Abstract
The study was conducted from October 2020 to March 2022 in a province in southern Thailand. The inpatients with community-acquired pneumonia (CAP) and more than 18 years old were enrolled. Of the 1511 inpatients with CAP, COVID-19 was the leading cause, accounting for 27%. Among the patients with COVID-19 CAP, mortalities, mechanical ventilators, ICU admissions, ICU stay, and hospital costs were significantly higher than of those with non-COVID-19 CAP. Household and workplace contact with COVID-19, co-morbidities, lymphocytopenia and peripheral infiltration in chest imaging were associated with CAP due to COVID-19. The delta variant yielded the most unfavorable clinical and non-clinical outcomes. While COVID-19 CAP due to B.1.113, Alpha and Omicron variants had relatively similar outcomes. Among those with CAP, COVID-19 infection as well as obesity, a higher Charlson comorbidity index (CCI) and APACHE II score were associated with in-hospital mortality. Among those with COVID-19 CAP, obesity, infection due to the Delta variant, a higher CCI and higher APACHE II score were associated with in-hospital mortality. COVID-19 had a great impact on the epidemiology and outcomes of CAP.
1. Introduction
Community-acquired pneumonia (CAP) is the leading cause of hospitalization and potentially life-threatening conditions worldwide [1,2]. CAP also yields relatively high medical costs for diagnosis and management [3,4,5]. Approximately 20% of patients with CAP are hospitalized due to the severity of the disease as well as their underlying conditions [6,7,8]. Most epidemiological studies of CAP based on patients who were hospitalized, address the high fatality rate with a reported 30-day mortality rate of 13.0%, a 6-month mortality rate of 23.4%, and a 1-year mortality rate of 30.6% [7]. Various pathogens associated with CAP cause a wide range of clinical characteristics and outcomes [9,10,11]. Thus, CAP cases, due to various causative pathogens, require specific treatments and different resource utilization [12,13]. Over the decades, the epidemiology of CAP was disrupted by the emergence of infectious diseases such as the influenza pandemic, severe respiratory distress syndrome (SARS), and Middle-East Respiratory Syndrome (MERS) [14,15,16]. However, the epidemiological change occurred within a short period [14].
In December 2019, cases of CAP were reported in Wuhan, China. The etiology of these infections was severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), a novel coronavirus [17]. The infection caused due to this virus resulted in a wide range of clinical manifestations from mild symptoms, such as fever, cough, myalgia, and diarrhea, to severe forms of pneumonia and respiratory disease syndrome [18]. The SARS-CoV-2 pandemic began in early 2020 with approximately 500 million confirmed cases and 6 million deaths through August 2022 [19]. The unprecedented situation has challenged the healthcare system with a large number of hospitalizations due to CAP because of the unfamiliarity of the disease [20]. The two major concerns regarding hospitalized patients with CAP due to SARS-CoV-2 are how to distinguish them from CAP due to other causes and how to stratify them based on the different risks of unfavorable outcomes [21]. Several factors including the underlying diseases, vaccination, and treatments have been explored for their potential association with the patients’ mortality [22,23,24]. However, due to multiple epidemic waves because of different variants of this virus, the relationship between the patient outcomes and the variants remains unclear. Hence, this observational study aimed to compare the clinical and non-clinical outcomes of hospitalized patients with CAP due to SARS-CoV-2 variants and those with CAP due to other causes.
2. Methodology
2.1. Study Design and Population
This prospective observational study was conducted during the SARS-CoV-2 outbreaks in Thailand, initially from 1 October 2020 to 30 September 2021, then extended to 31 March 2022 (a total of 1 year and 6 months) and included patients from Songkhla Province, southern Thailand, who were admitted to the following 21 hospitals due to pneumonia: one university hospital (Songklanagarind Hospital, PSU), two provincial central hospitals (Hatyai Hospital and Songkhla Hospital), three private hospitals (Bangkok Hospital Hat Yai, Rajyindee Hospital, and Sikarin Hat Yai Hospital), and 15 district primary care hospitals (Krasae Sin Hospital, Khlong Hoi Khong Hospital, Khuanniang Hospital, Chana Hospital, Thepha Hospital, Na Mom Hospital, Bang Klam Hospital, Padang Besar Hospital, Ranot Hospital, Rattaphum Hospital, Satingpra Hospital, Somdet Phraborom Rachineenat Hospital, Sadao Hospital, Saba Yoi Hospital, and Singha Nakhon Hospital).
2.2. Inclusion and Exclusion Criteria
The inclusion criteria of the patients included: (1) aged ≥18 years (2) admitted to the hospital from 1 October 2020 to 31 March 2022, and (3) diagnosed with CAP or healthcare-associated pneumonia. The exclusion criteria included patients with (1) <50% completeness of the data record, (2) an initial diagnosis of hospital-acquired pneumonia or ventilator-associated pneumonia and (3) SARS-CoV-2 co-infection with other pathogens.
2.3. Definition and Diagnosis Criteria
Pneumonia was diagnosed according to the modified criteria from the CDC/NHSN surveillance definition of healthcare-associated infection [25] (see Supplementary Materials), which included clinical and imaging criteria. CAP/healthcare-associated pneumonia, (HCAP)/hospital-acquired pneumonia and (HAP)/ventilator-associated pneumonia (VAP) was diagnosed according to the ATS/IDSA definition [26] (see Supplementary Materials).
2.4. Sample Collection and Pathogen Identification
The research protocol (REC.63-164-14-1) was approved by the local ethics committee and all participants provided written informed consent before enrollment in the study.
