Which Adverse Events and Which Drugs Are Implicated in Drug-Related Hospital Admissions? A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Reporting
2.2. Literature Search
2.3. Study Selection Process
2.4. Eligibility Criteria
2.5. Assessing Reporting Quality and Risk of Bias
2.6. Data Extraction
2.7. Statistical Analysis
2.7.1. Prevalence Estimates in Each Study
2.7.2. Meta-Analyses of Prevalence for Research Aims 1 to 3
2.7.3. Sensitivity Analyses
3. Results
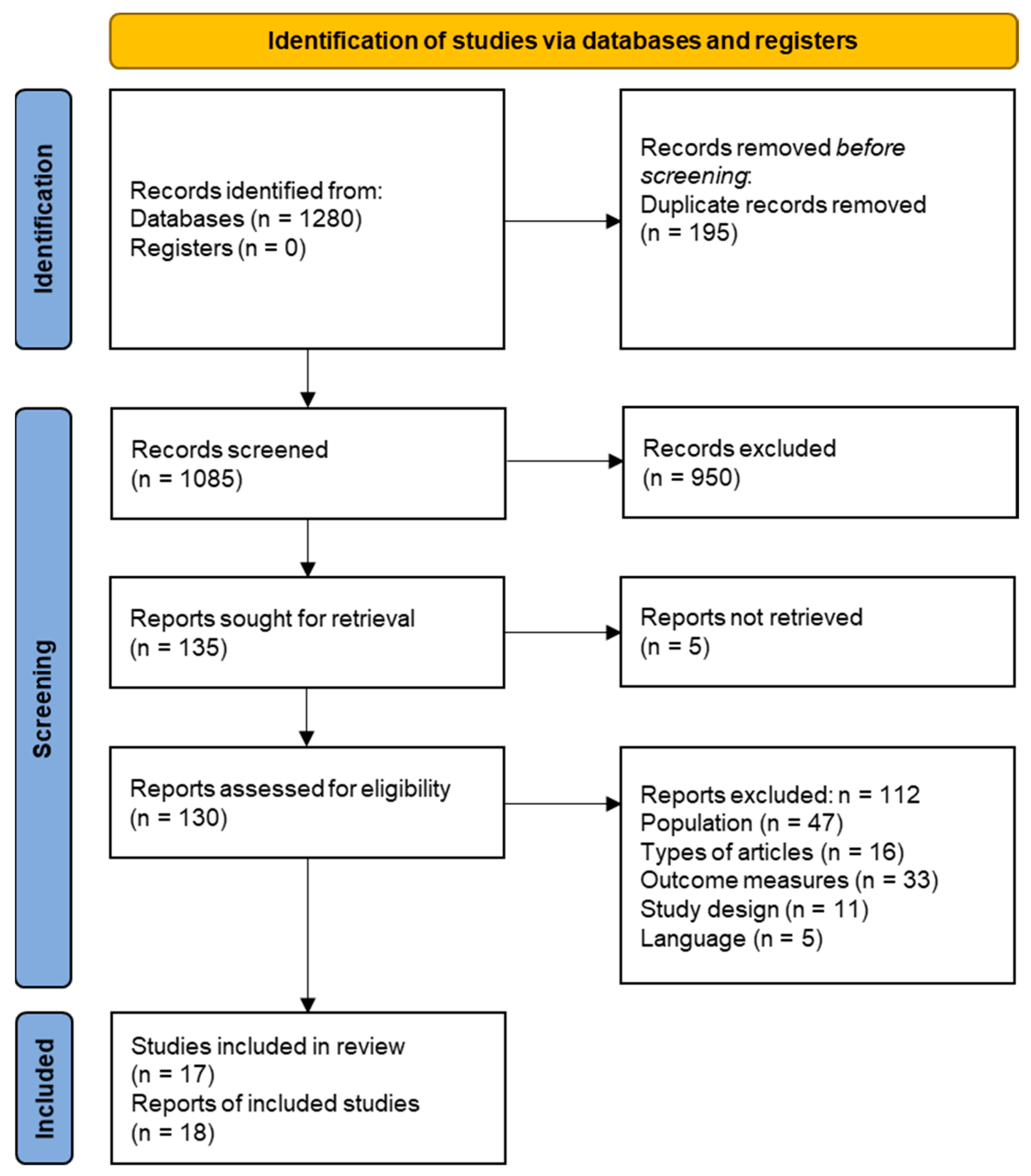
3.1. Literature Search and Study Selection
3.2. Study Characteristics
3.3. Quality of Reporting
3.4. Risk of Bias
3.5. Prevalence of Drug-Related Admissions
3.6. Preventability of ADRs/ADEs
3.7. Implicated ADRs
3.8. Implicated Drugs and Drug Groups
4. Discussion
4.1. Summary of Principal Findings
4.2. Comparison with Other Literature
4.3. Strengths and Limitations
4.4. Implications for Research and Practice
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Medication without Harm. Available online: https://www.who.int/initiatives/medication-without-harm (accessed on 17 April 2022).
