Transcatheter Tricuspid Valve Replacement: Illustrative Case Reports and Review of State-of-Art
Abstract
:1. Introduction
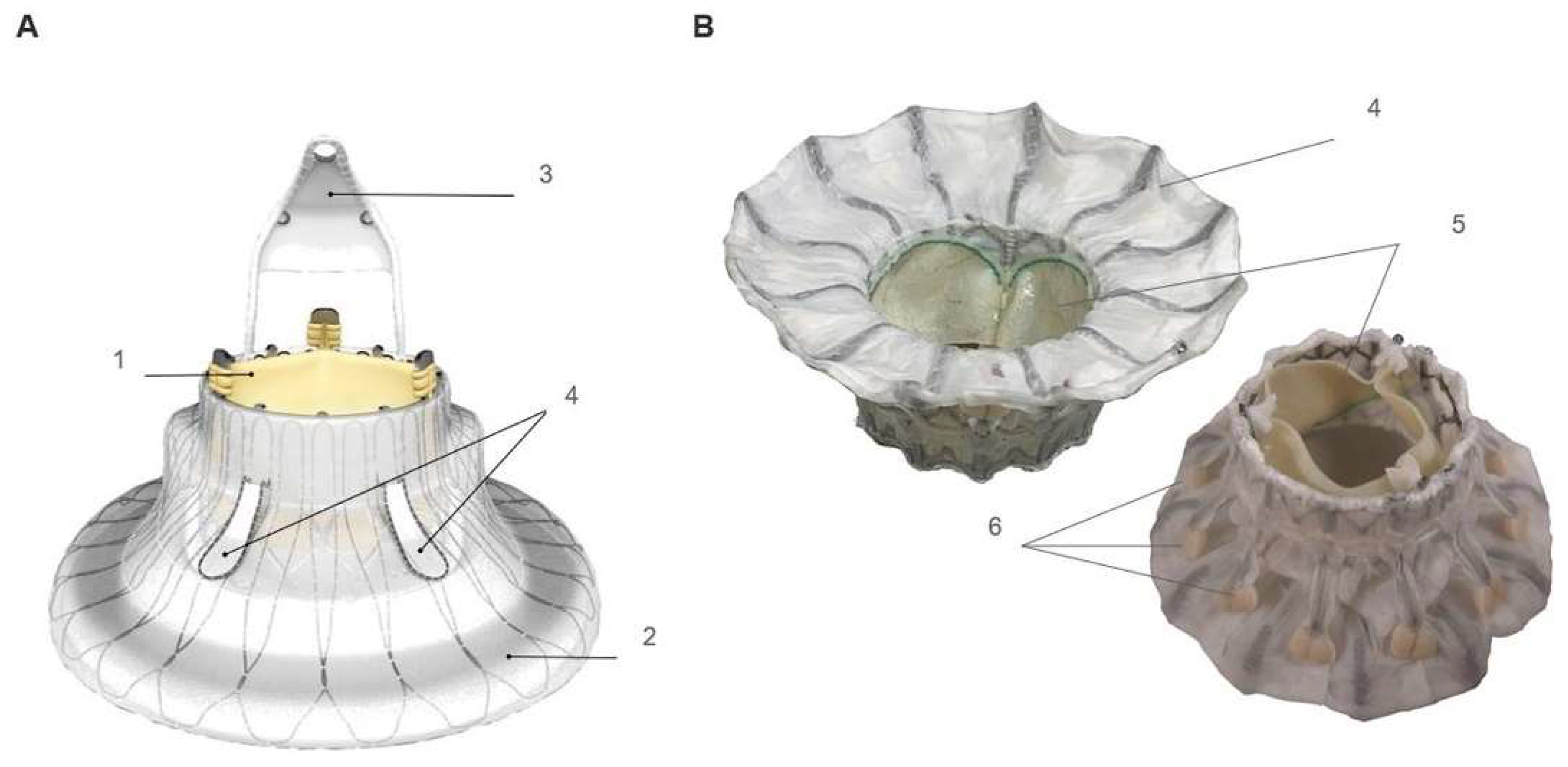
2. Cardiovalve Cases
3. Lux-Valve Plus Case
4. Ttvr Portfolio Review
4.1. Navigate
4.2. Cardiovalve
4.3. Evoque
4.4. LuX-Valve
4.5. Early Experience Phase with Other Devices
5. Ttvr Clinical Results Review
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| TR | tricuspid regurgitation |
| TV | tricuspid valve |
| TTVR | transcatheter tricuspid valve replacement |
| CT | computed tomography |
| TEE | transoesophageal echocardiography |
| TTE | transthoracic echocardiography |
References
- Topilsky, Y.; Maltais, S.; Medina-Inojosa, J.; Oguz, D.; Michelena, H.; Maalouf, J.; Mahoney, D.W.; Enriquez-Sarano, M. Burden of Tricuspid Regurgitation in Patients Diagnosed in the Community Setting. JACC Cardiovasc. Imaging 2019, 12, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Fulcher, J.; Abeysuriya, N.; McGrady, M.; Wilcox, I.; Celermajer, D.; Lal, S. Tricuspid regurgitation is associated with increased mortality independent of pulmonary pressures and right heart failure: A systematic review and meta-analysis. Eur. Heart J. 2019, 40, 476–484. [Google Scholar] [CrossRef]
- Taramasso, M.; Benfari, G.; van der Bijl, P.; Alessandrini, H.; Attinger-Toller, A.; Biasco, L.; Lurz, P.; Braun, D.; Brochet, E.; Connelly, K.A.; et al. Transcatheter Versus Medical Treatment of Patients With Symptomatic Severe Tricuspid Regurgitation. J. Am. Coll. Cardiol. 2019, 74, 2998–3008. [Google Scholar] [CrossRef] [PubMed]
- Dowling, C.; Gooley, R.; McCormick, L.; Firoozi, S.; Brecker, S.J. Patient-specific Computer Simulation: An Emerging Technology for Guiding the Transcatheter Treatment of Patients with Bicuspid Aortic Valve. Interv. Cardiol. 2021, 16, e26. [Google Scholar] [CrossRef] [PubMed]
- Pasta, S.; Cannata, S.; Gentile, G.; Di Giuseppe, M.; Cosentino, F.; Pasta, F.; Agnese, V.; Bellavia, D.; Raffa, G.M.; Pilato, M.; et al. Simulation study of transcatheter heart valve implantation in patients with stenotic bicuspid aortic valve. Med. Biol. Eng. Comput. 2020, 58, 815–829. [Google Scholar] [CrossRef]
- Maisano, F.; Benetis, R.; Rumbinaite, E.; Unikas, R.; Mizariene, V.; Jakuska, P.; Topilsky, Y.; Vahanian, A. 2-Year Follow-Up After Transseptal Transcatheter Mitral Valve Replacement With the Cardiovalve. JACC Cardiovasc. Interv. 2020, 13, e163–e164. [Google Scholar] [CrossRef]
- Aoi, S.; Wiley, J.; Ho, E.; Goldberg, Y.; Chau, M.; Latib, A. Transcatheter tricuspid valve implantation with the Cardiovalve system. Future Cardiol. 2021, 17, 963–969. [Google Scholar] [CrossRef]