The doctors in charge or medical staff in each of the participating hospitals were informed to notify the research team or the correspondent, within 72 h of any patient admission due to pneumonia. Written informed consent was provided prior to enrollment. The clinical and imaging findings of the consented patients were reviewed by the research team along with a radiologist if participants are diagnosed with CAP/HCAP according to the criteria. Expectorated/endotracheal-aspirated (intubated participants) sputum was collected within 24 h of enrollment. Nasopharyngeal throat swabs were used when sputum was not adequately collected. The clinical data of the patients were collected, and the patients were followed up until they were discharged or died. Sample collection, processing, and laboratory diagnostic testing followed the World Health Organization recommendations and CDC guidelines. The patient’s sputum was re-suspended in N-acetylcysteine (NAC) in a 1:1 ratio, and the nucleic acids were extracted from 200 µL of the samples using MagDEA® Dx reagents (Precision System Science, Chiba, Japan) and a fully automated nucleic acid extraction system, according to the manufacturer’s instructions. The presence of SARS-CoV-2 was detected via real-time polymerase chain reaction (RT-PCR) amplification using a SARS-CoV-2 Nucleic Acid Diagnostic Kit (Sansure, Changsha, China). ORF 1ab and N genes were used as the target regions, and human RNase P was used as an internal standard gene control with a lower limit of detection of 200 copies/mL. The respiratory pathogens were detected using the Allplex™ Respiratory Panel Assays (Seegene Inc., Seoul, South Korea), which is a multiplex one-step real-time PCR assay based on Seegene’s proprietary MuDT™ technology to identify 26 causative pathogens, including influenza virus (FluA, Flu A-H1, Flu A-H1pdm09, Flu A-H3, Flu B), respiratory syncytial virus (RSV-A, RSV-B), adenovirus, enterovirus, metapneumovirus, parainfluenza virus (PIV 1-4), bocavirus 1/2/3/4, coronavirus 229E, coronavirus NL63, coronavirus OC43, human rhinovirus, Bordetella parapertussis, Bordetella pertussis, Chlamydophila pneumoniae, Hemophilus influenzae, Legionella pneumophila, Mycoplasma pneumoniae, and Streptococcus pneumoniae.
2.5. Statistical Analyses
The prevalence of SARS-CoV-2 and other pathogens was reported as the proportion of participants. The continuous normally distributed data were reported as mean ± SD, the continuous skewed distributed data as median with IQRs, and the categorical data as percentages. Univariate analysis was used to compare the characteristics or clinical parameters between the two groups, and factors that showed significance (p value < 0.2) in the univariate analysis were then introduced into the multivariate analysis. Survival analysis was used to compare the survival among each CAP group. The statistical significance was set at a p value < 0.05. All statistical tests were performed using R language and environment (version 2.14.1) (Hatyai, Songkhla, Thailand).
2.6. Ethical Statement
This study was approved by the institutional review board of the Faculty of Medicine, Prince of Songkla University, Thailand (REC: 63-164-14-1). The researchers were given permission to retrieve clinical and microbiological data from the hospital database with a consent waiver. Before being assessed and used, all data were anonymized. The researchers confirm that this research was conducted in line with the Declaration of Helsinki principles.
3. Results
A total of 1526 patients were admitted to the participating hospitals in Songkhla Province due to CAP, from October 2020 to March 2022. The causative pathogens of CAP in the inpatients are shown in Table 1. SARS-CoV-2, which causes coronavirus disease 2019 (COVID-19), was the most common cause of CAP (n = 408), accounting for 27.0% of all the cases. The characteristics of patients with CAP due to SARS-CoV-2, its variants and those due to other causes (including unknown causes) are shown and compared in Table 2. The factors that showed a p value of < 0.2 were introduced into a multiple logistic regression model to differentiate between the groups (Table 3). Household or workplace contact with COVID-19 cases (p < 0.001), the presence of comorbidities such as diabetes mellitus (p = 0.002), chronic kidney disease (CKD) (p = 0.028), and malignancy (p < 0.001) were associated with the increased risk of CAP due to COVID-19. Of the 85 patients with malignancy and CAP due to COVID-19, 39 (45%) had lung cancer, 28 (33%) had hematologic malignancy, and 13 (15%) had cancer of the hepatobiliary tract. Laboratory characteristics, including lymphocytopenia (p < 0.001) and peripheral infiltration (p < 0.001) in the chest radiographic findings, were associated with CAP due to COVID-19.

Table 1.
Causative pathogens of community-acquired pneumonia (CAP) in the study cohort.

Table 2.
Characteristics of patients with community-acquired pneumonia (CAP) and comparison between CAP due to coronavirus disease 2019 (COVID-19) and CAP due to non-COVID-19.

Table 3.
Characteristics associated with community-acquired pneumonia (CAP) due to coronavirus disease 2019 (COVID-19).
COVID-19 vaccination negatively correlated with the risk of CAP due to COVID-19 (odds ratio (OR), 0.67; 95% confidential interval (CI), 0.51–0.88). Of the patients with CAP due to COVID-19, 69 (17%) were vaccinated against SARS-CoV-2 as follows: one dose of CoronaVac (n = 11), two doses of CoronaVac (n = 7), one dose of ChAdOx1 nCoV-19 (n = 5), two doses of ChAdOx1 nCoV-19 (n = 5), one dose of BNT162b2 mRNA (n = 6), two doses of BNT162b2 mRNA (n = 6), two doses of mRNA-Moderna (n = 2), one dose of CoronaVac/one dose of ChAdOx1 nCoV-19 (n = 5), one dose of CoronaVac/one dose of BNT162b2 mRNA (n = 4), two doses of CoronaVac/one dose of ChAdOx1 nCoV-19 (n = 3), two doses of CoronaVac/one dose of BNT162b2 mRNA (n = 3), two doses of CoronaVac/two doses of mRNA-Moderna (n = 3), one dose of ChAdOx1 nCoV-19/one dose of BNT162b2 mRNA (n = 3), two doses of ChAdOx1 nCoV-19/one dose of BNT162b2 mRNA (n = 2), one dose of ChAdOx1 nCoV-19/two doses of BNT162b2 mRNA (n = 2), two doses of CoronaVac/two doses of ChAdOx1 nCoV-19 (n = 1), two doses of CoronaVac/two doses of BNT162b2 mRNA (n = 1).
The outcomes of patients with CAP due to COVID-19 and those with CAP due to other causes are shown and compared in Table 4. The outcomes, including mortality, length of hospital stay, and hospital costs were significantly unfavorable in patients with CAP due to COVID-19. Table 4 presents the comparison of the outcomes of patients with infection due to different strains of SARS-CoV-2. The mortality rates, lengths of hospital stay, and hospital costs significantly differed among patients infected with various strains: B. 1.113, B. 1.1.7, B. 1.617.1/2, and B. 1.1.529. The mortality rate, length of hospital stay, and hospital costs were most unfavorable in patients infected with the B.1.617.1/2 strain than those infected with other strains. Survival analysis among patients with CAP due to other causes (non-COVID-19) and CAP due to the various variants of SARS-CoV-2 demonstrated significantly different survival outcomes (p < 0.001 by log-rank test) (Figure 1).

Table 4.
Comparisons of outcomes of patients with community-acquired pneumonia (CAP) due to coronavirus disease 2019 (COVID-19) and non-COVID-19 CAP and comparisons of outcomes among the patients with CAP due to the B.1.113, B.1.1.7, B.1.617.1/2 and B.1.1.529 variants.
