Retinal Pigment Epithelial Abnormality and Choroidal Large Vascular Flow Imbalance Are Associated with Choriocapillaris Flow Deficits in Age-Related Macular Degeneration in Fellow Eyes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Eye Examinations
2.3. OCT and OCTA
2.4. Fundus Autofluorescence (FAF)
2.5. FA, IA, and Red Laser Scanning Using an Ultra-Widefield Retinal Imaging System
2.6. Statistical Analyses
3. Results
3.1. Participants’ Characteristics
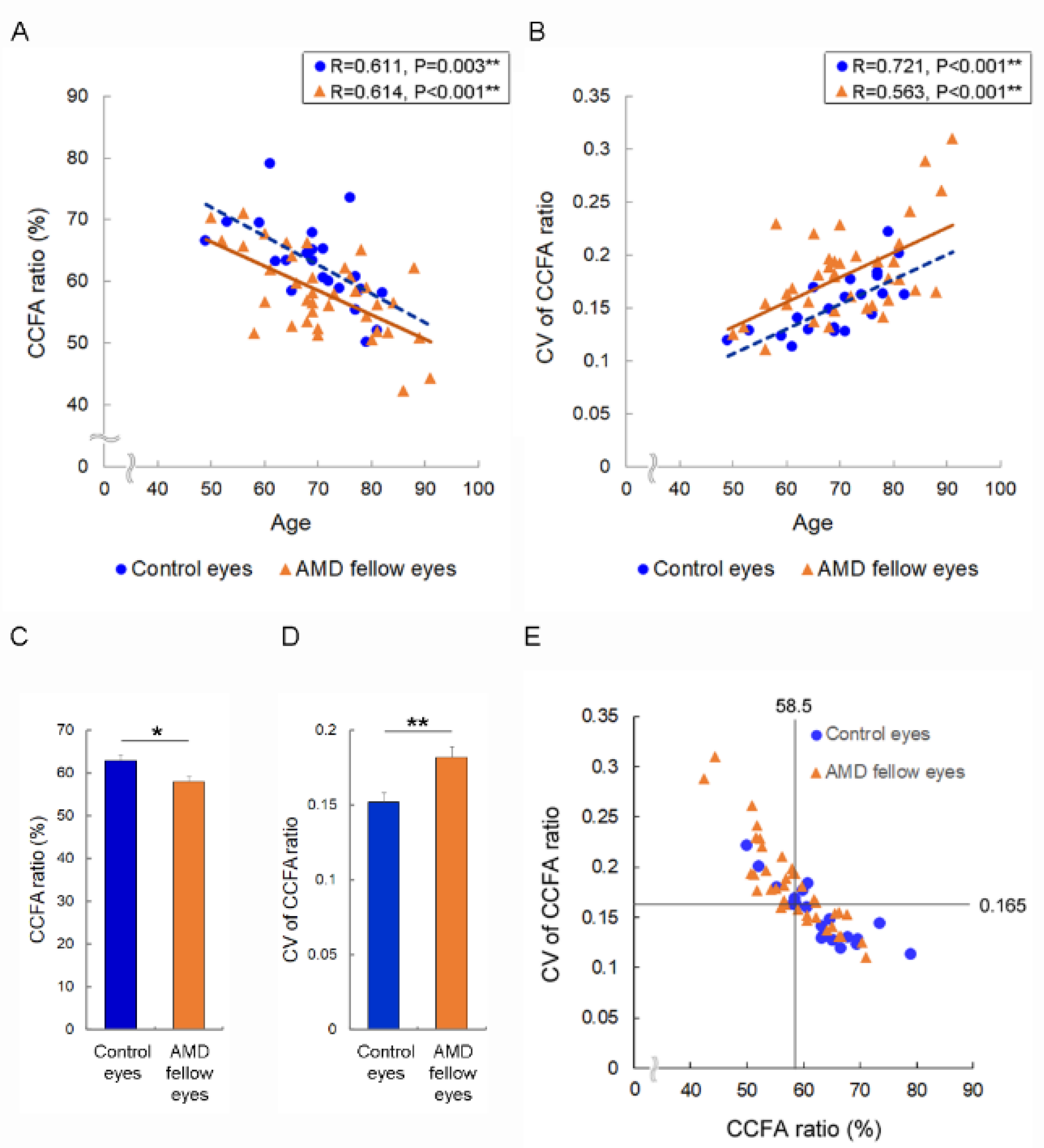
3.2. Risk of Accelerated Choriocapillaris Flow Deficits in AMD Fellow Eyes and AMD Risk Eyes
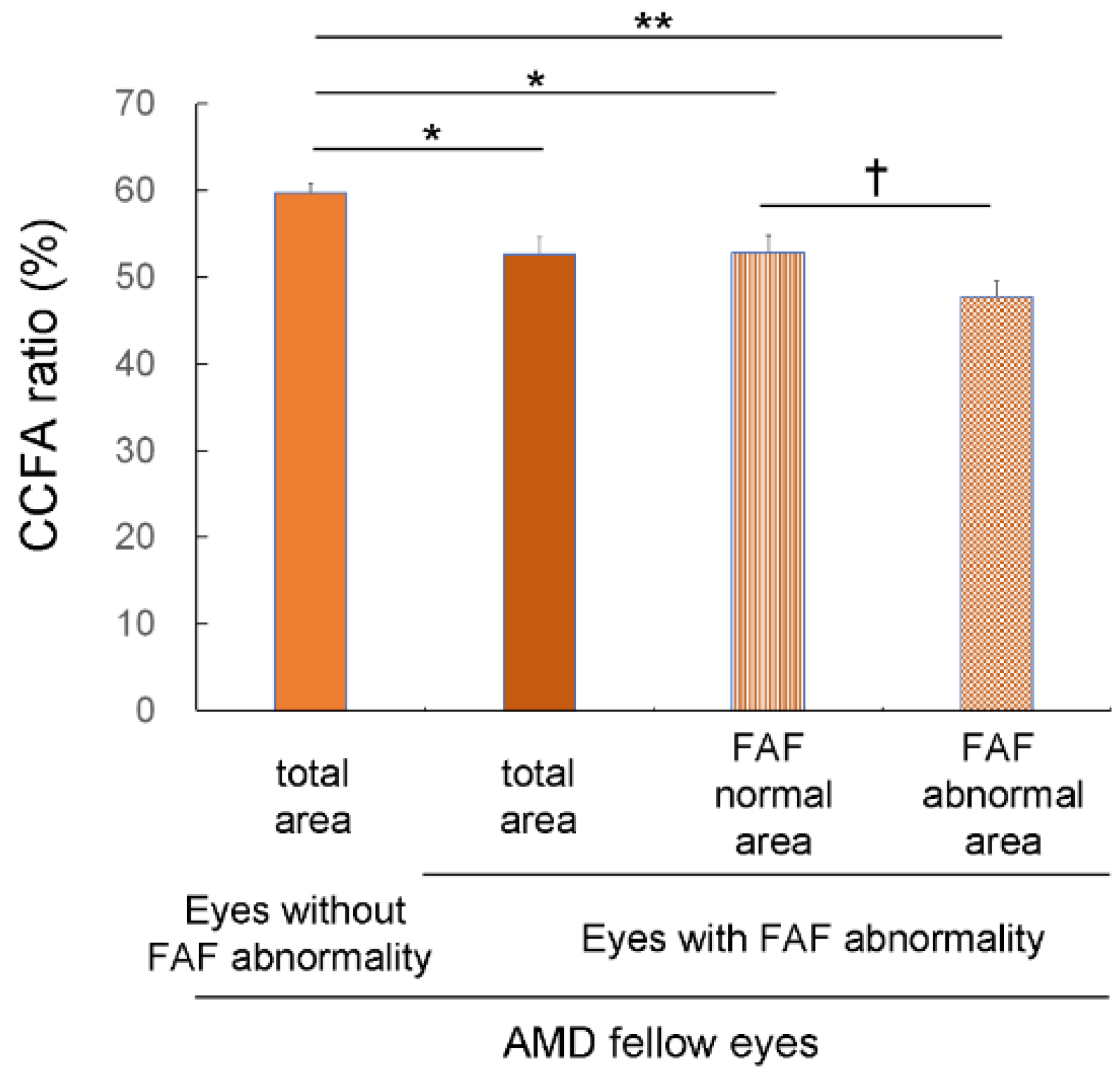
3.3. Choriocapillaris Flow Deficits in Eyes with FAF Abnormalities
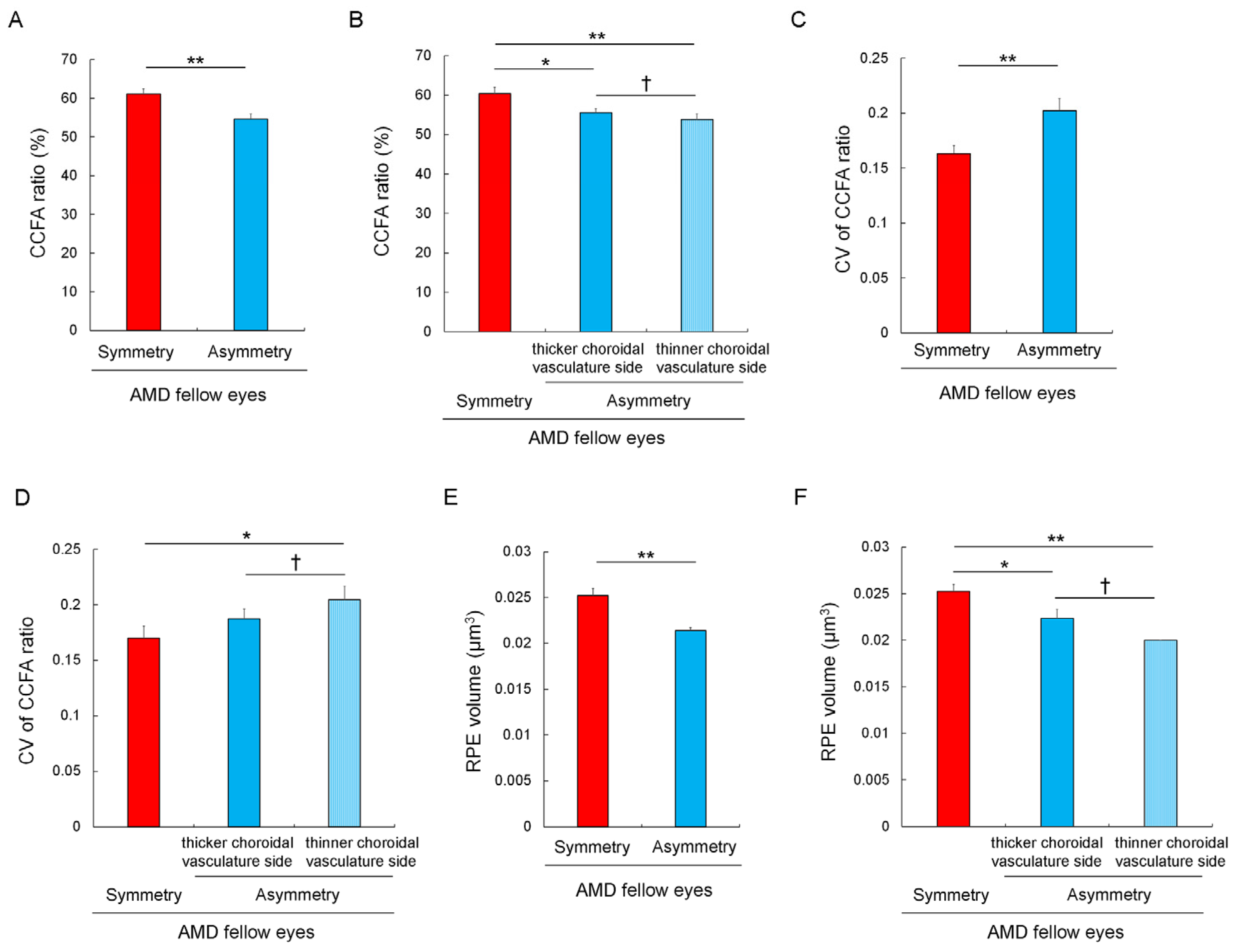
3.4. Choriocapillaris Flow Deficits and Their Heterogeneity in Eyes with Asymmetrically Dilated Choroidal Large Vasculature
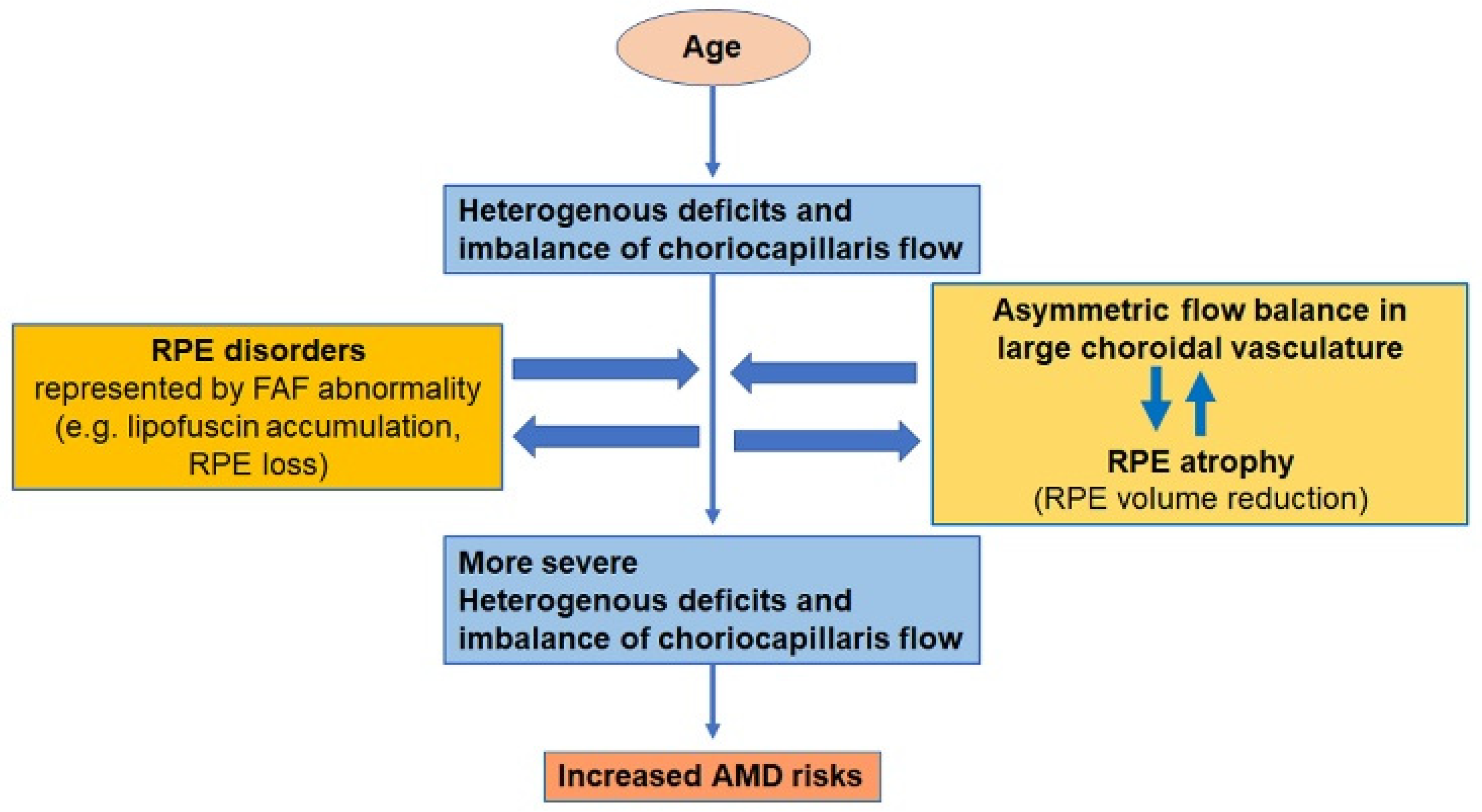
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bhutto, I.; Lutty, G. Understanding age-related macular degeneration (AMD): Relationships between the photoreceptor/retinal pigment epithelium/Bruch’s membrane/choriocapillaris complex. Mol. Asp. Med. 2012, 33, 295–317. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, P.; Liew, G.; Gopinath, B.; Wong, T.Y. Age-related macular degeneration. Lancet 2018, 392, 1147–1159. [Google Scholar] [CrossRef]
- Gillies, M.C.; Hunyor, A.P.; Arnold, J.J.; Guymer, R.H.; Wolf, S.; Pecheur, F.L.; Munk, M.R.; McAllister, I.L. Macular Atrophy in Neovascular Age-Related Macular Degeneration: A Randomized Clinical Trial Comparing Ranibizumab and Aflibercept (RIVAL Study). Ophthalmology 2020, 127, 198–210. [Google Scholar] [CrossRef]
- Holz, F.G.; Sadda, S.R.; Staurenghi, G.; Lindner, M.; Bird, A.C.; Blodi, B.A.; Bottoni, F.; Chakravarthy, U.; Chew, E.Y.; Csaky, K.; et al. Imaging Protocols in Clinical Studies in Advanced Age-Related Macular Degeneration: Recommendations from Classification of Atrophy Consensus Meetings. Ophthalmology 2017, 124, 464–478. [Google Scholar] [CrossRef]
