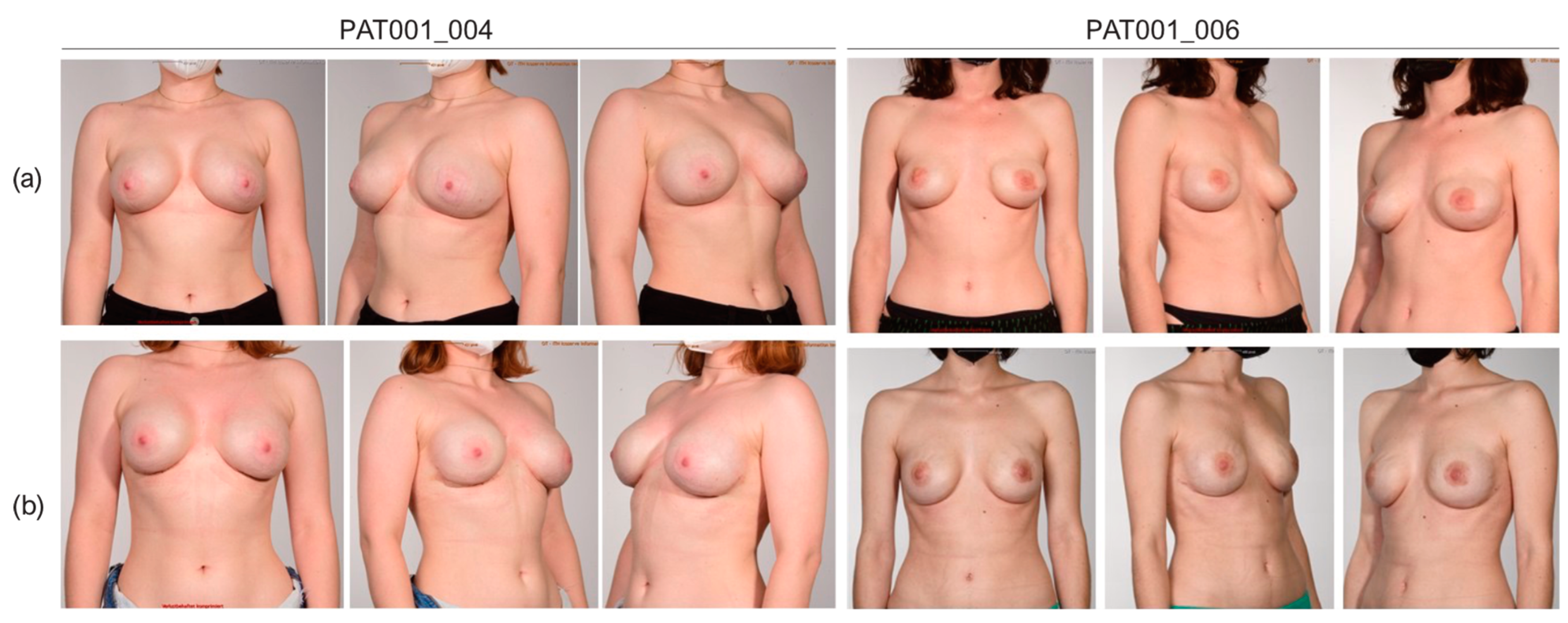
3.4. Cosmetic Results
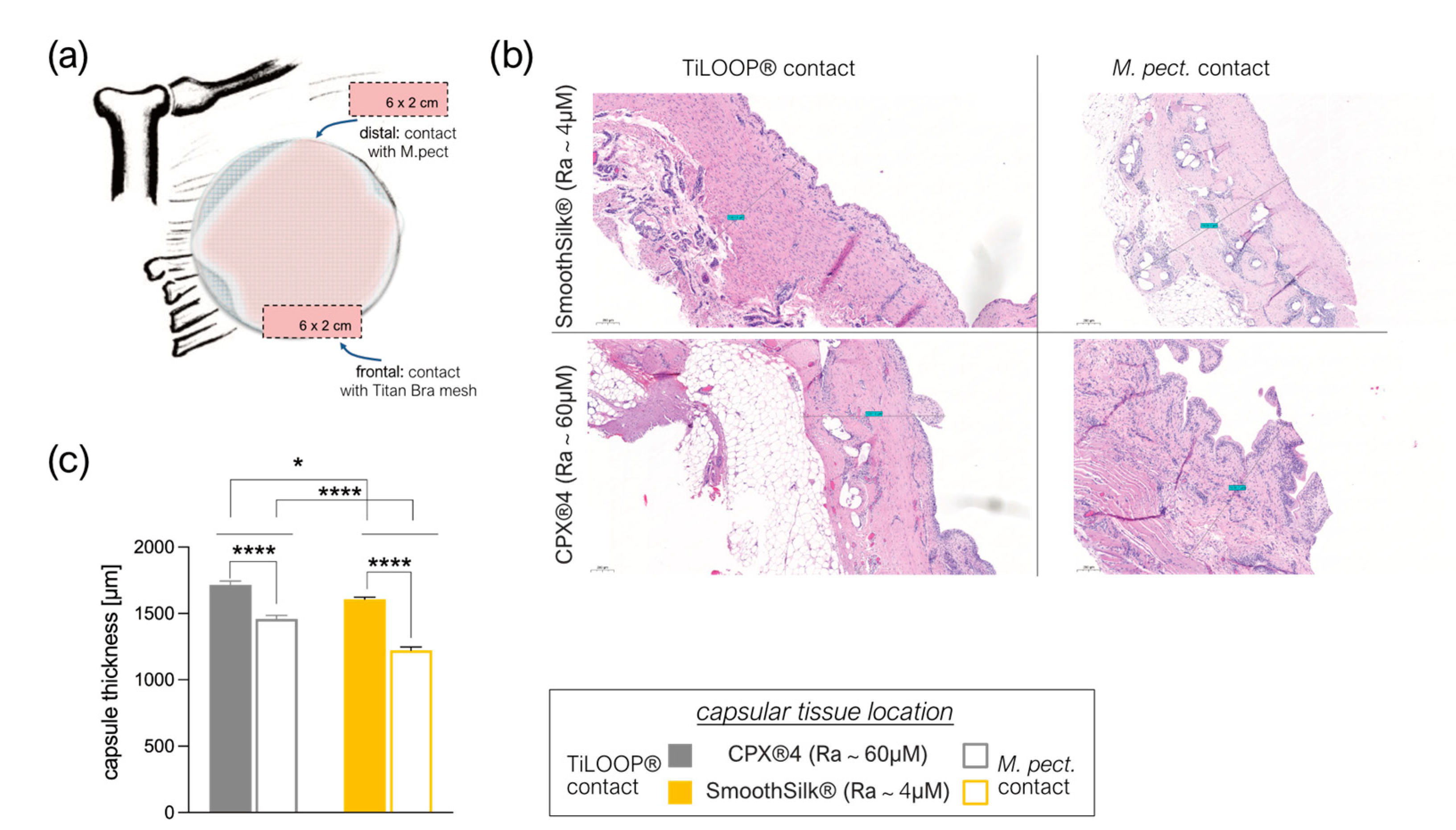
3.5. Intra- and Inter-Individual Comparison of the Fibrotic Capsule Thickness Formed around the CPX®4 and SmoothSilk® Tissue Expander
3.6. Titanium Debris from TiLoop® Bra Increases Histopathological Changes of the Capsule
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- American Society of Plastic Surgeons. Plastic Surgery Statistics. Available online: https://www.plasticsurgery.org/news/plastic-surgery-statistics (accessed on 21 November 2022).
- Collett, D.J.; Rakhorst, H.; Lennox, P.; Magnusson, M.; Cooter, R.; Deva, A. Current Risk Estimate of Breast Implant–Associated Anaplastic Large Cell Lymphoma in Textured Breast Implants. Plast. Reconstr. Surg. 2019, 143, 30S–40S. [Google Scholar] [CrossRef]
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef]
- OECD.Stat. Health Care Utilisation: Surgical procedures. Available online: https://stats.oecd.org (accessed on 7 November 2022).
- Fanakidou, I.; Zyga, S.; Alikari, V.; Tsironi, M.; Stathoulis, J.; Theofilou, P. Mental health, loneliness, and illness perception outcomes in quality of life among young breast cancer patients after mastectomy: The role of breast reconstruction. Qual. Life Res. 2018, 27, 539–543. [Google Scholar] [CrossRef]
- Fortunato, L.; Loreti, A.; Cortese, G.; Spallone, D.; Toto, V.; Cavaliere, F.; Farina, M.; La Pinta, M.; Manna, E.; Detto, L.; et al. Regret and Quality of Life after Mastectomy with or without Reconstruction. Clin. Breast Cancer 2021, 21, 162–169. [Google Scholar] [CrossRef]