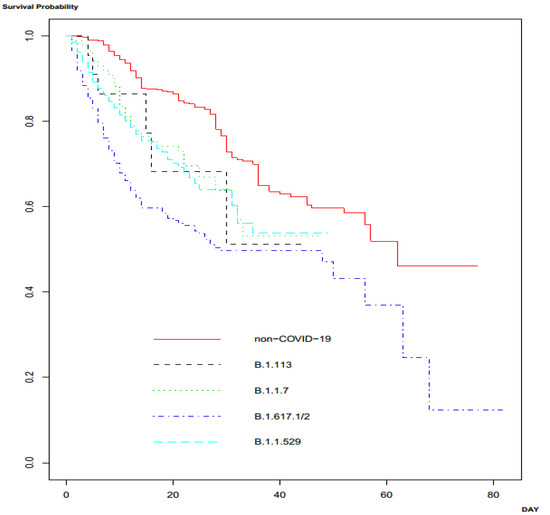
Figure 1.
Survival analysis among the patients with community-acquired pneumonia (CAP) due to SARS-CoV-2 variants and other pathogens.
Of the 408 patients with CAP due to COVID-19, 176 died during admission, accounting for 43% of the cases. The causes of death of these patients are shown in Table 5. The factors influencing mortality in patients with CAP (n = 1526) included obesity, Charlson comorbidity index, initial APACHE II score, and infection due to SARS-CoV-2 (Table 6). Among the patients with CAP due to COVID-19, receiving COVID-19 vaccination and infection due to the B.1.617.1/2 strain were significantly associated with in-hospital mortality (Table 7). In patients with CAP due to COVID-19 who died during admission (n = 176), 20 (11%) had received COVID-19 vaccines as follows: one dose of CoronaVac (n = 4), two doses of CoronaVac (n = 3), one dose of ChAdOx1 nCoV-19 (n = 3), two doses of ChAdOx1 nCoV-19 (n = 1), one dose of BNT162b2 mRNA (n = 2), two doses of BNT162b2 mRNA (n = 1), two doses of CoronaVac/one dose of ChAdOx1 nCoV-19 (n = 1), two doses of CoronaVac/one dose of BNT162b2 mRNA (n = 1), two doses of CoronaVac/two doses of mRNA-Moderna (n = 1), one dose of ChAdOx1 nCoV-19/one dose of BNT162b2 mRNA (n = 1), two doses of ChAdOx1 nCoV-19/one dose of BNT162b2 mRNA (n = 1), one dose of ChAdOx1 nCoV-19/two doses of BNT162b2 mRNA (n = 1).

Table 5.
Causes of in-hospital deaths in patients with community-acquired pneumonia (CAP) due to coronavirus disease 2019 (COVID-19).

Table 6.
Factors associated with in-hospital mortality among the patients with community-acquired pneumonia (CAP).

Table 7.
Factors associated with in-hospital mortality among the patients with community-acquired pneumonia (CAP) due to due to coronavirus disease 2019 (COVID-19).
4. Discussion
This study demonstrated that among inpatients with CAP admitted to the participating hospitals between October 2020 and March 2022, SARS-CoV-2 was the most common cause of infection. The factors associated with CAP due to SARS-CoV-2 included household or workplace contact with COVID-19, and the presence of comorbidities such as diabetes mellitus, CKD, and malignancy. Clinical manifestations, including lymphocytopenia and abnormal chest radiographic findings with peripheral infiltration, were also shown to be associated with CAP due to COVID-19. Comorbidities, such as malignancy and CKD, were predominant among those with infection due to the B.1.1.529 variant. The clinical outcomes and economic burdens among patients with CAP due to COVID-19 were significantly unfavorable compared to those with CAP due to other causes. The patients with CAP due to COVID-19 infected with the B.1.617.1/2 variant yielded the most unfavorable clinical and non-clinical outcomes. The most common cause of death among inpatients with CAP due to COVID-19 was ventilator-associated pneumonia; thus, the most common causative organism was Acinetobacter baumannii. The factors associated with in-hospital mortality among those with CAP were obesity, Charlson comorbidity index, initial APACHE II score, and infection due to COVID-19, and among those with CAP due to COVID-19 were obesity, Charlson comorbidity index, initial APACHE II score, and infection due to the B.1.617.1/2 variant.
Regarding the inclusion criteria, the current study focused only on inpatients and did not include those with mild symptoms, such as walking pneumonia or patients with CAP due to COVID-19 admitted in the community or home isolation. Therefore, several patient characteristics should be considered. First, the clinical condition of the enrolled patients was relatively severe with an initial APACHE score of 17 and a respiratory failure rate of 11% at admission, when compared to previous data on the patients with CAP [27]. Second, the percentage of identifiable causative pathogens were higher (75.2%) in our study cohort than in previous reports [9,10,11,28,29,30]. These can be explained by the obvious presenting symptoms of the patients; for example, a large amount of sputum or intubation for mechanical ventilation aiding specimen (sputum) collection and raised the sensitivity for diagnosis. Thus, the current study used an additional molecular method with a microarray technique in addition to conventional culture methods for the identification of the causative pathogens [31,32] Additionally, the major causative pathogen was SARS-CoV-2, identified using a molecular technique of PCR from sputum and nasal/nasopharyngeal swabs. Third, the proportion of pathogens causing non-severe or walking pneumonia, such as Mycoplasma spp. was comparatively low [33,34,35].
Several clinical characteristics of patients with CAP due to SARS-CoV-2 have been demonstrated and distinguished from those with CAP due to other pathogens. The comorbidities, including diabetes mellitus, CKD, and malignancy were significantly associated with CAP due to SARS-CoV-2 among those with CAP. In this study, the presence of diabetes mellitus was found to be significantly associated with CAP due to SARS-CoV-2. Although the prevalence of diabetes among COVID-19 patients varied from 7.7 to 35% (higher in the US compared with China) and is not higher than that in the general population, diabetes or uncontrolled hyperglycemia tends to have an unfavorable outcome [33] (e.g., severe symptoms, acute respiratory distress syndrome (ARDS), ICU admission, and mortality). Several mechanisms have been proposed to explain this phenomenon including hyperglycemia-induced lung dysfunction, immunosuppression, and increased oxidative stress (OS). Diabetes-induced OS plays a major role in COVID-19 pneumonia progression. Hyperglycemia promotes OS by elevating mitochondrial superoxide anion generation and increasing the glycosylation of proteins, as well as by activating various signaling pathways that may change pulmonary function and structure (e.g., decrease in volume, elastic recoil, and diffusion capacity) [34].