- Praz, F.; Muraru, D.; Kreidel, F.; Lurz, P.; Hahn, R.T.; Delgado, V.; Senni, M.; von Bardeleben, R.S.; Nickenig, G.; Hausleiter, J.; et al. Transcatheter treatment for tricuspid valve disease. EuroIntervention 2021, 17, 791–808. [Google Scholar] [CrossRef]
- Praz, F.; Khalique, O.K.; Macedo, L.G.D.R.; Pulerwitz, T.C.; Jantz, J.; Wu, I.Y.; Kantor, A.; Patel, A.; Vahl, T.; Bapat, V.; et al. Comparison between Three-Dimensional Echocardiography and Computed Tomography for Comprehensive Tricuspid Annulus and Valve Assessment in Severe Tricuspid Regurgitation: Implications for Tricuspid Regurgitation Grading and Transcatheter Therapies. J. Am. Soc. Echocardiogr. 2018, 31, 1190–1202.e3. [Google Scholar] [CrossRef]
- Lu, F.-L.; Ma, Y.; An, Z.; Cai, C.-L.; Li, B.-L.; Song, Z.-G.; Han, L.; Wang, J.; Qiao, F.; Xu, Z.-Y. First-in-Man Experience of Transcatheter Tricuspid Valve Replacement With LuX-Valve in High-Risk Tricuspid Regurgitation Patients. JACC Cardiovasc. Interv. 2020, 13, 1614–1616. [Google Scholar] [CrossRef]
- Sun, Z.; Li, H.; Zhang, Z.; Li, Y.; Zhang, L.; Xie, Y.; Han, Z.; Wang, J.; Chen, Y.; Yang, Y.; et al. Twelve-month outcomes of the LuX-Valve for transcatheter treatment of severe tricuspid regurgitation. EuroIntervention 2021, 17, 818–826. [Google Scholar] [CrossRef]
- Ning, X.P.; An, Z.; Qiao, F.; Cai, C.L.; Han, L.; Song, Z.G.; Li, B.L.; Zhou, G.W.; Wang, J.; Xu, Z.Y.; et al. Safety and efficacy of transcatheter tricuspid valve replacement with LuX-Valve in patients with severe tricuspid regurgitation. Zhonghua Xin Xue Guan Bing Za Zhi 2021, 49, 455–460. [Google Scholar] [CrossRef]
- Zhang, Y.; Lu, F.; Li, W.; Chen, S.; Li, M.; Zhang, X.; Pan, C.; Qiao, F.; Zhou, D.; Pan, W.; et al. A first-in-human study of transjugular transcatheter tricuspid valve replacement with the LuX-Valve Plus system. EuroIntervention 2022, 18, e1088–e1089. [Google Scholar] [CrossRef]
- Navia, J.L.; Kapadia, S.; Elgharably, H.; Harb, S.C.; Krishnaswamy, A.; Unai, S.; Mick, S.; Rodriguez, L.; Hammer, D.; Gillinov, A.M.; et al. First-in-Human Implantations of the NaviGate Bioprosthesis in a Severely Dilated Tricuspid Annulus and in a Failed Tricuspid Annuloplasty Ring. Circ. Cardiovasc. Interv. 2017, 10, e005840. [Google Scholar] [CrossRef]
- Hahn, R.T.; Kodali, S.; Fam, N.; Bapat, V.; Bartus, K.; Rodés-Cabau, J.; Dagenais, F.; Estevez-Loureiro, R.; Forteza, A.; Kapadia, S.; et al. Early Multinational Experience of Transcatheter Tricuspid Valve Replacement for Treating Severe Tricuspid Regurgitation. JACC Cardiovasc. Interv. 2020, 13, 2482–2493. [Google Scholar] [CrossRef]