- Panchal, H.M.; Matros, E.M. Current Trends in Postmastectomy Breast Reconstruction. Plast. Reconstr. Surg. 2017, 140, 7S–13S. [Google Scholar] [CrossRef]
- Santosa, K.B.; Qi, J.; Kim, H.M.; Hamill, J.B.; Wilkins, E.G.; Pusic, A.L. Long-term Patient-Reported Outcomes in Postmastectomy Breast Reconstruction. JAMA Surg. 2018, 153, 891. [Google Scholar] [CrossRef]
- Toyserkani, N.M.; Jørgensen, M.G.; Tabatabaeifar, S.; Damsgaard, T.; Sørensen, J.A. Autologous versus implant-based breast reconstruction: A systematic review and meta-analysis of Breast-Q patient-reported outcomes. J. Plast. Reconstr. Aesthetic Surg. 2020, 73, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Miseré, R.M.; van Kuijk, S.M.; Claassens, E.L.; Heuts, E.M.; Piatkowski, A.A.; van der Hulst, R.R. Breast-related and body-related quality of life following autologous breast reconstruction is superior to implant-based breast reconstruction—A long-term follow-up study. Breast 2021, 59, 176–182. [Google Scholar] [CrossRef]
- Lemaine, V.; Schilz, S.R.; Van Houten, H.K.; Zhu, L.; Habermann, E.B.; Boughey, J.C. Autologous Breast Reconstruction versus Implant-Based Reconstruction: How Do Long-Term Costs and Health Care Use Compare? Plast. Reconstr. Surg. 2020, 145, 303–311. [Google Scholar] [CrossRef] [PubMed]
- American Society of Plastic Surgeons. American Society of Plastic Surgeons Statistics Report2020: National Clearinghous of Plastic Suergery Procedural Statistics. Available online: https://www.plasticsurgery.org (accessed on 7 November 2022).