The present study also showed that patients with underlying malignancy had significantly more complications with CAP due to COVID-19 than those with non-COVID-19 CAP. Our results are concordant with those of previous studies demonstrating an increased risks of COVID-19-related severe events and mortality rates in patients with cancer [36,37,38,39]. Furthermore, the independent factors reported to be associated with increased 30-day mortality in cancer patients with COVID-19 included older age, male sex, former smoking habit, presence of comorbidities, an Eastern Cooperative Oncology Group performance status of 2 or higher, and receipt of azithromycin plus hydroxychloroquine [36]. A recent publication from India also demonstrated that advancing age, smoking history, concurrent comorbidities, and palliative intent of treatment were independently associated with severe COVID-19 or death [39]. In addition, patients with cancer might have a higher risk of COVID-19 than those without cancer in Chinese populations [38]. This finding might be potentially explained by the fact that the expression of angiotensin-converting enzyme 2 (ACE2) increases with age, and, in general, cancer patients tend to be older. Since ACE2 is a receptor for the virus permitting entry into the target cells, a higher expression of ACE2 would escalate the risk of SARS-CoV-2 infection in these patients [40]. Taken together, whether cancer itself or the aforementioned risks commonly observed in cancer patients increase the risk of COVID-19 remains unclear. This study also found that patients with lung cancer appeared to have a greater risk of CAP due to COVID-19 than those with other types of malignancies. Interestingly, Gottschalk et al. reported that ACE2, which is frequently detected in patients with lung cancer, is also strongly up-regulated in the lungs during SARS-CoV-2 infection. Therefore, the up-regulated expression of ACE2 in lung tumors might increase the susceptibility to SARS-CoV-2 infection in patients with lung cancer [41]. In contrast, another study reported no difference in severe events related to COVID-19 between patients with lung cancer and those with other cancers [38]. However, it is important to note that the number of patients with lung cancer in that study was quite small (n = 5); hence, it was unlikely to detect significant differences between the groups. Additionally, patients with lung cancer might be at an increased risk for SARS-CoV-2 infection due to difficulties in prolonged mask-wearing due to their compromised respiratory status [42].
Our study showed that CKD was associated with an increased risk of CAP due to SARS-CoV-2, which was corroborated by the large national wide analysis from China which reported that patients with COVID-19 and CKD had a high possibility of ICU admission and receiving invasive mechanical ventilation and thus a higher mortality rate [43]. In contrast, the studies discussed in a meta-analysis reported that CKD is not a predictor of severe SARS-CoV-2 infection. Nevertheless, after the pool estimation, the presence of CKD increased the risk of severe COVID-19 [44]. This might be due to the inclusion of underpowered studies that did not have a sufficient sample size to detect a true effect. Recently, a study from the US demonstrated that CKD is a significant predictor of mortality among patients with COVID-19 after adjusting for other risk factors [45]. To our knowledge, these associations could be explained by two main reasons: first, uremia-induced dysregulation of the immune system revealed inadequate CD4 T cell responses, which led to a delayed clearance of the virus [46,47,48]. Second, ACE2 receptor, used by SARS-CoV-2 for initial attachment and access into the cells, is highly expressed in the lungs and kidneys. Hence, this might increase a patient’s susceptibility to adverse outcomes [49,50]. Interestingly, this study found that CKD strongly correlated with CAP due to the B.1.159 SARS-CoV-2 variant, which was similar to a previous report that showed that renal disease was a common comorbidity among patients infected with SARS-CoV-2 [51]. However, they also found that patients with COVID-19 were less likely to have severe clinical consequences. Thus, we hypothesized that the effect of renal disease on CAP due to the B.1.159 variant could be related to the residual confounding by the increased number of COVID-19 CKD cases per day during the Omicron period. In fact, there was a decline in COVID-19 patient admissions during the B.1.159 SARS-CoV-2 variant outbreak. Hence, patients with CKD who were unvaccinated against SARS-CoV-2 showed severe symptoms, as demonstrated by CAP, and were more likely to seek medical care at the hospital during the Omicron wave. However, this approach can also lead to the overestimation of the number of patients.
Several investigative laboratories have distinguished patients with CAP due to SARS-CoV-2 from those with CAP due to other pathogens. Leukopenia and lymphopenia were higher in patients with COVID-19 than in non-COVID-19 pneumonia patients; however, after adjusting for OR with other parameters, only leukopenia was associated with COVID-19 pneumonia. A meta-analysis of data on 1995 patients with COVID-19 reported that 64.5% of the patients had lymphocytopenia and 29.4% had leukocytopenia [52]; however, the underlying mechanism is not well understood. Granulocytic myeloid-derived suppressor cells (G-MDSCs) have been proposed to play an important role in T lymphocyte suppression. The suppressive functions of G-MDSCs include (1) impaired T cell proliferation and suppressed IFN-γ cytokine production; (2) anergy of effector CD8+ cytotoxic T cells and CD4+ helper T cells, or (3) expansion of Treg cells [53]. This study showed that peripheral infiltration on chest radiographs was associated with CAP due to COVID-19. Multiple meta-analyses reported that the most common chest radiographic findings were ground-glass opacity and consolidation, which involve the bilateral lungs and have peripheral distribution [54,55,56], which could be due to the involvement of the SARS-CoV-2 infection site. SARS-CoV-2 is usually involved in gas exchange units, especially pneumocyte type II cells, which infect the peripheral and subpleural areas, resulting in the infiltration patterns [57].
The current study demonstrated that receiving a vaccination against COVID-19 was negatively associated with the risk of CAP due to COVID-19 and in-hospital mortality. The efficacy of COVID-19 vaccination in this study should be carefully considered owing to several limitations. First, there was relatively high heterogeneity in the types of vaccines administered with varying effectiveness in infection prevention and decreased the severity of the disease [58,59,60,61]. Second, the variation of completed vaccination (at least 2 dosages) course as well as the boosting dosage(s) among the patients receiving vaccines against COVID-19 might affect the effectiveness of each vaccination [58,59,60,61]. Third, in Thailand, there were several heterogeneous regimens for COVID-19 vaccination, which should be considered when analyzing the varying outcomes [62,63]. Finally, each variant of SARS-CoV-2 emerged in different periods with a wide range of rates of completed vaccination and boosting. Thus, recent influenza immunization provided borderline protective mortality against CAP due to COVID-19. It is well established that COVID-19 vaccination and infection reduce disease mortality by inducing antigen-specific T cell repertoires. Re-infection with different viral strains is mainly asymptotic, which could be explained by the well-conserved region of spike-specific T cells [64,65]. Influenza vaccines induce T cell responses specific to the influenza nucleocapsid, which can cross-counter the nucleocapsid of other common cold viruses, including corona species [66,67]. Therefore, pre-existing nucleocapsid-specific T cells could confer some protection against SARS-CoV-2 infection.