- Rottenkolber, D.; Schmiedl, S.; Rottenkolber, M.; Farker, K.; Saljé, K.; Mueller, S.; Hippius, M.; Thuermann, P.A.; Hasford, J. Adverse drug reactions in Germany: Direct costs of internal medicine hospitalizations. Pharmacoepidemiol. Drug Saf. 2011, 20, 626–634. [Google Scholar] [CrossRef] [PubMed]
- Bundesministerium für Gesundheit. Aktionsplan 2021–2024 zur Verbesserung der Arzneimitteltherapiesicherheit in Deutschland. Available online: https://www.akdae.de/AMTS/Aktionsplan/Aktionsplan-2021-2024/Aktionsplan-AMTS-2021-2024.pdf (accessed on 17 April 2022).
- Pirmohamed, M.; James, S.; Meakin, S.; Green, C.; Scott, A.K.; Walley, T.J.; Farrar, K.; Park, B.K.; Breckenridge, A.M. Adverse drug reactions as cause of admission to hospital: Prospective analysis of 18 820 patients. BMJ 2004, 329, 15–19. [Google Scholar] [CrossRef]
- Schurig, A.M.; Böhme, M.; Just, K.S.; Scholl, C.; Dormann, H.; Plank-Kiegele, B.; Seufferlein, T.; Gräff, I.; Schwab, M.; Stingl, J.C. Adverse Drug Reactions (ADR) and Emergencies. Dtsch. Arztebl. Int. 2018, 115, 251–258. [Google Scholar] [CrossRef]
- Howard, R.L.; Avery, A.J.; Slavenburg, S.; Royal, S.; Pipe, G.; Lucassen, P.; Pirmohamed, M. Which drugs cause preventable admissions to hospital? A systematic review. Br. J. Clin. Pharmacol. 2007, 63, 136–147. [Google Scholar] [CrossRef]
- Al Hamid, A.; Ghaleb, M.; Aljadhey, H.; Aslanpour, Z. A systematic review of hospitalization resulting from medicine-related problems in adult patients. Br. J. Clin. Pharmacol. 2014, 78, 202–217. [Google Scholar] [CrossRef]
- Schmiedl, S.; Rottenkolber, M.; Szymanski, J.; Drewelow, B.; Siegmund, W.; Hippius, M.; Farker, K.; Guenther, I.R.; Hasford, J.; Thuermann, P.A.; et al. Preventable ADRs leading to hospitalization—Results of a long-term prospective safety study with 6,427 ADR cases focusing on elderly patients. Expert Opin. Drug Saf. 2018, 17, 125–137. [Google Scholar] [CrossRef] [PubMed]
- Kongkaew, C.; Noyce, P.R.; Ashcroft, D.M. Hospital admissions associated with adverse drug reactions: A systematic review of prospective observational studies. Ann. Pharmacother. 2008, 42, 1017–1025. [Google Scholar] [CrossRef] [PubMed]
- Alhawassi, T.M.; Krass, I.; Bajorek, B.V.; Pont, L.G. A systematic review of the prevalence and risk factors for adverse drug reactions in the elderly in the acute care setting. Clin. Interv. Aging 2014, 9, 2079–2086. [Google Scholar] [CrossRef]
- Taché, S.V.; Sönnichsen, A.; Ashcroft, D.M. Prevalence of adverse drug events in ambulatory care: A systematic review. Ann. Pharmacother. 2011, 45, 977–989. [Google Scholar] [CrossRef]
- Leendertse, A.J.; Visser, D.; Egberts, A.C.; van den Bemt, P.M. The relationship between study characteristics and the prevalence of medication-related hospitalizations: A literature review and novel analysis. Drug Saf. 2010, 33, 233–244. [Google Scholar] [CrossRef]
- Brvar, M.; Fokter, N.; Bunc, M.; Mozina, M. The frequency of adverse drug reaction related admissions according to method of detection, admission urgency and medical department specialty. BMC Clin. Pharmacol. 2009, 9, 8. [Google Scholar] [CrossRef] [PubMed]
- Wallerstedt, S.M.; Hoffmann, M.; Lönnbro, J. Methodological issues in research on drug-related admissions: A meta-epidemiological review with focus on causality assessments. Br. J. Clin. Pharmacol. 2022, 88, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Nebeker, J.R.; Barach, P.; Samore, M.H. Clarifying adverse drug events: A clinician’s guide to terminology, documentation, and reporting. Ann. Intern. Med. 2004, 140, 795–801. [Google Scholar] [CrossRef] [PubMed]
- International drug monitoring: The role of national centres. Report of a WHO meeting. World Health Organ. Tech. Rep. Ser. 1972, 498, 1–25.