- Pondorfer, S.G.; Wintergerst, M.W.M.; Gorgi Zadeh, S.; Schultz, T.; Heinemann, M.; Holz, F.G.; Finger, R.P. Association of Visual Function Measures with Drusen Volume in Early Stages of Age-Related Macular Degeneration. Investig. Opthalmol. Vis. Sci. 2020, 61, 55. [Google Scholar] [CrossRef] [PubMed]
- Sassmannshausen, M.; Pfau, M.; Thiele, S.; Fimmers, R.; Steinberg, J.S.; Fleckenstein, M.; Holz, F.G.; Schmitz-Valckenberg, S. Longitudinal Analysis of Structural and Functional Changes in Presence of Reticular Pseudodrusen Associated With Age-Related Macular Degeneration. Investig. Opthalmol. Vis. Sci. 2020, 61, 19. [Google Scholar] [CrossRef]
- Seddon, J.M.; McLeod, D.S.; Bhutto, I.A.; Villalonga, M.B.; Silver, R.E.; Wenick, A.S.; Edwards, M.M.; Lutty, G.A. Histopathological Insights Into Choroidal Vascular Loss in Clinically Documented Cases of Age-Related Macular Degeneration. JAMA Ophthalmol. 2016, 134, 1272–1280. [Google Scholar] [CrossRef] [PubMed]
- Borrelli, E.; Uji, A.; Sarraf, D.; Sadda, S.R. Alterations in the Choriocapillaris in Intermediate Age-Related Macular Degeneration. Investig. Opthalmol. Vis. Sci. 2017, 58, 4792–4798. [Google Scholar] [CrossRef]
- Borrelli, E.; Shi, Y.; Uji, A.; Balasubramanian, S.; Nassisi, M.; Sarraf, D.; Sadda, S.R. Topographic Analysis of the Choriocapillaris in Intermediate Age-related Macular Degeneration. Am. J. Ophthalmol. 2018, 196, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Rai, B.B.; Essex, R.W.; Sabeti, F.; Maddess, T.; Rohan, E.M.F.; van Kleef, J.P.; Carle, C.F. An Objective Perimetry Study of Central Versus Peripheral Sensitivities and Delays in Age-Related Macular Degeneration. Transl. Vis. Sci. Technol. 2021, 10, 24. [Google Scholar] [CrossRef]
- Sabeti, F.; Lane, J.; Rohan, E.M.F.; Rai, B.B.; Essex, R.W.; McKone, E.; Maddess, T. Correlation of Central Versus Peripheral Macular Structure-Function With Acuity in Age-Related Macular Degeneration. Transl. Vis. Sci. Technol. 2021, 10, 10. [Google Scholar] [CrossRef]
- Age-Related Eye Disease Study Research, G. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch. Ophthalmol. 2001, 119, 1417–1436. [Google Scholar] [CrossRef]
- Harada, N.; Nagai, N.; Mushiga, Y.; Ozawa, Y. Choriocapillaris Flow Imbalance in Fellow Eyes in Age-Related Macular Degeneration. Investig. Opthalmol. Vis. Sci. 2022, 63, 13. [Google Scholar] [CrossRef]