The present study also found that the severity and mortality of patients with COVID-19 differed among the variants. The B.1.617.1/2 (Delta) variant was a risk factor for unfavorable outcomes, whereas pre-Delta and its predecessors compared with Omicron had similar ICU admission rates and mortality, similar to previous studies that reported the association of the B.1.617.1/2 variant with poor outcomes and the Omicron variant with a better outcome [68,69,70,71,72,73]. The disease severity associated with Alpha, Gamma, and Delta variants was reported to be comparable, while Omicron infections were significantly less severe, but the breakthrough disease was significantly more common in patients with Omicron infection [74]. While in this study, the mortality among patients infected with the Omicron variant was similar to that of the B.1.113 and B.1.1.7 (Alpha) variants. Magazine et al. reported more related mutations located in the S1 subunit of the spike protein such as 69-70del, 144del, and 156del, including N501Y and D614G, between the Alpha and Omicron variants when compared with other variants, which play an essential role in enhancing viral replication and increasing transmission [75,76,77]. The 1.617.1/2 variant developed several mutations in the viral genome that resulted in many significant features [78]. The Delta spike mutation P681R, located at the furin cleavage site, enhances the cleavage of the full-length spike to S1 and S2 subunits, which improves cell-surface-mediated viral entry [79]. The L452R and T478K mutations enhance ACE receptor affinity by altering the receptor-binding domain (RBD) and developing immune invasion (both immune and vaccine escape) due to changes in the epitope of the spike protein [78]. Immune invasion results in a higher viral load and a longer viral shedding time [73]. When compared to other earlier studies, this one found a relatively low incidence of COVID-19-associated pulmonary aspergillosis (CAPA), with an estimated incidence of 8.6% in patients receiving mechanical ventilation [80]. This could be explained by the fact that COVID-19 CAP patients were admitted in a newly constructed isolated or negative pressure room instead of an old one; before the COVID-19 pandemic, hospitals had a very low number of negative pressure or isolated rooms. Therefore, the new one may have less fungal contamination and reduce the incidence of aspergillosis and mucormycosis.
Although previous studies demonstrated the efficacy of systemic corticosteroids, remdesivir, interleukin-6 inhibitors, and Janus kinase inhibitors for patients with severe CAP due to SARS-CoV-2 [81,82,83,84,85], those efficacies were not demonstrated in this current study. It can be explained as follows: (1) due to the wide range of severity of patients in this study, patients with mild disease might not receive the benefits from those treatments. (2) Most of the patients were treated according to the standard guidelines, which recommend using these treatments based on the severity of the patient.
This study has some limitations that should be acknowledged. First, the patients with CAP and mild symptoms were not enrolled in this study. Second, this study population could have included patients with healthcare-associated pneumonia, such as those undergoing regular hemodialysis or receiving chemotherapy. Third, the potential misclassification bias might be secondary to tertiary data extraction. Fourth, in relation to non-interventional studies, the reason for clinical decisions has not been well explored. Lastly, there were several interferences from the dynamics of diseases, including changes in major variants involved in outbreaks over time, changes in the national policy for the standard of treatment, and availability of those recommended treatments, which potentially might alter the clinical outcomes of infection.
In conclusion, during the COVID-19 pandemic, SARS-CoV-2 was the major cause of CAP and caused unfavorable clinical and non-clinical outcomes, particularly due to the B.1.617.1/2 variant. Regarding to the wide range of vaccine regimens and dosages, even this study demonstrated that the COVID-19 vaccination tended to be protective factor; thus, the efficacy of vaccination should be further explored.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm12041388/s1. The definition of pneumonia case according to ATS/IDSA guideline.
Author Contributions
Conceptualization, N.T., W.T., P.S., N.K., S.K., A.N., T.H. and S.C.; Methodology, S.S., W.T., P.S., N.K., S.K., A.N. and S.C.; Software, S.C.; Validation, S.C.; Formal analysis, S.C.; Investigation, N.T., S.S., T.H., B.C. and S.C.; Resources, W.T., P.S., N.K., S.K., A.N. and S.C.; Data curation, B.C. and S.C.; Writing—original draft, N.T., N.P., A.D., A.P. and S.C.; Writing—review & editing, N.T. and S.C.; Visualization, S.C.; Supervision, S.C.; Project administration, S.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Science and Technology Development Agency (NSTDA) grant number [P-20-50620] and Songklanagarind Hospital Foundation (no grant number). The APC was funded by Faculty of Medicine, Prince of Songkla University.
Institutional Review Board Statement
This study was approved by the institutional review board of the Faculty of Medicine, Prince of Songkla University, Thailand (REC: 63-164-14-1).
Informed Consent Statement
The researchers were given permission to retrieve clinical and microbiological data from the hospital database with a consent waiver to avoid the transmission of COVID-19. Before being assessed and used, all data were anonymized. The researchers confirm that this research was conducted in line with the Declaration of Helsinki principles.
Data Availability Statement
Data is contained within the article.
Acknowledgments
The authors are appreciate for the cooperation of the staffs from Songklanagarind Hospital, Hatyai Hospital, Songkhla Hospital, Bangkok Hospital Hat Yai, Rajyindee Hospital, and Sikarin Hat Yai Hospital, Krasae Sin Hospital, Khlong Hoi Khong Hospital, Khuanniang Hospital, Chana Hospital, Thepha Hospital, Na Mom Hospital, Bang Klam Hospital, Padang Be-sar Hospital, Ranot Hospital, Rattaphum Hospital, Satingpra Hospital, Somdet Phrab-orom Rachineenat Hospital, Sadao Hospital, Saba Yoi Hospital, and Singha Nakhon Hospital. The authors are appreciate for data management of Division of Digital Innovation and Data Analytics (DIDA), Faculty of Medicine, Prince of Songkhla University.
Conflicts of Interest
The authors declare no conflict of interest.
References
- GBD Results Tool. GHDx. Available online: http://ghdx.healthdata.org/gbd-results-tool (accessed on 17 June 2020).