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Naranjo, C.A.; Busto, U.; Sellers, E.M.; Sandor, P.; Ruiz, I.; Roberts, E.A.; Janecek, E.; Domecq, C.; Greenblatt, D.J. A method for estimating the probability of adverse drug reactions. Clin. Pharmacol. Ther. 1981, 30, 239–245. [Google Scholar] [CrossRef]
- World Health Organization (WHO). The WHO-UMC System. Available online: https://www.who.int/publications/m/item/WHO-causality-assessment (accessed on 17 April 2022).
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. PLoS Med. 2007, 4, e296. [Google Scholar] [CrossRef]
- Clopper, C.J.; Pearson, E.S. The Use of Confidence or Fiducial Limits Illustrated in the Case of the Binomial. Biometrika 1934, 26, 404–413. [Google Scholar] [CrossRef]
- WHO International Classification of Diseases, Version 10. Available online: https://icd.who.int/browse10/2019/en (accessed on 17 April 2022).
- Brown, E.G.; Wood, L.; Wood, S. The Medical Dictionary for Regulatory Activities (MedDRA). Drug Saf. 1999, 20, 109–117. [Google Scholar] [CrossRef]
- WHO Collaborating Centre for Drug Statistics Methodology. ATC/DDD Index. Available online: https://www.whocc.no/atc_ddd_index/ (accessed on 17 April 2022).
- Lin, L.; Chu, H. Meta-analysis of Proportions Using Generalized Linear Mixed Models. Epidemiology 2020, 31, 713–717. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahern, F.; Sahm, L.J.; Lynch, D.; McCarthy, S. Determining the frequency and preventability of adverse drug reaction-related admissions to an Irish University Hospital: A cross-sectional study. Emerg. Med. J. 2014, 31, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Lönnbro, J.; Holmqvist, L.; Persson, E.; Thysell, P.; Åberg, N.D.; Wallerstedt, S.M. Inter-rater reliability of assessments regarding the quality of drug treatment, and drug-related hospital admissions. Br. J. Clin. Pharmacol. 2021, 87, 3825–3834. [Google Scholar] [CrossRef] [PubMed]
- Mejía, G.; Saiz-Rodríguez, M.; Gómez de Olea, B.; Ochoa, D.; Abad-Santos, F. Urgent Hospital Admissions Caused by Adverse Drug Reactions and Medication Errors-A Population-Based Study in Spain. Front. Pharmacol. 2020, 11, 734. [Google Scholar] [CrossRef] [PubMed]
- Pedros, C.; Quintana, B.; Rebolledo, M.; Porta, N.; Vallano, A.; Arnau, J.M. Prevalence, risk factors and main features of adverse drug reactions leading to hospital admission. Eur. J. Clin. Pharmacol. 2014, 70, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Rydberg, D.M.; Holm, L.; Engqvist, I.; Fryckstedt, J.; Lindh, J.D.; Stiller, C.-O.; Asker-Hagelberg, C. Adverse Drug Reactions in a Tertiary Care Emergency Medicine Ward—Prevalence, Preventability and Reporting. PLoS ONE 2016, 11, e0162948. [Google Scholar] [CrossRef] [PubMed]
- Girgin, M.C.; Yanturali, S.; Arici, M.A.; Çolak Oray, N.; Doylan, Ö.; Demiral, Y.; Tunçok, Y. Emergency department visits caused by adverse drug reactions: Results of aTurkish university hospital. Turk. J. Med. Sci. 2016, 46, 945–952. [Google Scholar] [CrossRef]
- Just, K.S.; Dormann, H.; Schurig, M.; Böhme, M.; Steffens, M.; Plank-Kiegele, B.; Ettrich, K.; Seufferlein, T.; Gräff, I.; Igel, S.; et al. The phenotype of adverse drug effects: Do emergency visits due to adverse drug reactions look different in older people? Results from the ADRED study. Br. J. Clin. Pharmacol. 2020, 86, 2144–2154. [Google Scholar] [CrossRef]