- Eriksen, C.; Lindgren, E.N.; Frisell, J.; Stark, B. A Prospective Randomized Study Comparing Two Different Expander Approaches in Implant-Based Breast Reconstruction. Plast. Reconstr. Surg. 2012, 130, 254e–264e. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-T.; Mun, G.-H. Comparison of one-stage vs two-stage prosthesis-based breast reconstruction: A systematic review and meta-analysis. Am. J. Surg. 2016, 212, 336–344. [Google Scholar] [CrossRef] [PubMed]
- Nahabedian, M.Y.; Jacobson, S.R. Two-stage prepectoral breast reconstruction. Gland. Surg. 2019, 8, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.B.; Eldred, D.E.; Kim, G.; Curtis, J.M.; Brandon, H.J.; Klykken, P.C. Assessment of silicone gel breast implant biodurability by NMR and EDS techniques. J. Biomed. Mater. Res. Part A 2008, 85, 684–691. [Google Scholar] [CrossRef] [PubMed]
- Stevens, W.G.; Pacella, S.J.; Gear, A.J.; Freeman, M.E.; McWhorter, C.; Tenenbaum, M.J.; Stoker, D.A. Clinical Experience with a Fourth-Generation Textured Silicone Gel Breast Implant: A Review of 1012 Mentor MemoryGel Breast Implants. Aesthetic Surg. J. 2008, 28, 642–647. [Google Scholar] [CrossRef] [PubMed]
- Bengtson, B.P.; Eaves, F.F. High-Resolution Ultrasound in the Detection of Silicone Gel Breast Implant Shell Failure: Background, In Vitro Studies, and Early Clinical Results. Aesthetic Surg. J. 2012, 32, 157–174. [Google Scholar] [CrossRef]
- Cole, N.M. Consequences of the U.S. Food and Drug Administration–Directed Moratorium on Silicone Gel Breast Implants: 1992 to 2006. Plast. Reconstr. Surg. 2018, 141, 1137–1141. [Google Scholar] [CrossRef] [PubMed]
- Cohen, B.E.; Biggs, T.M.; Cronin, E.D.; Collins, D.R. Assessment and Longevity of the Silicone Gel Breast Implant. Plast. Reconstr. Surg. 1997, 99, 1597–1601. [Google Scholar] [CrossRef]
- Lam, M.C.; Walgenbach-Brünagel, G.; Pryalukhin, A.; Vorhold, J.; Pech, T.; Kalff, J.C.; Kristiansen, G.; Walgenbach, K.J. Management of Capsular Contracture in Cases of Silicone Gel Breast Implant Rupture with Use of Pulse Lavage and Open Capsulotomy. Aesthetic Plast. Surg. 2019, 43, 1173–1185. [Google Scholar] [CrossRef]
- Jasanoff, S. Expert Games in Silicone Gel Breast Implant Litigation. In Science in Court; Routledge: London, UK, 2019. [Google Scholar] [CrossRef]
- Henning, C.; Wang, J.; Swift, R.; Eades, B.; Spektor, T.M.; Berenson, J.R. Removal of a Silicone Gel Breast Implant in a Multiple Myeloma Patient Improved Disease Status: A Case Report. Case Rep. Oncol. 2020, 13, 1103–1108. [Google Scholar] [CrossRef]
- Glazebrook, K.N.; Doerge, S.; Leng, S.; Drees, T.A.; Hunt, K.N.; Zingula, S.N.; Pruthi, S.; Geske, J.R.; Carter, R.E.; McCollough, C.H.; et al. Ability of Dual-Energy CT to Detect Silicone Gel Breast Implant Rupture and Nodal Silicone Spread. Am. J. Roentgenol. 2019, 212, 933–942. [Google Scholar] [CrossRef]
- Brown, S.L.; Pennello, G.; Berg, W.A.; Soo, M.S.; Middleton, M.S. Silicone gel breast implant rupture, extracapsular silicone, and health status in a population of women. J. Rheumatol. 2001, 28, 996–1003. [Google Scholar] [PubMed]
- Alfano, C.; Mazzocchi, M. Mammary Compliance: An Objective Measurement of Capsular Contracture. Aesthetic Plast. Surg. 2004, 28, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Henriksen, T.F.; Fryzek, J.P.; Hölmich, L.R.; McLaughlin, J.K.; Kjøller, K.; Høyer, A.P.; Olsen, J.H.; Friis, S. Surgical Intervention and Capsular Contracture After Breast Augmentation: A prospective study of risk factors. Ann. Plast. Surg. 2005, 54, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Brody, G.S. On the Safety of Breast Implants. Plast. Reconstr. Surg. 1997, 100, 1314–1321. [Google Scholar] [CrossRef] [PubMed]
- Henriksen, T.F.; Hölmich, L.R.; Fryzek, J.P.; Friis, S.; McLaughlin, J.K.; Høyer, A.P.; Kjøller, K.; Olsen, J.H. Incidence and Severity of Short-Term Complications After Breast Augmentation: Results from a Nationwide Breast Implant Registry. Ann. Plast. Surg. 2003, 51, 531–539. [Google Scholar] [CrossRef]
- Handel, N.; Cordray, T.; Gutierrez, J.; Jensen, J.A. A Long-Term Study of Outcomes, Complications, and Patient Satisfaction with Breast Implants. Plast. Reconstr. Surg. 2006, 117, 757–767. [Google Scholar] [CrossRef]
- Wick, G.; Grundtman, C.; Mayerl, C.; Wimpissinger, T.-F.; Feichtinger, J.; Zelger, B.; Sgonc, R.; Wolfram, D. The Immunology of Fibrosis. Annu. Rev. Immunol. 2013, 31, 107–135. [Google Scholar] [CrossRef]
- Ji, L.; Wang, T.; Tian, L.; Song, H.; Gao, M. Roxatidine inhibits fibrosis by inhibiting NF-κB and MAPK signaling in macrophages sensing breast implant surface materials. Mol. Med. Rep. 2019, 21, 161–172. [Google Scholar] [CrossRef]