- Salah, H.M.; Minhas, A.M.K.; Khan, M.S.; Pandey, A.; Michos, E.D.; Mentz, R.J.; Fudim, M. Causes of hospitalization in the USA between 2005 and 2018. Eur. Heart J. Open. 2021, 1, oeab001. [Google Scholar] [CrossRef] [PubMed]
- Campling, J.; Wright, H.F.; Hall, G.C.; Mugwagwa, T.; Vyse, A.; Mendes, D.; Slack, M.P.E.; Ellsbury, G.F. Hospitalization costs of adult community-acquired pneumonia in England. J. Med. Econ. 2022, 25, 912–918. [Google Scholar] [CrossRef] [PubMed]
- Angus, D.C.; Marrie, T.J.; Obrosky, D.S.; Clermont, G.; Dremsizov, T.T.; Coley, C.; Fine, M.J.; Singer, D.E.; Kapoor, W.N. Severe community-acquired pneumonia: Use of intensive care services and evaluation of American and British Thoracic Society Diagnostic criteria. Am. J. Respir. Crit. Care Med. 2002, 166, 717–723. [Google Scholar] [CrossRef] [PubMed]
- Pfuntner, A.; Wier, L.M.; Steiner, C. Costs for Hospital Stays in the United States, 2011. In Healthcare Cost and Utilization Project (HCUP) Statistical Briefs; Agency for Healthcare Research and Quality (US): Rockville, MD, USA, 2006. Available online: http://www.ncbi.nlm.nih.gov/books/NBK179289 (accessed on 24 October 2022).
- Jain, S.; Self, W.H.; Wunderink, R.G.; Fakhran, S.; Balk, R.; Bramley, A.M.; Chappell, J.D. Community-acquired pneumonia requiring hospitalization among U.S. adults. N. Engl. J. Med. 2015, 373, 415–427. [Google Scholar] [CrossRef]
- Ramirez, J.A.; Wiemken, T.L.; Peyrani, P.; Arnold, F.W.; Kelley, R.; A Mattingly, W.; Nakamatsu, R.; Pena, S.; Guinn, B.E.; Furmanek, S.P.; et al. Adults Hospitalized with Pneumonia in the United States: Incidence, Epidemiology, and Mortality. Clin. Infect. Dis. 2017, 65, 1806–1812. [Google Scholar] [CrossRef]
- Griffin, M.R.; Zhu, Y.; Moore, M.R.; Whitney, C.G.; Grijalva, C.G.U.S. Hospitalizations for Pneumonia after a Decade of Pneumococcal Vaccination. N. Engl. J. Med. 2013, 369, 155–163. [Google Scholar] [CrossRef]
- Eshwara, V.; Mukhopadhyay, C.; Rello, J. Community-acquired bacterial pneumonia in adults: An update. Indian J. Med. Res. 2020, 151, 287–302. [Google Scholar] [CrossRef]
- Mandell, L.A. Community-acquired pneumonia: An overview. Postgrad. Med. 2015, 127, 607–615. [Google Scholar] [CrossRef]
- Apisarnthanarak, A.; Mundy, L.M. Etiology of community-acquired pneumonia. Clin. Chest Med. 2005, 26, 47–55. [Google Scholar] [CrossRef]
- Metlay, J.P.; Waterer, G.W.; Long, A.C.; Anzueto, A.; Brozek, J.; Crothers, K.; Cooley, L.A.; Dean, N.C.; Fine, M.J.; Flanders, S.A.; et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the american thoracic society and infectious diseases society of America. Am. J. Respir. Crit. Care Med. 2019, 200, e45–e67. [Google Scholar] [CrossRef]
- Woodhead, M.; Blasi, F.; Ewig, S.; Garau, J.; Huchon, G.; Ieven, M.; Ortqvist, A.; Schaberg, T.; Torres, A.; van der Heijden, G.; et al. Guidelines for the management of adult lower respiratory tract infections—Full version. Clin. Microbiol. Infect. 2011, 17, E1–E59. [Google Scholar] [CrossRef] [PubMed]
- Summary of Probable SARS Cases with Onset of Illness from 1 November 2002 to 31 July 2003. Available online: https://www.who.int/publications/m/item/summary-of-probable-sars-cases-with-onset-of-illness-from-1-november-2002-to-31-july-2003 (accessed on 27 October 2022).
- CDC Novel H1N1 Flu. The 2009 H1N1 Pandemic: Summary Highlights, April 2009–April 2010. Available online: https://www.cdc.gov/h1n1flu/cdcresponse.htm (accessed on 27 October 2022).
- CSR. MERS Outbreaks. World Health Organization—Regional Office for the Eastern Mediterranean. Available online: http://www.emro.who.int/health-topics/mers-cov/mers-outbreaks.html (accessed on 27 October 2022).
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Guan, W.J.; Ni, Z.Y.; Hu, Y.; Liang, W.H.; Qu, C.Q.; He, J.X.; Liu, L.; Shan, H.; Lei, C.L.; Hui, D.S.C.; et al. Clinical Characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef]
- Weekly Epidemiological Update on COVID-19, 31 August 2022. Available online: https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19---31-august-2022 (accessed on 28 October 2022).