- Just, K.S.; Dormann, H.; Böhme, M.; Schurig, M.; Schneider, K.L.; Steffens, M.; Dunow, S.; Plank-Kiegele, B.; Ettrich, K.; Seufferlein, T.; et al. Personalising drug safety-results from the multi-centre prospective observational study on Adverse Drug Reactions in Emergency Departments (ADRED). Eur. J. Clin. Pharmacol. 2020, 76, 439–448. [Google Scholar] [CrossRef]
- Laureau, M.; Vuillot, O.; Gourhant, V.; Perier, D.; Pinzani, V.; Lohan, L.; Faucanie, M.; Macioce, V.; Marin, G.; Giraud, I.; et al. Adverse Drug Events Detected by Clinical Pharmacists in an Emergency Department: A Prospective Monocentric Observational Study. J. Patient Saf. 2021, 17, e1040–e1049. [Google Scholar] [CrossRef]
- Alayed, N.; Alkhalifah, B.; Alharbi, M.; Alwohaibi, N.; Farooqui, M. Adverse Drug Reaction (ADR) as a Cause of Hospitalization at a Government Hospital in Saudi Arabia: A Prospective Observational Study. Curr. Drug Saf. 2019, 14, 192–198. [Google Scholar] [CrossRef]
- Aldardeer, N.; Benslimane, N.; Khalifa, M. Medications Related Emergency Admissions: Causes and Recommendations. Stud. Health Technol. Inform. 2017, 238, 169–172. [Google Scholar]
- Chan, S.L.; Ang, X.; Sani, L.L.; Ng, H.Y.; Winther, M.D.; Liu, J.J.; Brunham, L.R.; Chan, A. Prevalence and characteristics of adverse drug reactions at admission to hospital: A prospective observational study. Br. J. Clin. Pharmacol. 2016, 82, 1636–1646. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jin, L.; Zhang, X.; Bai, R.; Chen, D.; Ma, Y.; Zhai, X. Emergency hospitalizations for adverse drug events in China: Clinical pharmacists’ approach to assessment and categorization. Pharmacoepidemiol. Drug Saf. 2021, 30, 636–643. [Google Scholar] [CrossRef]
- Jatau, A.I.; Aung, M.M.T.; Kamauzaman, T.H.T.; Rahman, A.F.A. Prevalence of Drug-Related Emergency Department Visits at a Teaching Hospital in Malaysia. Drugs Real World Outcomes 2015, 2, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Curtis, K.; Zaidi, S.T.R.; Van, C.; Thomson, A.; Castelino, R. Prevalence, characteristics, and reporting of adverse drug reactions in an Australian hospital: A retrospective review of hospital admissions due to adverse drug reactions. Expert Opin. Drug Saf. 2021, 20, 1267–1274. [Google Scholar] [CrossRef] [PubMed]
- Phillips, A.L.; Nigro, O.; Macolino, K.A.; Scarborough, K.C.; Doecke, C.J.; Angley, M.T.; Shakib, S. Hospital admissions caused by adverse drug events: An Australian prospective study. Aust. Health Rev. 2014, 38, 51–57. [Google Scholar] [CrossRef]
- Hohl, C.M.; Badke, K.; Zhao, A.; Wickham, M.E.; Woo, S.A.; Sivilotti, M.L.A.; Perry, J.J. Prospective Validation of Clinical Criteria to Identify Emergency Department Patients at High Risk for Adverse Drug Events. Acad. Emerg. Med. 2018, 25, 1015–1026. [Google Scholar] [CrossRef]
- Karch, F.E.; Lasagna, L. Toward the operational identification of adverse drug reactions. Clin. Pharmacol. Ther. 1977, 21, 247–254. [Google Scholar] [CrossRef]
- Gallagher, R.M.; Kirkham, J.J.; Mason, J.R.; Bird, K.A.; Williamson, P.R.; Nunn, A.J.; Turner, M.A.; Smyth, R.L.; Pirmohamed, M. Development and inter-rater reliability of the Liverpool adverse drug reaction causality assessment tool. PLoS ONE 2011, 6, e28096. [Google Scholar] [CrossRef]