- Apte, R.S. Age-Related Macular Degeneration. N. Engl. J. Med. 2021, 385, 539–547. [Google Scholar] [CrossRef]
- Kim, J.; Lee, Y.J.; Won, J.Y. Molecular Mechanisms of Retinal Pigment Epithelium Dysfunction in Age-Related Macular Degeneration. Int. J. Mol. Sci. 2021, 22, 12298. [Google Scholar] [CrossRef]
- Simo, R.; Villarroel, M.; Corraliza, L.; Hernandez, C.; Garcia-Ramirez, M. The retinal pigment epithelium: Something more than a constituent of the blood-retinal barrier—Implications for the pathogenesis of diabetic retinopathy. J. Biomed. Biotechnol. 2010, 2010, 190724. [Google Scholar] [CrossRef]
- Hayreh, S.S. Segmental nature of the choroidal vasculature. Br. J. Ophthalmol. 1975, 59, 631–648. [Google Scholar] [CrossRef] [PubMed]
- Hiroe, T.; Kishi, S. Dilatation of Asymmetric Vortex Vein in Central Serous Chorioretinopathy. Ophthalmol. Retin. 2018, 2, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Terao, N.; Imanaga, N.; Wakugawa, S.; Sawaguchi, S.; Tamashiro, T.; Yamauchi, Y.; Koizumi, H. Short Axial Length Is Related to Asymmetric Vortex Veins in Central Serous Chorioretinopathy. Ophthalmol. Sci. 2021, 1, 100071. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, H.; Hoshino, J.; Mukai, R.; Nakamura, K.; Kishi, S.; Akiyama, H. Clinical characteristics and pachychoroid incidence in Japanese patients with neovascular age-related macular degeneration. Sci. Rep. 2022, 12, 4492. [Google Scholar] [CrossRef] [PubMed]
- Gemmy Cheung, C.M.; Teo, K.Y.C.; Spaide, R.F. Pulsatile Filling of Dilated Choroidal Vessels in Macular Watershed Zones. Retina 2021, 41, 2370–2377. [Google Scholar] [CrossRef] [PubMed]
- Sampson, D.M.; Gong, P.; An, D.; Menghini, M.; Hansen, A.; Mackey, D.A.; Sampson, D.D.; Chen, F.K. Axial Length Variation Impacts on Superficial Retinal Vessel Density and Foveal Avascular Zone Area Measurements Using Optical Coherence Tomography Angiography. Investig. Opthalmol. Vis. Sci. 2017, 58, 3065–3072. [Google Scholar] [CrossRef] [PubMed]