- Kuehlmann, B.A.; Bonham, C.A.; Gurtner, G.C. Abstract 114. Plast. Reconstr. Surg.-Glob. Open 2019, 7, 80. [Google Scholar] [CrossRef]
- Kuo, Y.-L.; Jou, I.-M.; Jeng, S.-F.; Chu, C.-H.; Huang, J.-S.; Hsu, T.-I.; Chang, L.-R.; Huang, P.-W.; Chen, J.-A.; Chou, T.-M. Hypoxia-induced epithelial-mesenchymal transition and fibrosis for the development of breast capsular contracture. Sci. Rep. 2019, 9, 10269. [Google Scholar] [CrossRef]
- Biernacka, A.; Dobaczewski, M.; Frangogiannis, N.G. TGF-β signaling in fibrosis. Growth Factors 2011, 29, 196–202. [Google Scholar] [CrossRef]
- Chaikuad, A.; Bullock, A.N. Structural Basis of Intracellular TGF-β Signaling: Receptors and Smads. Cold Spring Harb. Perspect. Biol. 2016, 8, a022111. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.-M.; Nikolic-Paterson, D.J.; Lan, H.Y. TGF-β: The master regulator of fibrosis. Nat. Rev. Nephrol. 2016, 12, 325–338. [Google Scholar] [CrossRef]
- Lan, H.Y. Diverse Roles of TGF-β/Smads in Renal Fibrosis and Inflammation. Int. J. Biol. Sci. 2011, 7, 1056–1067. [Google Scholar] [CrossRef]
- Margadant, C.; Sonnenberg, A. Integrin–TGF-β crosstalk in fibrosis, cancer and wound healing. EMBO Rep. 2010, 11, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.-H.; Chen, D.-Q.; Wang, Y.-N.; Feng, Y.-L.; Cao, G.; Vaziri, N.D.; Zhao, Y.-Y. New insights into TGF-β/Smad signaling in tissue fibrosis. Chem. Interact. 2018, 292, 76–83. [Google Scholar] [CrossRef] [PubMed]
- ISO 14607:2018; Non-Active Surgical Implants—Mammary Implants—Particular Requirements. ISO: London, UK, 2018. Available online: https://www.iso.org/standard/63973.html (accessed on 17 November 2022).
- Stevens, W.G.; Nahabedian, M.Y.; Calobrace, M.B.; Harrington, J.L.; Capizzi, P.J.; Cohen, R.; D’Incelli, R.C.; Beckstrand, M. Risk Factor Analysis for Capsular Contracture: A 5-year sientra study analysis using round, smooth, and textured implants for breast augmentation. Plast. Reconstr. Surg. 2013, 132, 1115–1123. [Google Scholar] [CrossRef] [PubMed]
- Webb, L.H.; Aime, V.L.; Do, A.; Mossman, K.; Mahabir, R.C. Textured Breast Implants: A Closer Look at the Surface Debris Under the Microscope. Plast. Surg. 2017, 25, 179–183. [Google Scholar] [CrossRef]
- Martin, S.V.; Ho, W.; Khan, K. An extended 7-year review of textured breast implants for primary breast augmentation: Allergan versus Mentor. Ann. Breast Surg. 2019, 3, 14. [Google Scholar] [CrossRef]
- Doloff, J.C.; Veiseh, O.; de Mezerville, R.; Sforza, M.; Perry, T.A.; Haupt, J.; Jamiel, M.; Chambers, C.; Nash, A.; Aghlara-Fotovat, S.; et al. The surface topography of silicone breast implants mediates the foreign body response in mice, rabbits and humans. Nat. Biomed. Eng. 2021, 5, 1115–1130. [Google Scholar] [CrossRef] [PubMed]
- Cappellano, G.; Ploner, C.; Lobenwein, S.; Sopper, S.; Hoertnagl, P.; Mayerl, C.; Wick, N.; Pierer, G.; Wick, G.; Wolfram, D. Immunophenotypic characterization of human T cells after in vitro exposure to different silicone breast implant surfaces. PLoS ONE 2018, 13, e0192108. [Google Scholar] [CrossRef] [PubMed]
- Lam, M.; Migonney, V.; Falentin-Daudre, C. Review of silicone surface modification techniques and coatings for antibacterial/antimicrobial applications to improve breast implant surfaces. Acta Biomater. 2020, 121, 68–88. [Google Scholar] [CrossRef] [PubMed]
- Atlan, M.; Nuti, G.; Wang, H.; Decker, S.; Perry, T. Breast implant surface texture impacts host tissue response. J. Mech. Behav. Biomed. Mater. 2018, 88, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Shurer, C.R.; Schmidt, S.; Gupta, V.K.; Chuang, G.; Su, J.; Watkins, A.R.; Shetty, A.; Spector, J.A.; Hui, C.-Y.; et al. The surface stress of biomedical silicones is a stimulant of cellular response. Sci. Adv. 2020, 6, eaay0076. [Google Scholar] [CrossRef] [PubMed]
- Kyle, D.J.; Oikonomou, A.; Hill, E.; Bayat, A. Development and functional evaluation of biomimetic silicone surfaces with hierarchical micro/nano-topographical features demonstrates favourable in vitro foreign body response of breast-derived fibroblasts. Biomaterials 2015, 52, 88–102. [Google Scholar] [CrossRef]
- Valencia-Lazcano, A.A.; Alonso-Rasgado, T.; Bayat, A. Characterisation of breast implant surfaces and correlation with fibroblast adhesion. J. Mech. Behav. Biomed. Mater. 2013, 21, 133–148. [Google Scholar] [CrossRef]
- McLaughlin, C.; Hughes, A.J.B.; Parham, C.S.; Fritsche, M.B.; Potochny, J.D.; Kunselman, A.M.; Ravnic, D.J.D. Smooth Versus Textured Tissue Expander Breast Reconstruction: Complications and Efficacy. Ann. Plast. Surg. 2022, 88, S288–S292. [Google Scholar] [CrossRef]
- Giot, J.-P.; Paek, L.S.; Nizard, N.; El-Diwany, M.; Gaboury, L.A.; Nelea, M.; Bou-Merhi, J.S.; Harris, P.G.; Danino, M.A. The double capsules in macro-textured breast implants. Biomaterials 2015, 67, 65–72. [Google Scholar] [CrossRef]