- Asmarats, L.; Dagenais, F.; Bédard, E.; Pasian, S.; Hahn, R.T.; Navia, J.L.; Rodés-Cabau, J. Transcatheter Tricuspid Valve Replacement for Treating Severe Tricuspid Regurgitation: Initial Experience with the NaviGate Bioprosthesis. Can. J. Cardiol. 2018, 34, 1370.e5–1370.e7. [Google Scholar] [CrossRef]
- Caneiro-Queija, B.; Estévez-Loureiro, R.; Piñón-Esteban, M.; Barreiro-Pérez, M.; Baz-Alonso, J.A.; Íñiguez-Romo, A. Transfemoral transcatheter tricuspid valve replacement with the Cardiovalve system. Rev. Esp. Cardiol. 2022, in press. [Google Scholar] [CrossRef]
- Barreiro-Perez, M.; Estevez-Loureiro, R.; Baz, J.A.; Piñón, M.A.; Maisano, F.; Puga, L.; Caneiro-Queija, B.; Iñiguez-Romo, A. Cardiovalve Transfemoral Tricuspid Valve Replacement Assisted With CT-Fluoroscopy Fusion Imaging. JACC Cardiovasc. Interv. 2022, 15, e197–e199. [Google Scholar] [CrossRef]
- Webb, J.; Hensey, M.; Fam, N.; Rodés-Cabau, J.; Daniels, D.; Smith, R.; Szeto, W.; Boone, R.; Ye, J.; Moss, R.; et al. Transcatheter Mitral Valve Replacement with the Transseptal EVOQUE System. JACC Cardiovasc. Interv. 2020, 13, 2418–2426. [Google Scholar] [CrossRef]
- Webb, J.G.; Chuang, A.-Y.; Meier, D.; von Bardeleben, R.S.; Kodali, S.K.; Smith, R.L.; Hausleiter, J.; Ong, G.; Boone, R.; Ruf, T.; et al. Transcatheter Tricuspid Valve Replacement with the EVOQUE System: 1-Year Outcomes of a Multicenter, First-in-Human Experience. JACC Cardiovasc. Interv. 2022, 15, 481–491. [Google Scholar] [CrossRef]
- McCarthy, P.M.; Kislitsina, O.N.; Malaisrie, S.C.; Davidson, C.J. Transcatheter Mitral Valve Replacement with Intrepid. Interv. Cardiol. Clin. 2019, 8, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Fam, N.P.; von Bardeleben, R.S.; Hensey, M.; Kodali, S.K.; Smith, R.L.; Hausleiter, J.; Ong, G.; Boone, R.; Ruf, T.; George, I.; et al. Transfemoral Transcatheter Tricuspid Valve Replacement With the EVOQUE System: A Multicenter, Observational, First-in-Human Experience. JACC Cardiovasc. Interv. 2021, 14, 501–511. [Google Scholar] [CrossRef] [PubMed]
- Vaturi, M.; Vaknin-Assa, H.; Shapira, Y.; Perl, L.; Levi, A.; Koren, A.; Kornowski, R. First-in-Human Percutaneous Transcatheter Tricuspid Valve Replacement with a Novel Valve. JACC Case Rep. 2021, 3, 1281–1286. [Google Scholar] [CrossRef] [PubMed]
- Teiger, E.; Nejjari, M.; Lim, P.; Ruf, T.; Blanke, P.; Schäfer, U.; Treede, H.; Gallet, R.; Dreyfus, J. First-in-human implantation of the Topaz transcatheter tricuspid valve replacement system. EuroIntervention 2022, 18, 862–864. [Google Scholar] [CrossRef]