- Mehta, N.; Liu, K.; Alibhai, A.Y.; Gendelman, I.; Braun, P.X.; Ishibazawa, A.; Sorour, O.; Duker, J.S.; Waheed, N.K. Impact of Binarization Thresholding and Brightness/Contrast Adjustment Methodology on Optical Coherence Tomography Angiography Image Quantification. Am. J. Ophthalmol. 2019, 205, 54–65. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Early Treatment Diabetic Retinopathy Study Research Group. Early Treatment Diabetic Retinopathy Study design and baseline patient characteristics: ETDRS report number 7. Ophthalmology 1991, 98, 741–756. [Google Scholar] [CrossRef]
- Xu, Q.; Li, Y.; Cheng, Y.; Qu, Y. Assessment of the effect of age on macular layer thickness in a healthy Chinese cohort using spectral-domain optical coherence tomography. BMC Ophthalmol. 2018, 18, 169. [Google Scholar] [CrossRef]
- Curcio, C.A.; Millican, C.L.; Allen, K.A.; Kalina, R.E. Aging of the human photoreceptor mosaic: Evidence for selective vulnerability of rods in central retina. Investig. Opthalmol. Vis. Sci. 1993, 34, 3278–3296. [Google Scholar]
- Curcio, C.A. Photoreceptor topography in ageing and age-related maculopathy. Eye 2001, 15, 376–383. [Google Scholar] [CrossRef]
- Jackson, G.R.; Owsley, C.; Curcio, C.A. Photoreceptor degeneration and dysfunction in aging and age-related maculopathy. Ageing Res. Rev. 2002, 1, 381–396. [Google Scholar] [CrossRef]
- Song, H.; Chui, T.Y.; Zhong, Z.; Elsner, A.E.; Burns, S.A. Variation of cone photoreceptor packing density with retinal eccentricity and age. Investig. Opthalmol. Vis. Sci. 2011, 52, 7376–7384. [Google Scholar] [CrossRef]
- Minami, S.; Nagai, N.; Suzuki, M.; Kurihara, T.; Sonobe, H.; Watanabe, K.; Shinoda, H.; Takagi, H.; Tsubota, K.; Ozawa, Y. Spatial-sweep steady-state pattern electroretinography can detect subtle differences in visual function among healthy adults. Sci. Rep. 2019, 9, 18119. [Google Scholar] [CrossRef] [PubMed]
- Nagai, N.; Izumi-Nagai, K.; Suzuki, M.; Shinoda, H.; Koto, T.; Uchida, A.; Mochimaru, H.; Tomita, Y.; Miyake, S.; Kobayashi, S.; et al. Association of macular pigment optical density with serum concentration of oxidized low-density lipoprotein in healthy adults. Retina 2015, 35, 820–826. [Google Scholar] [CrossRef] [PubMed]