- Arimone, Y.; Bidault, I.; Dutertre, J.P.; Gérardin, M.; Guy, C.; Haramburu, F.; Hillaire-Buys, D.; Meglio, C.; Penfornis, C.; Théophile, H.; et al. Updating the French method for the causality assessment of adverse drug reactions. Therapie 2013, 68, 69–76. [Google Scholar] [CrossRef]
- Jones, J.K. Adverse drug reactions in the community health setting: Approaches to recognizing, counseling, and reporting. Fam. Community Health 1982, 5, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Hallas, J.; Harvald, B.; Gram, L.F.; Grodum, E.; Brøsen, K.; Haghfelt, T.; Damsbo, N. Drug related hospital admissions: The role of definitions and intensity of data collection, and the possibility of prevention. J. Intern. Med. 1990, 228, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Schumock, G.T.; Thornton, J.P. Focusing on the preventability of adverse drug reactions. Hosp. Pharm. 1992, 27, 538. [Google Scholar] [PubMed]
- Chan, M.; Nicklason, F.; Vial, J.H. Adverse drug events as a cause of hospital admission in the elderly. Intern. Med. J. 2001, 31, 199–205. [Google Scholar] [CrossRef]
- Nelson, K.M.; Talbert, R.L. Drug-related hospital admissions. Pharmacotherapy 1996, 16, 701–707. [Google Scholar]
- Queneau, P.; Trombert, B.; Carpentier, F.; Trinh-Duc, A.; Bannwarth, B.; Bouget, J. Adverse drug effects: A prospective study by Apnet performed in seven emergency care units in France: Propositions for preventive measures. Ann. Pharm. Fr. 2005, 63, 131–142. [Google Scholar] [CrossRef]
- Hakkarainen, K.M.; Hedna, K.; Petzold, M.; Hägg, S. Percentage of patients with preventable adverse drug reactions and preventability of adverse drug reactions--a meta-analysis. PLoS ONE 2012, 7, e33236. [Google Scholar] [CrossRef]
- Patel, N.S.; Patel, T.K.; Patel, P.B.; Naik, V.N.; Tripathi, C.B. Hospitalizations due to preventable adverse reactions-a systematic review. Eur. J. Clin. Pharmacol. 2017, 73, 385–398. [Google Scholar] [CrossRef]
- Angamo, M.T.; Chalmers, L.; Curtain, C.M.; Bereznicki, L.R. Adverse-Drug-Reaction-Related Hospitalisations in Developed and Developing Countries: A Review of Prevalence and Contributing Factors. Drug Saf. 2016, 39, 847–857. [Google Scholar] [CrossRef]
- American Geriatrics Society 2019 Updated AGS Beers Criteria® for Potentially Inappropriate Medication Use in Older Adults. J. Am. Geriatr. Soc. 2019, 67, 674–694. [CrossRef] [PubMed]
- Gallagher, P.; Ryan, C.; Byrne, S.; Kennedy, J.; O’Mahony, D. STOPP (Screening Tool of Older Person’s Prescriptions) and START (Screening Tool to Alert doctors to Right Treatment). Consensus validation. Int. J. Clin. Pharmacol. Ther. 2008, 46, 72–83. [Google Scholar] [CrossRef]
- Dreischulte, T.; Donnan, P.; Grant, A.; Hapca, A.; McCowan, C.; Guthrie, B. Safer Prescribing—A Trial of Education, Informatics, and Financial Incentives. N. Engl. J. Med. 2016, 374, 1053–1064. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Avery, A.J.; Rodgers, S.; Cantrill, J.A.; Armstrong, S.; Cresswell, K.; Eden, M.; Elliott, R.A.; Howard, R.; Kendrick, D.; Morris, C.J.; et al. A pharmacist-led information technology intervention for medication errors (PINCER): A multicentre, cluster randomised, controlled trial and cost-effectiveness analysis. Lancet 2012, 379, 1310–1319. [Google Scholar] [CrossRef] [PubMed]