- Hui, D.S.; Azhar, E.I.; Madani, T.A.; Ntoumi, F.; Kock, R.; Dar, O.; Ippolito, G.; McHugh, T.D.; Memish, Z.A.; Drosten, C.; et al. The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health—The latest 2019 novel coronavirus outbreak in Wuhan, China. Int. J. Infect. Dis. IJID Off. Publ. Int. Soc. Infect. Dis. 2020, 91, 264–266. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y.; et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus—Infected Pneumonia in Wuhan, China. JAMA 2020, 323, 1061–1069. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Peng, F.; Xu, B.; Zhao, J.; Liu, H.; Peng, J.; Li, Q.; Jiang, C.; Zhou, Y.; Liu, S.; et al. Risk factors of critical & mortal COVID-19 cases: A systematic literature review and meta-analysis. J. Infect. 2020, 81, e16–e25. [Google Scholar] [CrossRef] [PubMed]
- Thakur, B.; Dubey, P.; Benitez, J.; Torres, J.P.; Reddy, S.; Shokar, N.; Aung, K.; Mukherjee, D.; Dwivedi, A.K. A systematic review and meta-analysis of geographic differences in comorbidities and associated severity and mortality among individuals with COVID-19. Sci. Rep. 2021, 11, 8562. [Google Scholar] [CrossRef]
- Parohan, M.; Yaghoubi, S.; Seraji, A.; Javanbakht, M.H.; Sarraf, P.; Djalali, M. Risk factors for mortality in patients with Coronavirus disease 2019 (COVID-19) infection: A systematic review and meta-analysis of observational studies. Aging Male 2020, 23, 1416–1424. [Google Scholar] [CrossRef]
- Horan, T.C.; Andrus, M.; Dudeck, M.A. CDC/NHSN surveillance definition of health care–associated infection and criteria for specific types of infections in the acute care setting. Am. J. Infect. Control. 2008, 36, 309–332. [Google Scholar] [CrossRef]
- American Thoracic Society; Infectious Diseases Society of America. Guidelines for the Management of Adults with Hospital-acquired, Ventilator-associated, and Healthcare-associated Pneumonia. Am. J. Respir. Crit. Care Med. 2005, 171, 388–416. [Google Scholar] [CrossRef]
- Reechaipichitkul, W.; Thavornpitak, Y.; Sutra, S. Burden of adult pneumonia in Thailand: A nationwide hospital admission data 2010. J. Med. Assoc. Thail. 2014, 97, 283–292. [Google Scholar]
- Cilloniz, C.; Ewig, S.; Polverino, E.; Marcos, M.A.; Esquinas, C.; Gabarrús, A.; Mensa, J.; Torres, A. Microbial aetiology of community-acquired pneumonia and its relation to severity. Thorax 2011, 66, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Restrepo, M.I.; Mortensen, E.M.; Velez, J.A.; Frei, C.; Anzueto, A. A Comparative Study of Community-Acquired Pneumonia Patients Admitted to the Ward and the ICU. Chest 2008, 133, 610–617. [Google Scholar] [CrossRef] [PubMed]
- Poovieng, J.; Sakboonyarat, B.; Nasomsong, W. Bacterial etiology and mortality rate in community-acquired pneumonia, healthcare-associated pneumonia and hospital-acquired pneumonia in Thai university hospital. Sci. Rep. 2022, 12, 9004. [Google Scholar] [CrossRef] [PubMed]
- Rand, K.H.; Beal, S.G.; Cherabuddi, K.; Couturier, B.; Lingenfelter, B.; Rindlisbacher, C.; Jones, J.; Houck, H.J.; Lessard, K.J.; Tremblay, E.E. Performance of a Semiquantitative Multiplex Bacterial and Viral PCR Panel Compared with Standard Microbiological Laboratory Results: 396 Patients Studied with the BioFire Pneumonia Panel. Open Forum Infect. Dis. 2021, 8, ofaa560. [Google Scholar] [CrossRef] [PubMed]
- Edin, A.; Eilers, H.; Allard, A. Evaluation of the Biofire Filmarray Pneumonia panel plus for lower respiratory tract infections. Infect. Dis. 2020, 52, 479–488. [Google Scholar] [CrossRef]
- Huh, K.; Chung, D.R.; Song, J.-H. Community-Acquired Pneumonia in the Asia-Pacific Region. Semin. Respir. Crit. Care Med. 2016, 37, 839–854. [Google Scholar] [CrossRef]
- Marchello, C.; Dale, A.; Thai, T.N.; Han, D.S.; Ebell, M.H. Prevalence of Atypical Pathogens in Patients with Cough and Community-Acquired Pneumonia: A Meta-Analysis. Ann. Fam. Med. 2016, 14, 552–566. [Google Scholar] [CrossRef]
- Prapphal, N.; Suwanjutha, S.; Durongkaveroj, P.; Lochindarat, S.; Kunakorn, M.; Deerojanawong, J.; Chantarojanasiri, T.; Supanitayaonon, Y.; Janedittakarn, P. Prevalence and clinical presentations of atypical pathogens infection in community acquired pneumonia in Thailand. J. Med. Assoc. Thail. 2006, 89, 1412–1419. [Google Scholar]
- Kuderer, N.M.; Choueiri, T.K.; Shah, D.P.; Shyr, Y.; Rubinstein, S.M.; Rivera, D.R.; Shete, S.; Hsu, C.-Y.; Desai, A.; de Lima Lopes, G., Jr.; et al. Clinical impact of COVID-19 on patients with cancer (CCC19): A cohort study. Lancet 2020, 395, 1907–1918. [Google Scholar] [CrossRef]
- Lee, L.Y.W.; Cazier, J.-B.; Starkey, T.; Briggs, S.E.W.; Arnold, R.; Bisht, V.; Booth, S.; Campton, N.A.; Cheng, V.W.T.; Collins, G.; et al. COVID-19 prevalence and mortality in patients with cancer and the effect of primary tumour subtype and patient demographics: A prospective cohort study. Lancet Oncol. 2020, 21, 1309–1316. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Guan, W.; Chen, R.; Wang, W.; Li, J.; Xu, K.; Li, C.; Ai, Q.; Lu, W.; Liang, H.; et al. Cancer patients in SARS-CoV-2 infection: A nationwide analysis in China. Lancet Oncol. 2020, 21, 335–337. [Google Scholar] [CrossRef] [PubMed]
- Sengar, M.; Chinnaswamy, G.; Ranganathan, P.; Ashok, A.; Bhosale, S.; Biswas, S.; Chaturvedi, P.; Dhamne, C.; Divatia, J.; D’Sa, K.; et al. Outcomes of COVID-19 and risk factors in patients with cancer. Nat. Cancer 2022, 3, 547–551. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, X.; Wang, W. The Impact of COVID-19 on Cancer. Infect. Drug Resist. 2021, 14, 3809–3816. [Google Scholar] [CrossRef]
- Gottschalk, G.; Knox, K.; Roy, A. ACE2: At the crossroad of COVID-19 and lung cancer. Gene Rep. 2021, 23, 101077. [Google Scholar] [CrossRef]