- Ozawa, Y.; Shigeno, Y.; Nagai, N.; Suzuki, M.; Kurihara, T.; Minami, S.; Hirano, E.; Shinoda, H.; Kobayashi, S.; Tsubota, K. Absolute and estimated values of macular pigment optical density in young and aged Asian participants with or without age-related macular degeneration. BMC Ophthalmol. 2017, 17, 161. [Google Scholar] [CrossRef]
- Obana, A.; Hiramitsu, T.; Gohto, Y.; Ohira, A.; Mizuno, S.; Hirano, T.; Bernstein, P.S.; Fujii, H.; Iseki, K.; Tanito, M.; et al. Macular carotenoid levels of normal subjects and age-related maculopathy patients in a Japanese population. Ophthalmology 2008, 115, 147–157. [Google Scholar] [CrossRef]
- Zekavat, S.M.; Sekimitsu, S.; Ye, Y.; Raghu, V.; Zhao, H.; Elze, T.; Segre, A.V.; Wiggs, J.L.; Natarajan, P.; Del Priore, L.; et al. Photoreceptor Layer Thinning Is an Early Biomarker for Age-Related Macular Degeneration: Epidemiologic and Genetic Evidence from UK Biobank OCT Data. Ophthalmology 2022, 129, 694–707. [Google Scholar] [CrossRef]
- Chakravarthy, U.; Wong, T.Y.; Fletcher, A.; Piault, E.; Evans, C.; Zlateva, G.; Buggage, R.; Pleil, A.; Mitchell, P. Clinical risk factors for age-related macular degeneration: A systematic review and meta-analysis. BMC Ophthalmol. 2010, 10, 31. [Google Scholar] [CrossRef] [PubMed]
- Hirooka, K.; Saito, M.; Yamashita, Y.; Hashimoto, Y.; Terao, N.; Koizumi, H.; Noda, K.; Ishida, S. Imbalanced choroidal circulation in eyes with asymmetric dilated vortex vein. Jpn. J. Ophthalmol. 2022, 66, 14–18. [Google Scholar] [CrossRef]
- Saito, M.; Saito, W.; Hashimoto, Y.; Yoshizawa, C.; Fujiya, A.; Noda, K.; Ishida, S. Macular choroidal blood flow velocity decreases with regression of acute central serous chorioretinopathy. Br. J. Ophthalmol. 2013, 97, 775–780. [Google Scholar] [CrossRef] [PubMed]
- Saito, M.; Saito, W.; Hirooka, K.; Hashimoto, Y.; Mori, S.; Noda, K.; Ishida, S. Pulse Waveform Changes in Macular Choroidal Hemodynamics With Regression of Acute Central Serous Chorioretinopathy. Investig. Opthalmol. Vis. Sci. 2015, 56, 6515–6522. [Google Scholar] [CrossRef]
- Clemons, T.E.; Milton, R.C.; Klein, R.; Seddon, J.M.; Ferris, F.L., 3rd; Age-Related Eye Disease Study Research, G. Risk factors for the incidence of Advanced Age-Related Macular Degeneration in the Age-Related Eye Disease Study (AREDS) AREDS report no. 19. Ophthalmology 2005, 112, 533–539. [Google Scholar] [CrossRef]