- Kodali, S.; Hahn, R.T.; George, I.; Davidson, C.J.; Narang, A.; Zahr, F.; Chadderdon, S.; Smith, R.; Grayburn, P.A.; O’Neill, W.W.; et al. Transfemoral Tricuspid Valve Replacement in Patients with Tricuspid Regurgitation: TRISCEND Study 30-Day Results. JACC Cardiovasc. Interv. 2022, 15, 471–480. [Google Scholar] [CrossRef]
- Mao, Y.; Li, L.; Liu, Y.; Zhai, M.; Ma, Y.; Xu, C.; Jin, P.; Yang, J. Safety, efficacy, and clinical outcomes of transcatheter tricuspid valve replacement: One-year follow-up. Front. Cardiovasc. Med. 2022, 9, 1019813. [Google Scholar] [CrossRef]








| Device Name (Manufacturer) | Design | Access | Delivery Sheath Size | Anchoring Mechanism | Pacemaker Implant | Procedural Success in Trials | Conversion to Open Heart Surgery | Reduction of TR to Mild or Less |
|---|---|---|---|---|---|---|---|---|
| Gate (NaviGate Cardiac Structures, Inc.) | nitinol self-expanding conical stent with a tri-leaflet equine pericardial valve | Transjugular or transatrial | 42 Fr | Native leaflets grasping | N/A | 87% | 5% | 76% |
| Cardiovalve (Cardiovalve, Lda) | self-expanding nitinol frame with 3 bovine pericardial leaflets | transfemoral | 28 Fr | Native leaflets grasping | N/A | N/A | N/A | N/A |
| Evoque TV (Edwards Lifesciences) | self-expanding nitinol frame, bovine pericardial leaflets, and an intra-annular sealing skirt | transfemoral | 28 Fr | Annulus, leaflets and chords | 13% | 94% | 1% | 99% |
| LuX-Valve (Jenscare Biotechnology) | self-expanding stented valve, composed by a tri-leaflet bovine pericardium valve, with an external nitinol stent covered with a layer of polyethylene terephthalate | Transapical or transjugular | 33 Fr | Anterior leaflet grasping and septal anchor | 0% | 100% | 0% | 83% |
| Navigate | Evoque | LuX-Valve | LuX-Valve Plus | ||
|---|---|---|---|---|---|
| Hahn et al. | Fam et al. | Kodali et al. | Mao et al. | Zhang et al. | |
| Baseline characteristics | |||||
| Number of patients | 30 | 25 | 56 | 15 | 10 |
| Age, years (IQR) | 78 (70–80) | 76 (73–79) | 79.3 | 62 (56–78) | 70 |
| NYHA III or IV, nº (%) | 85.7% | 88.0% | 87.5% | 15 (100) | 10 (100) |
| TR severity | |||||
| Moderate | 2 (6.6) | 4 (16.0) | 8.9% | 0 (0.0) | 0 (0.0) |
| ≥Severe | 28 (92.4) | 25 (100) | 91.1% | 15 (100) | 10 (100) |
| Procedure | |||||
| Procedural success, nº (%) | 26 (87.0) | 23 (92.0) | NA | 15 (100) | 10 (100) |
| Conversion to open surgery, nº (%) | 2 (6.6) | 0 | 1 (1.8) | 0 (0.0) | 0 (0.0) |
| Device embolization or malposition, nº (%) | 4 (13.3) | 1 (4.0) | 2 (3.6) | 0 (0.0) | 0 (0.0) |
| 30-day outcomes | |||||
| Mortality | 3 (10) | 0 (0.0) | 1 (1.8) | 1 (6.6) | 0 (0.0) |
| Stroke | 1 (3.3) | 0 (0.0) | 0 (0) | 0 (0.0) | 0 (0.0) |