- Beijer, H.J.; de Blaey, C.J. Hospitalisations caused by adverse drug reactions (ADR): A meta-analysis of observational studies. Pharm. World Sci. 2002, 24, 46–54. [Google Scholar] [CrossRef]
- Tannenbaum, C.; Martin, P.; Tamblyn, R.; Benedetti, A.; Ahmed, S. Reduction of inappropriate benzodiazepine prescriptions among older adults through direct patient education: The EMPOWER cluster randomized trial. JAMA Intern. Med. 2014, 174, 890–898. [Google Scholar] [CrossRef] [Green Version]




| Author, Year | Location | Data Collection (P/R) | Duration (Months) | Sample Size (n) | Age (Years) | Age (years) Mean (SD)/ * Median (IQR or Range) | Gender n Female (%) | ADR/ADE Definition | Causality Method | Preventability Method Rate | ADR or ADE-Related / All Admissions % [95 % CI] | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adverse drug reactions (ADRs) | ||||||||||||
| ADR-related hospital admissions (through the ED) | ||||||||||||
| Ahern et al., 2014 [27] | Ireland | P | 1 | 856 | all ages | ADR: 68.8 (18.0) No ADR: 44.8 (25.8) | ADR: 37 (49.3) No ADR: 366 (46.9) | WHO | Naranjo | Hallas | 43/75 (57.3) | 75/856 8.8 [7.0–10.9] |
| Alayed et al., 2019 [36] | Saudi Arabia | P | 6 | unclear | > 12 | 49.1 (22.4) | 18 (47.4) | WHO | Naranjo | - | - | 38/- |
| Aldardeer et al., 2017 [37] | Saudi Arabia | R | 6 | 698 | all ages | MREA: 55 No MREA: 54.3 | 326 (46.7) | Author defined | Naranjo | - | - | 43/698 6.2 [4.5–8.2] |
| Chan et al., 2016 [38] | Singapore | P | 3 | 1000 | ≥ 21 | 62.8 (16.9) | 474 (47.4) | Edwards/ Aronson | LCAT | Hallas | 81/83 (97.6) | 81/1000 8.1 [6.5–10.0] |
| Li et al., 2021 [41] | Australia | R | 3 | 5521 | > 18 | * ADR: 74 (IQR: 58–83) No ADR: 55 (IQR: 35–72) | ADR: 264 (53.2) No ADR: 2760 (54.9) | Not reported | WHO-UMC | Hallas | 302/496 (60.9) | 496/5521 9.0 [8.2–9.8] |
| Lönnbro et al., 2021 [28] | Sweden | R | 0.5 | 30 | ≥ 18 | * 72 (range: 25–93) | 15 (50.0) | Not reported | WHO-UMC | Hallas | 3/9 (33.3) | 9/30 30.0 [14.7–49.4] |
| Mejía et al., 2020 [29] △ | Spain | R | 1 | 847 | all ages | * 75 (range: 26–100) | 46 (48.4) | Spanish Ministry of Health | SPhVS Algorithm | - | - | 71/847 8.4 [6.5–10.3] |
| Pedrós et al., 2014 [30] △ | Spain | P | 4 | 4403 | all ages | * ADR: 75 (range: 28–97) No ADR: 66 (range: 16–102) | ADR: 82 (44.1) No ADR: 1660 (39.4) | EU | SPhVS Algorithm | - | - | 186/4403 4.2 [3.7–4.8] |
| Rydberg et al., 2016 [31] | Sweden | P | 13 | 706 | ≥ 18 | * 71 (IQR: 58–82) | 351 (49.7) | Nebeker | Naranjo | Hallas | 31/129 (24.0) | 129/706 18.3 [15.5–21.3] |
| ADR-related ED visits and hospital admissions | ||||||||||||
| Girgin et al., 2016 [32] | Turkey | P | 1 | 1838 | ≥ 17 | 51.5 | 59 (54.6) | WHO | WHO-UMC | - | - | 108/1838 5.9 [4.8–7.1] |
| Hohl et al., 2018 [43] | Canada | P | 12 | 1529 | ≥ 19 | 59.3 (20.9) | 851 (55.7) | WHO | Naranjo (adapted) | - | - | 91/1529 6.0 [4.8–7.3] |
| Jatau et al., 2015 [40] | Malaysia | P | 1.5 | 434 | all ages | 41.0 (21.6) Age distribution: ≤ 19 (6.0), ≥ 20 (94.0) | 83 (62.0) | WHO | French method (Queneau et al. [52]) | Nelson and Talbert criteria | Not reported | 43/434 9.9 [7.3–13.1] |
| Just et al., 2020 [33,34] | Germany | P | 27 | NA | ≥ 18 | * 73 (IQR: 58–80) | 1100 (49.7) | ICH | WHO-UMC | - | - | 2215/- |