- Schmitz-Valckenberg, S.; Pfau, M.; Fleckenstein, M.; Staurenghi, G.; Sparrow, J.R.; Bindewald-Wittich, A.; Spaide, R.F.; Wolf, S.; Sadda, S.R.; Holz, F.G. Fundus autofluorescence imaging. Prog. Retin. Eye Res. 2021, 81, 100893. [Google Scholar] [CrossRef] [PubMed]
- Lindblad, A.S.; Lloyd, P.C.; Clemons, T.E.; Gensler, G.R.; Ferris, F.L., 3rd; Klein, M.L.; Armstrong, J.R.; Age-Related Eye Disease Study Research, G. Change in area of geographic atrophy in the Age-Related Eye Disease Study: AREDS report number 26. Arch Ophthalmol. 2009, 127, 1168–1174. [Google Scholar] [CrossRef]
- McLeod, D.S.; Grebe, R.; Bhutto, I.; Merges, C.; Baba, T.; Lutty, G.A. Relationship between RPE and choriocapillaris in age-related macular degeneration. Investig. Opthalmol. Vis. Sci. 2009, 50, 4982–4991. [Google Scholar] [CrossRef] [PubMed]
- Saint-Geniez, M.; Kurihara, T.; Sekiyama, E.; Maldonado, A.E.; D’Amore, P.A. An essential role for RPE-derived soluble VEGF in the maintenance of the choriocapillaris. Proc. Natl. Acad. Sci. USA 2009, 106, 18751–18756. [Google Scholar] [CrossRef]
- Strauss, O. The retinal pigment epithelium in visual function. Physiol. Rev. 2005, 85, 845–881. [Google Scholar] [CrossRef]
- Pang, C.E.; Shah, V.P.; Sarraf, D.; Freund, K.B. Ultra-widefield imaging with autofluorescence and indocyanine green angiography in central serous chorioretinopathy. Am. J. Ophthalmol. 2014, 158, 362–371 e362. [Google Scholar] [CrossRef]
- Dansingani, K.K.; Balaratnasingam, C.; Naysan, J.; Freund, K.B. En Face Imaging of Pachychoroid Spectrum Disorders with Swept-Source Optical Coherence Tomography. Retina 2016, 36, 499–516. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, H.; Hoshino, J.; Mukai, R.; Nakamura, K.; Kikuchi, Y.; Kishi, S.; Akiyama, H. Vortex Vein Anastomosis at the Watershed in Pachychoroid Spectrum Diseases. Ophthalmol. Retin. 2020, 4, 938–945. [Google Scholar] [CrossRef]
- Matsumoto, H.; Hoshino, J.; Arai, Y.; Mukai, R.; Nakamura, K.; Kikuchi, Y.; Kishi, S.; Akiyama, H. Quantitative measures of vortex veins in the posterior pole in eyes with pachychoroid spectrum diseases. Sci. Rep. 2020, 10, 19505. [Google Scholar] [CrossRef]