- Hall-Findlay, E.J. Breast Implant Complication Review: Double Capsules and Late Seromas. Plast. Reconstr. Surg. 2011, 127, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Loch-Wilkinson, A.; Beath, K.; Knight, R.J.W.; Wessels, W.L.F.; Magnusson, M.; Papadopoulos, T.; Connell, T.; Lofts, J.; Locke, M.; Hopper, I.; et al. Breast Implant–Associated Anaplastic Large Cell Lymphoma in Australia and New Zealand: High-Surface-Area Textured Implants Are Associated with Increased Risk. Plast. Reconstr. Surg. 2017, 140, 645–654. [Google Scholar] [CrossRef]
- Hu, H.; Jacombs, A.; Vickery, K.; Merten, S.L.; Pennington, D.G.; Deva, A.K. Chronic Biofilm Infection in Breast Implants Is Associated with an Increased T-Cell Lymphocytic Infiltrate: Implications for Breast Implant–Associated Lymphoma. Plast. Reconstr. Surg. 2015, 135, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.R.; Chien, P.N.; Trinh, X.-T.; Nam, S.-Y.; Heo, C.-Y. Comparison of Formation of Capsule among Different Breast Silicone Implants. In Vivo 2022, 36, 2756–2766. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-T.; Park, H.Y.; Jeon, B.-J.; Mun, G.-H.; Bang, S.I.; Pyon, J.K. Does the Textured-Type Tissue Expander Affect the Outcomes of Two-Stage Prosthetic Breast Reconstruction? A Propensity Score Matching Analysis between Macrotextured and Microtextured Expanders. Plast. Reconstr. Surg. 2021, 147, 545–555. [Google Scholar] [CrossRef] [PubMed]
- Fairchild, B.; Ellsworth, W.; Selber, J.C.; Bogue, D.P.; Zavlin, D.; Nemir, S.; Checka, C.M.; Clemens, M.W. Safety and Efficacy of Smooth Surface Tissue Expander Breast Reconstruction. Aesthetic Surg. J. 2020, 40, 53–62. [Google Scholar] [CrossRef]
- Chiu, W.-K.; Fracol, M.; Feld, L.N.; Qiu, C.S.; Kim, J.Y.S. Judging an Expander by Its Cover: A Propensity-Matched Analysis of the Impact of Tissue Expander Surface Texture on First-Stage Breast Reconstruction Outcomes. Plast. Reconstr. Surg. 2021, 147, 1e–6e. [Google Scholar] [CrossRef]
- Dolores, W.; Christian, R.; Harald, N.; Hildegunde, P.; Georg, W. Cellular and molecular composition of fibrous capsules formed around silicone breast implants with special focus on local immune reactions. J. Autoimmun. 2004, 23, 81–91. [Google Scholar] [CrossRef]
- Wolfram, D.; Oberreiter, B.; Mayerl, C.; Soelder, E.; Ulmer, H.; Piza-Katzer, H.; Wick, G.; Backovic, A. Altered systemic serologic parameters in patients with silicone mammary implants. Immunol. Lett. 2008, 118, 96–100. [Google Scholar] [CrossRef]
- Duraes, E.F.R.M.; Durand, P.M.; Morisada, M.M.; Scomacao, I.M.; Duraes, L.C.M.; de Sousa, J.M.B.; Abedi, N.M.; Djohan, R.S.M.; Bernard, S.M.; Moreira, A.M.; et al. A Novel Validated Breast Aesthetic Scale. Plast. Reconstr. Surg. 2022, 149, 1297–1308. [Google Scholar] [CrossRef]
- Prantl, L.; Schreml, S.; Fichtner-Feigl, S.; Pöppl, N.; Eisenmann-Klein, M.; Schwarze, H.; Füchtmeier, B. Clinical and Morphological Conditions in Capsular Contracture Formed around Silicone Breast Implants. Plast. Reconstr. Surg. 2007, 120, 275–284. [Google Scholar] [CrossRef]
- Siggelkow, W.; Gescher, D.; Klee, D.; Malik, E.; Rath, W.; Faridi, A. In Vitro Analysis of Modified Surfaces of Silicone Breast Implants. Int. J. Artif. Organs 2004, 27, 1100–1108. [Google Scholar] [CrossRef]
- Siggelkow, W.; Faridi, A.; Spiritus, K.; Klinge, U.; Rath, W.; Klosterhalfen, B. Histological analysis of silicone breast implant capsules and correlation with capsular contracture. Biomaterials 2003, 24, 1101–1109. [Google Scholar] [CrossRef] [PubMed]
- Handel, N.M.; Jensen, J.A.M.; Black, Q.B.; Waisman, J.R.M.; Silverstein, M.J.M. The Fate of Breast Implants. Plast. Reconstr. Surg. 1995, 96, 1521–1533. [Google Scholar] [CrossRef] [PubMed]
- Mempin, M.; Hu, H.; Chowdhury, D.; Deva, A.; Vickery, K. The A, B and C’s of Silicone Breast Implants: Anaplastic Large Cell Lymphoma, Biofilm and Capsular Contracture. Materials 2018, 11, 2393. [Google Scholar] [CrossRef]
- Bizjak, M.; Selmi, C.; Praprotnik, S.; Bruck, O.; Perricone, C.; Ehrenfeld, M.; Shoenfeld, Y. Silicone implants and lymphoma: The role of inflammation. J. Autoimmun. 2015, 65, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhang, S. T Cells in Fibrosis and Fibrotic Diseases. Front. Immunol. 2020, 11, 1142. [Google Scholar] [CrossRef]
- Shin, B.H.; Kim, B.H.; Kim, S.; Lee, K.; Bin Choy, Y.; Heo, C.Y. Silicone breast implant modification review: Overcoming capsular contracture. Biomater. Res. 2018, 22, 37. [Google Scholar] [CrossRef]
- FDA. Risks and Complications of Breast Implants. Available online: https://www.fda.gov/medical-devices/breast-implants/risks-and-complications-breast-implants. (accessed on 21 November 2022).