| Schurig et al. 2018 [5] | Germany | P | 1 | 10174 | ≥ 18 | * 74.5 (range: 18–97) | 55 % | EMA | WHO-UMC | (Schumock and Thornton) | Not reported | 665/10174 6.5 [6.1–7.0] |
| Adverse drug events (ADEs) | ||||||||||||
| ADE-related hospital admissions | ||||||||||||
| Phillips et al., 2014 [42] | Australia | P | 2 | 370 | all ages | * 64 (IQR: 46–80) | 171 (46.2) | American Society of Health-System Pharmacists | Jones‘ algorithm | Chan et al. [50] | 47/72 (65.3) | 59/370 16.0 [12.4–20.1] |
| Zhang et al., 2021 [39] | China | P | 33 | 4020 | all ages | Age distribution: ≤ 39 (4.0), 40–69 (28.3), ≥ 70 (67.7) | 99 (50.0) | Not reported | Naranjo | Schumock and Thornton | 146/198 (73.7) | 198/4020 4.9 [4.3–5.6] |
| ADE-related ED visits and hospital admissions | ||||||||||||
| Hohl et al., 2018 [43] | Canada | P | 12 | 1529 | ≥ 19 | 59.3 (20.9) | 851 (55.7) | Author defined | Naranjo (adapted) | - | - | 184/1529 12.0 [10.4–13.8] |
| Jatau et al., 2015 [40] | Malaysia | P | 1.5 | 434 | all ages | 41.0 (21.6) Age distribution: ≤ 19 (6.0), ≥ 20 (94.0) | 83 (62.0) | Author defined | French method (Queneau et al. [52]) | Nelson and Talbert criteria | 89/133 (66.9) | 133/434 30.7 [26.3–35.2] |
| Laureau et al., 2021 [35] | France | P | 6 | 8275 | > 18 | 59.7 (22.9) | 4282 (51.7) | Nebeker | French method and Naranjo (modified) | - | - | 1299/8275 15.7 [14.9–16.5] |
| ADR Categories | ADR Frequency as a Proportion of All Cases with ADRs | |||
|---|---|---|---|---|
| Girgin et al. 2016 [32] | Hohl et al. 2018 [43] | Lönnbro et al. 2021 [28] | Prevalence % [95% CI] | |
| Gastrointestinal disorders | X | X | X | 28.5 [21.6; 36.6] |
| Electrolyte disturbances | X | 16.5 [9.5; 25.7] | ||
| Bleeding | X | X | X | 13.5 [9.5; 18.8] |
| Renal and urinary disorders | X | X | X | 11.8 [5.4; 24.1] |
| Skin reactions | X | X | 9.7 [2.2; 33.9] | |
| Cardiac and vascular disorders | X | X | X | 8.7 [5.5; 13.3] |
| Infection | X | X | 5.7 [1.0; 27.4] | |
| Nervous system disorders | X | X | 5.5 [3.1; 9.7] | |
| Blood dyscrasias | X | X | 5.0 [2.1; 11.5] | |
| Musculoskeletal disorders | X | 4.4 [1.2; 10.9] | ||
| Metabolism and nutrition disorders | X | 2.2 [0.3; 7.7] | ||
| Liver disorders | X | 1.1 [0.0; 6.0] | ||
| ATC Code | Drug Groups | Drug Frequency as a Proportion of All Cases with ADRs | ||||||
|---|---|---|---|---|---|---|---|---|
| ATC Level 1 | Alayed et al. 2019 [36] | Girgin et al. 2016 [32] | Hohl et al. 2018 [43] | Just et al. 2020 [34] | Li et al. 2021 [41] | Lönnbro et al. 2021 [28] | Prevalence % [95% CI] | |
| ATC Level 2 | ||||||||
| N | Nervous system | X | X | X | X | 21.2 [13.2; 32.4] | ||
| N02 | Analgesics | X | X | 9.0 [4.8; 16.4] | ||||
| N05 | Psycholeptics | X | X | 7.0 [3.4; 14.0] | ||||
| N03 | Antiepileptics | X | X | X | 3.3 [2.7; 4.1] | |||
| N06 | Psychoanaleptics | X | 3.3 [0.7; 9.3] | |||||
| N07 | Other nervous system drugs | X | 2.2 [0.3; 7.7] | |||||
| N04 | Anti-parkinson drugs | X | X | 1.6 [1.2; 2.2] | ||||
| C | Cardiovascular system | X | X | X | X | 19.9 [10.2; 35.0] | ||
| C09 | Agents acting on the renin-angiotensin system | X | X | X | 11.6 [7.2; 18.1] | |||
| C03 | Diuretics | X | X | X | X | 11.4 [7.2; 17.6] | ||
| C07 | Beta blocking agents | X | X | X | X | 5.7 [2.1; 14.6] | ||
| C08 | Calcium channel blockers | X | X | 3.9 [3.2; 4.7] | ||||
| C10 | Lipid modifying agents | X | 1.2 [0.8; 1.7] | |||||