- Matsumoto, H.; Mukai, R.; Hoshino, J.; Oda, M.; Matsuzaki, T.; Ishizaki, Y.; Shibasaki, K.; Akiyama, H. Choroidal congestion mouse model: Could it serve as a pachychoroid model? PLoS ONE 2021, 16, e0246115. [Google Scholar] [CrossRef]
- Imanaga, N.; Terao, N.; Nakamine, S.; Tamashiro, T.; Wakugawa, S.; Sawaguchi, K.; Koizumi, H. Scleral Thickness in Central Serous Chorioretinopathy. Ophthalmol. Retin. 2021, 5, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Kishi, S.; Matsumoto, H.; Sonoda, S.; Hiroe, T.; Sakamoto, T.; Akiyama, H. Geographic filling delay of the choriocapillaris in the region of dilated asymmetric vortex veins in central serous chorioretinopathy. PLoS ONE 2018, 13, e0206646. [Google Scholar] [CrossRef] [PubMed]
- Kishi, S.; Matsumoto, H. A new insight into pachychoroid diseases: Remodeling of choroidal vasculature. Graefe’s Arch. Clin. Exp. Ophthalmol. 2022, 260, 3405–3417. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, H.; Kishi, S.; Mukai, R.; Akiyama, H. Remodeling of macular vortex veins in pachychoroid neovasculopathy. Sci. Rep. 2019, 9, 14689. [Google Scholar] [CrossRef] [PubMed]
- Nagai, N.; Kawashima, H.; Toda, E.; Homma, K.; Osada, H.; Guzman, N.A.; Shibata, S.; Uchiyama, Y.; Okano, H.; Tsubota, K.; et al. Renin-angiotensin system impairs macrophage lipid metabolism to promote age-related macular degeneration in mouse models. Commun. Biol. 2020, 3, 767. [Google Scholar] [CrossRef]
- Narimatsu, T.; Ozawa, Y.; Miyake, S.; Kubota, S.; Hirasawa, M.; Nagai, N.; Shimmura, S.; Tsubota, K. Disruption of cell-cell junctions and induction of pathological cytokines in the retinal pigment epithelium of light-exposed mice. Investig. Opthalmol. Vis. Sci. 2013, 54, 4555–4562. [Google Scholar] [CrossRef]
- Narimatsu, T.; Ozawa, Y.; Miyake, S.; Kubota, S.; Yuki, K.; Nagai, N.; Tsubota, K. Biological effects of blocking blue and other visible light on the mouse retina. Clin. Exp. Ophthalmol. 2014, 42, 555–563. [Google Scholar] [CrossRef]