- Sforza, M.; Zaccheddu, R.; Alleruzzo, A.; Seno, A.; Mileto, D.; Paganelli, A.; Sulaiman, H.; Payne, M.; Maurovich-Horvat, L. Preliminary 3-Year Evaluation of Experience with SilkSurface and VelvetSurface Motiva Silicone Breast Implants: A Single-Center Experience with 5813 Consecutive Breast Augmentation Cases. Aesthetic Surg. J. 2017, 38, S62. [Google Scholar] [CrossRef]
- Wu, S.S.B.; Duraes, E.F.R.M.; Scomacao, I.M.; Morisada, M.B.; Djohan, R.S.M.; Bernard, S.L.M.; Moreira, A.M.; Schwarz, G.S.M. Beauty Is in the Eye of the Beholder: Factors Influencing Disparity in Perceptions of Breast Reconstruction Aesthetic Outcomes. Plast. Reconstr. Surg. 2022, 150, 42e–50e. [Google Scholar] [CrossRef]
- Buck, D.W.; Shenaq, D.; Heyer, K.; Kato, C.; Kim, J.Y. Patient-subjective cosmetic outcomes following the varying stages of tissue expander breast reconstruction: The importance of completion. Breast 2010, 19, 521–526. [Google Scholar] [CrossRef]
- Munhoz, A.M.; Chala, L.; de Melo, G.G.; Filho, A.D.A.M.; Tucunduva, T.; Gemperli, R. Usefulness of Radio Frequency Identification Device in Diagnosing Rotation of Motiva SmoothSilk Implants after Augmentation Mammoplasty. Plast. Reconstr. Surg.-Glob. Open 2019, 7, e2497. [Google Scholar] [CrossRef]
- Hallab, N.J.; Samelko, L.; Hammond, D. The Inflammatory Effects of Breast Implant Particulate Shedding: Comparison with Orthopedic Implants. Aesthetic Surg. J. 2019, 39, S36–S48. [Google Scholar] [CrossRef] [PubMed]
- Kappel, R.M.; Klunder, A.J.H.; Pruijn, G.J.M. Silicon chemistry and silicone breast implants. Eur. J. Plast. Surg. 2013, 37, 123–128. [Google Scholar] [CrossRef]
- Institute of Medicine (US) Committee on the Safety of Silicone Breast Implants. Immunology of Silicone; Bondurant, S., Ernster, V., Herdman, R., Eds.; National Academies Press: Washington, DC, USA, 1999. [Google Scholar]
- Dieterich, M.; Paepke, S.; Zwiefel, K.; Dieterich, H.; Blohmer, J.; Faridi, A.; Klein, E.; Gerber, B.; Nestle-Kraemling, C. Implant-Based Breast Reconstruction Using a Titanium-Coated Polypropylene Mesh (TiLOOP Bra). Plast. Reconstr. Surg. 2013, 132, 8e–19e. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.X.; Cheng, L.; Xu, L.Y.; Zhu, Y.L. A short follow-up of prosthesis-based breast reconstruction using TiLOOP Bra surgical mesh. Niger. J. Clin. Pract. 2019, 22, 1115–1119. [Google Scholar] [PubMed]
- Casella, D.; Bernini, M.; Bencini, L.; Roselli, J.; Lacaria, M.T.; Martellucci, J.; Banfi, R.; Calabrese, C.; Orzalesi, L. TiLoop Bra mesh used for immediate breast reconstruction: Comparison of retropectoral and subcutaneous implant placement in a prospective single-institution series. Eur. J. Plast. Surg. 2014, 37, 599–604. [Google Scholar] [CrossRef]

| Inclusion Criteria | Exclusion Criteria | |
|---|---|---|
| Female sex | 1 | Sever coagulation disorder, representing a potential contraindication for the elective surgery |
| Age > 18 years | 2 | Rheumatic disease accompanied by oblkigatory intake of immunomodulating therapeutic agents |
| High-risk family history for breast and/or ovarian cancer and/or BRCA1/2 gene mutation carrier | 3 | Severe renal functional disorder: renal insufficiency status IV or V (estimated glomerulary filtration rate (GFR) < 30 mL/min) |
| Planned bilateral mastectomy with simultaneous breast reconstruction | 4 | Active hematological or oncological disease |
| Signed Informed consent form. | 5 | HIV-Infection |
| 6 | Hepatitis-Infection | |
| 7 | Pregnancy or breast-feeding | |
| 8 | Intake of anti-inflammatory drugs | |
| 9 | Carrier of silicone implants (e.g., gastric banding, mammary implants) |
| Surgeon Scale | Time Point Post Op | Numeric Scale |
|---|---|---|
| How satisfied are you with the lower pole expansion of the expander? | 6–8 M | not satisfied: 0 to 3 satisfied 4 to 7 very satisfied: 8 to 10 |