| B | Blood and blood forming organs | X | X | X | X | 18.0 [13.0; 24.4] | ||
| B01 | Antithrombotic agents | X | X | X | X | 18.2 [11.0; 28.6] | ||
| B03 | Antianemic preparations | X | X | 0.2 [0.1; 0.5] | ||||
| L | Antineoplastic and immunomodulating agents | X | X | X | X | 9.7 [5.4; 16.6] | ||
| L04 | Immunosuppressants | X | X | 10.6 [4.5; 23.1] | ||||
| L01 | Antineoplastic agents | X | X | X | 5.8 [3.0; 11.2] | |||
| J | Antiinfectives for systemic use | X | X | X | 9.4 [2.0; 35.2] | |||
| J01 | Antibacterials for systemic use | X | X | X | 7.2 [3.9; 12.9] | |||
| J02 | Antimycotics for systemic use | X | 1.1 [0.0; 6.0] | |||||
| A | Alimentary tract and metabolism | X | X | X | X | 8.7 [4.4; 16.3] | ||
| A10 | Drugs used in diabetes | X | X | X | 2.7 [2.1; 3.4] | |||
| A02 | Drugs for acid related disorders | X | 1.9 [1.3; 2.5] | |||||
| A06 | Drugs for constipation | X | X | 0.8 [0.5; 1.3] | ||||
| A11 | Vitamins | X | X | 0.4 [0.2; 0.8] | ||||
| A12 | Mineral supplements | X | 0.3 [0.1; 0.6] | |||||
| M | Musculo-skeletal system | X | X | X | 4.8 [2.0; 11.0] | |||
| M01 | Antiinflammatory and antirheumatic products | X | X | 6.2 [3.1; 11.9] | ||||
| M03 | Muscle relaxants | X | 1.1 [0.0; 6.0] | |||||
| M04 | Antigout preparations | X | 0.5 [0.2; 0.8] | |||||
| V | Various | X | X | 3.0 [1.4; 6.6] | ||||
| V08 | Contrast media | X | 2.2 [0.3; 7.7] | |||||
| H | Systemic hormonal preparations | X | X | X | 1.9 [1.1; 3.2] | |||
| H02 | Corticosteroids for systemic use | X | X | 3.8 [3.1; 4.7] | ||||
| H01 | Pituitary and hypothalamic hormones and analogues | X | 1.1 [0.0; 6.0] | |||||
| H03 | Thyroid therapy | X | 0.9 [0.5; 1.3] | |||||
| R | Respiratory system | X | X | X | 1.5 [0.4; 5.0] | |||
| R01 | Nasal preparations | X | 1.1 [0.0; 6.0] | |||||
| R05 | Cough and cold preparations | X | 1.1 [0.0; 6.0] | |||||
| R06 | Antihistamines for systemic use | X | 1.1 [0.0; 6.0] | |||||
| R03 | Drugs for obstructive airway diseases | X | 1.0 [0.6; 1.4] | |||||
| P | Antiparasitic products, insecticides and repellents | X | 1.1 [0.0; 6.0] | |||||
| D | Dermatologicals | X | 0.9 [0.0; 5.1] | |||||
| G | Genito urinary system and sex hormones | X | X | X | 0.7 [0.3; 1.7] | |||
| G04 | Urologicals | X | X | 0.6 [0.4; 1.0] | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haerdtlein, A.; Debold, E.; Rottenkolber, M.; Boehmer, A.M.; Pudritz, Y.M.; Shahid, F.; Gensichen, J.; Dreischulte, T. Which Adverse Events and Which Drugs Are Implicated in Drug-Related Hospital Admissions? A Systematic Review and Meta-Analysis. J. Clin. Med. 2023, 12, 1320. https://doi.org/10.3390/jcm12041320
Haerdtlein A, Debold E, Rottenkolber M, Boehmer AM, Pudritz YM, Shahid F, Gensichen J, Dreischulte T. Which Adverse Events and Which Drugs Are Implicated in Drug-Related Hospital Admissions? A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2023; 12(4):1320. https://doi.org/10.3390/jcm12041320
Chicago/Turabian StyleHaerdtlein, Annette, Elisabeth Debold, Marietta Rottenkolber, Anna Maria Boehmer, Yvonne Marina Pudritz, Faiza Shahid, Jochen Gensichen, and Tobias Dreischulte. 2023. "Which Adverse Events and Which Drugs Are Implicated in Drug-Related Hospital Admissions? A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 12, no. 4: 1320. https://doi.org/10.3390/jcm12041320