- Narimatsu, T.; Ozawa, Y.; Miyake, S.; Nagai, N.; Tsubota, K. Angiotensin II type 1 receptor blockade suppresses light-induced neural damage in the mouse retina. Free Radic Biol. Med. 2014, 71, 176–185. [Google Scholar] [CrossRef]
| Control Eyes | AMD Fellow Eyes | p | |
|---|---|---|---|
| n (eyes) | 22 | 38 | |
| Age (years) | 69.4 ± 1.8 (49–82) | 71.7 ± 1.9 (50–91) | 0.618 |
| Sex (men; eyes (%)) | 11 (50.0) | 26 (64.8) | 0.128 |
| BCVA (logMAR) | −0.05 ± 0.01 (−0.18–0.10) | −0.05 ± 0.02 (−0.18–0.40) | 0.202 |
| Axial length (mm) | 24.10 ± 0.23 22.46–26.02) | 24.10 ± 0.21 (21.80–26.43) | 0.908 |
| Central retinal thickness (μm) | 223 ± 4 (192−269) | 227 ± 4 (177–264) | 0.988 |
| Central choroidal thickness (μm) | 217 ± 23 (76−535) | 224 ± 18 (75–489) | 0.914 |
| Choroidal vascular diameter (μm) | 172 ± 15 (70−346) | 192 ± 13 (55–4381) | 0.365 |
| FAF abnormality | 0 (0) | 9 (23.7) | 0.011 * |
| Asymmetry in choroidal vasculature | 7 (31.8) | 18 (47.3) | 0.183 |
| RPE volume (μm3) | 0.102 ± 0.002 (0.09–0.14) | 0.096 ± 0.003 (0.09–0.13) | 0.323 |
| OR | p Value | 95% Confidence Interval | |
|---|---|---|---|
| FAF Abnormality | 16.440 | 0.033 * | 1.262 to 214.240 |
| Asymmetry in choroidal vasculature | 4.176 | 0.042 * | 1.057 to 16.503 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nagai, N.; Mushiga, Y.; Ozawa, Y. Retinal Pigment Epithelial Abnormality and Choroidal Large Vascular Flow Imbalance Are Associated with Choriocapillaris Flow Deficits in Age-Related Macular Degeneration in Fellow Eyes. J. Clin. Med. 2023, 12, 1360. https://doi.org/10.3390/jcm12041360
Nagai N, Mushiga Y, Ozawa Y. Retinal Pigment Epithelial Abnormality and Choroidal Large Vascular Flow Imbalance Are Associated with Choriocapillaris Flow Deficits in Age-Related Macular Degeneration in Fellow Eyes. Journal of Clinical Medicine. 2023; 12(4):1360. https://doi.org/10.3390/jcm12041360
Chicago/Turabian StyleNagai, Norihiro, Yasuaki Mushiga, and Yoko Ozawa. 2023. "Retinal Pigment Epithelial Abnormality and Choroidal Large Vascular Flow Imbalance Are Associated with Choriocapillaris Flow Deficits in Age-Related Macular Degeneration in Fellow Eyes" Journal of Clinical Medicine 12, no. 4: 1360. https://doi.org/10.3390/jcm12041360