| How satisfied are you with the footprint created by the expander? | 6–8 M | |
| Patient Scale | Time Point Post Op | |
| How satisfied are you with the expander? | 4 W | not satisfied: 0 to 3 satisfied 4 to 7 very satisfied: 8 to 10 |
| Please indicate how comfortable the expander was? | 6–8 M | not comfortable: 0 to 3 comfortable 4 to 7 very comfortable: 8 to 10 |
| IMPLANTATION SITE | Left: SmoothSilk®; Right: Mentor CPX4 | Left: Mentor CPX4; Right: SmoothSilk® | |||||
|---|---|---|---|---|---|---|---|
| PAT 001_001 | PAT 001_002 | PAT 001_007 | PAT 001_003 | PAT 001_004 | PAT 001_005 | PAT 001_006 | |
| Vital Parameters | |||||||
| age (y) | 34 | 41 | 30 | 31 | 26 | 60 | 33 |
| weight (kg) | 86,3 | 54 | 108 | 54 | 58 | 105 | 57 |
| size (cm) | 186,5 | 155,5 | 172 | 167 | 159 | 176 | 166 |
| BMI | 24.8 | 22.3 | 36.5 | 19.4 | 22.9 | 33.9 | 20.7 |
| body surface area | 2.12 | 1.52 | 2.9 | 1.6 | 1.59 | 2.2 | 2.7 |
| Status of natural breast | |||||||
| asymmetry | no | no | no | no | no | no | no |
| scars | no | no | no | no | no | no | no |
| diseases | no | no | no | no | no | no | no |
| active smoker | yes | no | no | no | no | no | no |
| allergies | no | no | no | no | no | no | no |
| Chronic diseases | |||||||
| diabetes | no | yes | no | no | no | no | no |
| Other | |||||||
| job | manual job | office job | office job | office job | office job | manual job | office job |
| physical training (h/week) | >2 | >2 | 0.5–2 | >2 | >2 | 0.5–2 | 0.5–2 |
| dominant hand | right | right | right | right | right | right | right |
| 1st operation: tissue expander implantation | |||||||
| Bilateral prophylactic NSME resection weight [g] | |||||||
| right breast | 449 | 187 | 980 | 200 | 400 | 750 | 208 |
| left breast | 471 | 167 | 1060 | 200 | 450 | 575 | 252 |
| Prepectoral reconstruction volume [cc] | |||||||
| Motiva Flora® SmoothSilk® | 450 | 260 | 570 | 260 | 440 | 570 | 260 |
| Mentor CPX4 | 440 | 250 | 550 | 250 | 450 | 550 | 250 |
| Intrapoerative filling (both devices) | 250 | 150 | 550 | 150 | 300 | 500 | 150 |
| SmoothSilkⓇ | Mentor CPX4 | ||||
|---|---|---|---|---|---|
| Surface Roughness | Ra ~ 4 µM | Ra ~ 60 µM | |||
| Mean | (±std) | Mean | (±std) | p Value | |
| age (y) | 35.2 | 11.4 | 35.2 | 11.4 | intraindividual comparison >0.9999 |
| weight (kg) | 71.4 | 24.5 | 71.4 | 24.5 | |
| size (cm) | 168.6 | 10.5 | 168.6 | 10.5 | |
| BMI | 25.1 | 6.7 | 25.1 | 6.7 | |
| Bilateral prophylactic NSME resection weight [g] | |||||
| left breast | 434.9 | 404.0 | 436.9 | 454.0 | 0.993196 |
| right breast | 334.2 | 257.5 | 337.9 | 174.4 | 0.975407 |
| Prepectoral reconstruction volume [cc] | |||||
| left breast | 405.5 | 156.3 | 392.6 | 151.8 | 0.877595 |
| right breast | 360.8 | 151.1 | 352.7 | 150.0 | 0.920737 |
| intaoperative filling [mL] | 254.7 | 169.4 | 254.7 | 169.4 | intraindividual comparison >0.9999 |
| exchange time point [d] | 204.8 | 25.8 | 204.8 | 25.8 | |
| SmoothSilk® | Mentor CPX4 | |||||
|---|---|---|---|---|---|---|
| Surface Roughness | Ra ~ 4 µM | Ra ~ 60 µM | ||||
| Time Point Post Op | Mean | (±std) | Mean | (±std) | p Value | |
| Wound dehiscence | 2 W, 4 W, 16 W | no | no | >0.9999 | ||
| Signs of inflammation | 2 W, 4 W, 16 W | no | no | >0.9999 | ||
| symmetry | 2 W, 4 W, 16 W | yes | yes | >0.9999 | ||
| Breast structure swelling | 2 W, 4 W, 16 W | no | no | >0.9999 | ||
| Expander malposition | 2 W, 4 W, 16 W | no | no | >0.9999 | ||
| filling visits | 6–8 M | 0.86 | 0.90 | 0.86 | 0.90 | >0.9999 |
| Palpation | ||||||
| firm | 2 W, 4 W, 16 W | 1 (14.30%) | 0 (0%) | |||
| normal | 2 W, 4W, 16 W | 3 (42.85%) | 6 (85.70%) | |||
| soft | 2 W, 4 W, 16 W | 3 (42.85%) | 1 (14.30%) | |||
| SmoothSilk® | Mentor CPX4 | |||||
|---|---|---|---|---|---|---|
| Surface Roughness | Ra ~ 4 µM | Ra ~ 60 µM | ||||
| Surgeon scale | time point post op | Mean | (±std) | Mean | (±std) | p value |