| Major or life-threatening bleeding | 10 (30) | 3 (12.0) | 1 (1.8) | 1 (8) | 0 (0.0) |
| Others | 1 death for uncontrolled bleeding, 1 multiorgan failure, 1 after surgical conversion | 2 pacemaker implantations | 1 cardiovascular death, 2 reinterventions, 1 major access site or vascular complication | 1 death related to pulmonary infection | 1 pacemaker implantation for a complete AV block |
| TR post-procedural | |||||
| Mild or less | 67.0% | 88.0% | 98.1% | 85.7% | 10 (100) |
| Moderate | 14.0% | 8.0% | 1.9% | 7.1% | 0 (0.0) |
| ≥Severe | 19.0% | 4.0% | 0 (0.0) | NA | 0 (0.0) |
| 1 year outcomes | Webb et al. | ||||
| Mortality (30d-1 year) | 7.4% | 0 (0.0) | |||
| Stroke | 0 (0.0) | ||||
| Major or life-threatening bleeding | 0 (0.0) | ||||
| NYHA I or II, nº (%) | 70.0% | 76.8% | |||
| Others | 2 HF hospitalizations, 1 patient new pacemaker implantation | 1 device thrombosis | |||
| TR 1 year | |||||
| Mild or less | 87.0% | 85.7% | |||
| Moderate | 9.0% | NA | |||
| ≥Severe | 4.0% | NA | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barreiro-Pérez, M.; González-Ferreiro, R.; Caneiro-Queija, B.; Tavares-Silva, M.; Puga, L.; Parada-Barcia, J.A.; Rodriguez-Perez, A.; Baz-Alonso, J.A.; Pinon-Esteban, M.A.; Estevez-Loureiro, R.; et al. Transcatheter Tricuspid Valve Replacement: Illustrative Case Reports and Review of State-of-Art. J. Clin. Med. 2023, 12, 1371. https://doi.org/10.3390/jcm12041371
Barreiro-Pérez M, González-Ferreiro R, Caneiro-Queija B, Tavares-Silva M, Puga L, Parada-Barcia JA, Rodriguez-Perez A, Baz-Alonso JA, Pinon-Esteban MA, Estevez-Loureiro R, et al. Transcatheter Tricuspid Valve Replacement: Illustrative Case Reports and Review of State-of-Art. Journal of Clinical Medicine. 2023; 12(4):1371. https://doi.org/10.3390/jcm12041371
Chicago/Turabian StyleBarreiro-Pérez, Manuel, Rocío González-Ferreiro, Berenice Caneiro-Queija, Marta Tavares-Silva, Luis Puga, Jose A Parada-Barcia, Alvaro Rodriguez-Perez, Jose A Baz-Alonso, Miguel A Pinon-Esteban, Rodrigo Estevez-Loureiro, and et al. 2023. "Transcatheter Tricuspid Valve Replacement: Illustrative Case Reports and Review of State-of-Art" Journal of Clinical Medicine 12, no. 4: 1371. https://doi.org/10.3390/jcm12041371
APA StyleBarreiro-Pérez, M., González-Ferreiro, R., Caneiro-Queija, B., Tavares-Silva, M., Puga, L., Parada-Barcia, J. A., Rodriguez-Perez, A., Baz-Alonso, J. A., Pinon-Esteban, M. A., Estevez-Loureiro, R., & Iniguez-Romo, A. (2023). Transcatheter Tricuspid Valve Replacement: Illustrative Case Reports and Review of State-of-Art. Journal of Clinical Medicine, 12(4), 1371. https://doi.org/10.3390/jcm12041371