| How satisfied are you with the lower pole expansion of the expander? | 6–8 M | 9.00 | 0.63 | 7.33 | 1.37 | 0.021872 * |
| How satisfied are yopu with the footprint created by the expander? | 6–8 M | 8.67 | 0.52 | 7.17 | 1.72 | 0.068264 |
| Patient scale | time point post op | Mean | (±std) | Mean | (±std) | pvalue |
| How satisfied are you with the expander? | 4W | 9.50 | 1.22 | 7.67 | 1.21 | 0.026164 * |
| Please indicate how comfortable the expander was? | 6-8M | 8.83 | 0.98 | 5.67 | 2.88 | 0.028731 * |
| numeric scale | very satisfied: 10 to 7 | satisfied 7 to 4 | not satisfied: 3 to 0 | |||
| very comfortable: 10 to 7 | comfortable 7 to 4 | not comfortable: 3 to 0 | ||||
| First Stage of Reconstruction | Second Stage of Reconstruction | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Expander Type | Mentor CPX4 * | SmoothSilk® * | Mentor CPX4 * | SmoothSilk® * | ||||||
| Surface Roughness | Ra ~ 60 µM | Ra ~ 4 µM | Ra ~ 60 µM | Ra ~ 4 µM | ||||||
| Questions | Mean | (±std) | Mean | (±std) | p-Value | Mean | (std) | Mean | (std) | p-Value |
| Breast | ||||||||||
| Breast position | 4.04 | (0.57) | 4.07 | (0.59) | 0.9173 | 4.25 | (0.50) | 4.25 | (0.58) | >0.999 |
| Inframammary fold | 4.50 | (0.44) | 4.21 | (0.56) | 0.3454 | 4.38 | (0.49) | 4.50 | (0.48) | 0.6933 |
| Volume | 4.36 | (0.35) | 4.36 | (0.35) | >0.999 | 4.38 | (0.66) | 4.29 | (0.68) | 0.8482 |
| Shape and contour | 3.79 | (0.85) | 3.75 | (0.81) | 0.9419 | 4.29 | (0.53) | 4.17 | (0.61) | 0.7355 |
| Scar | ||||||||||
| Appearance | 4.54 | (0.47) | 4.46 | (0.59) | 0.8205 | 4.50 | (0.52) | 4.54 | (0.44) | 0.8943 |
| Nipple-Areola Complex | ||||||||||
| Nipple position | 4.29 | (0.51) | 4.29 | (0.63) | >0.999 | 4.58 | (0.47) | 4.29 | (0.73) | 0.4693 |
| First Stage | Second Stage | ||||
|---|---|---|---|---|---|
| Questions | Mean | (±std) | Mean | (±std) | p-Value |
| Breast | |||||
| Symmetry | 4.29 | (0.54) | 4.42 | (0.49) | 0.6850 |
| Breast position | 4.05 | (0.58) | 4.25 | (0.54) | 0.4035 |
| Inframammary fold | 4.36 | (0.52) | 4.44 | (0.49) | 0.7031 |
| Volume | 4.36 | (0.35) | 4.33 | (0.67) | 0.9125 |
| Shape and contour | 3.77 | (0.83) | 4.23 | (0.57) | 0.1326 |
| Scar | |||||
| Appearance | 4.50 | (0.53) | 4.52 | (0.48) | 0.9216 |
| Nipple-Areola Complex | |||||
| Nipple position | 4.29 | (0.57) | 4.44 | (0.63) | 0.5428 |
| Overall Appearance | 4.04 | (0.65) | 4.31 | (0.71) | 0.3271 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schoberleitner, I.; Augustin, A.; Egle, D.; Brunner, C.; Amort, B.; Zelger, B.; Brunner, A.; Wolfram, D. Is It All about Surface Topography? An Intra-Individual Clinical Outcome Analysis of Two Different Implant Surfaces in Breast Reconstruction. J. Clin. Med. 2023, 12, 1315. https://doi.org/10.3390/jcm12041315
Schoberleitner I, Augustin A, Egle D, Brunner C, Amort B, Zelger B, Brunner A, Wolfram D. Is It All about Surface Topography? An Intra-Individual Clinical Outcome Analysis of Two Different Implant Surfaces in Breast Reconstruction. Journal of Clinical Medicine. 2023; 12(4):1315. https://doi.org/10.3390/jcm12041315
Chicago/Turabian StyleSchoberleitner, Ines, Angela Augustin, Daniel Egle, Christine Brunner, Birgit Amort, Bettina Zelger, Andrea Brunner, and Dolores Wolfram. 2023. "Is It All about Surface Topography? An Intra-Individual Clinical Outcome Analysis of Two Different Implant Surfaces in Breast Reconstruction" Journal of Clinical Medicine 12, no. 4: 1315. https://doi.org/10.3390/jcm12041315
APA StyleSchoberleitner, I., Augustin, A., Egle, D., Brunner, C., Amort, B., Zelger, B., Brunner, A., & Wolfram, D. (2023). Is It All about Surface Topography? An Intra-Individual Clinical Outcome Analysis of Two Different Implant Surfaces in Breast Reconstruction. Journal of Clinical Medicine, 12(4), 1315. https://doi.org/10.3390/jcm12041315