- Walter, J.; Sellmer, L.; Kahnert, K.; Kiefl, R.; Syunyaeva, Z.; Kauffmann-Guerrero, D.; Manapov, F.; Schneider, C.; Behr, J.; Tufman, A. Consequences of the COVID-19 pandemic on lung cancer care and patient health in a German lung cancer center: Results from a cross-sectional questionnaire. Respir. Res. 2022, 23, 18. [Google Scholar] [CrossRef]
- Guan, W.-J.; Liang, W.-H.; Zhao, Y.; Liang, H.-R.; Chen, Z.-S.; Li, Y.-M.; Liu, X.-Q.; Chen, R.-C.; Tang, C.-L.; Wang, T.; et al. Comorbidity and its impact on 1590 patients with COVID-19 in China: A nationwide analysis. Eur. Respir. J. 2020, 55, 2000547. [Google Scholar] [CrossRef]
- Henry, B.M.; Lippi, G. Chronic kidney disease is associated with severe coronavirus disease 2019 (COVID-19) infection. Int. Urol. Nephrol. 2020, 52, 1193–1194. [Google Scholar] [CrossRef]
- Mohamed, N.E.; Benn, E.K.T.; Astha, V.; Okhawere, K.E.; Korn, T.G.; Nkemdirim, W.; Rambhia, A.; Ige, O.A.; Funchess, H.; Mihalopoulos, M.; et al. Association between chronic kidney disease and COVID-19-related mortality in New York. World J. Urol. 2021, 39, 2987–2993. [Google Scholar] [CrossRef]
- Cohen, G. Immune Dysfunction in Uremia 2020. Toxins 2020, 12, 439. [Google Scholar] [CrossRef]
- Koelman, L.; Pivovarova-Ramich, O.; Pfeiffer, A.F.H.; Grune, T.; Aleksandrova, K. Cytokines for evaluation of chronic inflammatory status in ageing research: Reliability and phenotypic characterisation. Immun. Ageing 2019, 16, 11. [Google Scholar] [CrossRef]
- Moderbacher, C.R.; Ramirez, S.I.; Dan, J.M.; Grifoni, A.; Hastie, K.M.; Weiskopf, D.; Belanger, S.; Abbott, R.K.; Kim, C.; Choi, J.; et al. Antigen-Specific Adaptive Immunity to SARS-CoV-2 in Acute COVID-19 and Associations with Age and Disease Severity. Cell 2020, 183, 996–1012.e19. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Shang, J.; Graham, R.; Baric, R.S.; Li, F. Receptor Recognition by the Novel Coronavirus from Wuhan: An Analysis Based on Decade-Long Structural Studies of SARS Coronavirus. J. Virol. 2020, 94, e00127-20. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Karakiulakis, G.; Roth, M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir. Med. 2020, 8, e21. [Google Scholar] [CrossRef] [PubMed]
- Skarbinski, J.; Wood, M.S.; Chervo, T.C.; Schapiro, J.M.; Elkin, E.P.; Valice, E.; Amsden, L.B.; Hsiao, C.; Quesenberry, C.; Corley, D.A.; et al. Risk of severe clinical outcomes among persons with SARS-CoV-2 infection with differing levels of vaccination during widespread Omicron (B.1.1.529) and Delta (B.1.617.2) variant circulation in Northern California: A retrospective cohort study. Lancet Reg. Heal. Am. 2022, 12, 100297. [Google Scholar] [CrossRef]
- Li, L.-Q.; Huang, T.; Wang, Y.-Q.; Wang, Z.-P.; Liang, Y.; Huang, T.-B.; Zhang, H.-Y.; Sun, W.; Wang, Y. COVID-19 patients’ clinical characteristics, discharge rate, and fatality rate of meta-analysis. J. Med. Virol. 2020, 92, 577–583. [Google Scholar] [CrossRef]
- Peñaloza, H.F.; Lee, J.S.; Ray, P. Neutrophils and lymphopenia, an unknown axis in severe COVID-19 disease. PLOS Pathog. 2021, 17, e1009850. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, L.; Ben, S. Meta-analysis of chest CT features of patients with COVID-19 pneumonia. J. Med. Virol. 2021, 93, 241–249. [Google Scholar] [CrossRef]
- Bao, C.; Liu, X.; Zhang, H.; Li, Y.; Liu, J. Coronavirus Disease 2019 (COVID-19) CT Findings: A Systematic Review and Meta-analysis. J. Am. Coll. Radiol. 2020, 17, 701–709. [Google Scholar] [CrossRef]
- Yang, H.; Lan, Y.; Yao, X.; Lin, S.; Xie, B. The chest CT features of coronavirus disease 2019 (COVID-19) in China: A meta-analysis of 19 retrospective studies. Virol. J. 2020, 17, 159. [Google Scholar] [CrossRef]
- Mason, R.J. Pathogenesis of COVID-19 from a cell biology perspective. Eur. Respir. J. 2020, 55, 2000607. [Google Scholar] [CrossRef]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Voysey, M.; Clemens, S.A.C.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E.; et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: An interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2021, 397, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Jara, A.; Undurraga, E.A.; González, C.; Paredes, F.; Fontecilla, T.; Jara, G.; Pizarro, A.; Acevedo, J.; Leo, K.; Leon, F.; et al. Effectiveness of an Inactivated SARS-CoV-2 Vaccine in Chile. N. Engl. J. Med. 2021, 385, 875–884. [Google Scholar] [CrossRef] [PubMed]
- Sritipsukho, P.; Khawcharoenporn, T.; Siribumrungwong, B.; Damronglerd, P.; Suwantarat, N.; Satdhabudha, A.; Chaiyakulsil, C.; Sinlapamongkolkul, P.; Tangsathapornpong, A.; Bunjoungmanee, P.; et al. Comparing real-life effectiveness of various COVID-19 vaccine regimens during the delta variant-dominant pandemic: A test-negative case-control study. Emerg. Microbes Infect. 2022, 11, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Wichaidit, M.; Nopsopon, T.; Sunan, K.; Phutrakool, P.; Ruchikachorn, P.; Wanvarie, D.; Pratanwanich, P.N.; Cheewaruangroj, N.; Punyabukkana, P.; Pongpirul, K. Breakthrough infections, hospital admissions, and mortality after major COVID-19 vaccination profiles: A prospective cohort study. Lancet Reg. Heal. Southeast Asia 2023, 8, 100106. [Google Scholar] [CrossRef]
- Keller, M.D.; Harris, K.M.; Jensen-Wachspress, M.A.; Kankate, V.V.; Lang, H.; Lazarski, C.A.; Durkee-Shock, J.; Lee, P.-H.; Chaudhry, K.; Webber, K.; et al. SARS-CoV-2–specific T cells are rapidly expanded for therapeutic use and target conserved regions of the membrane protein. Blood 2020, 136, 2905–2917. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Wang, G.; Wang, Y.; Zhang, Q.; Ren, L.; Gu, X.; Huang, T.; Zhong, J.; Wang, X.; Huang, L.; et al. SARS-CoV-2-specific antibody and T-cell responses 1 year after infection in people recovered from COVID-19: A longitudinal cohort study. Lancet Microbe 2022, 3, e348–e356. [Google Scholar] [CrossRef]
- Capoor, M.N.; Ahmed, F.S.; McDowell, A.; Slaby, O. Is the “Common Cold” Our Greatest Ally in the Battle Against SARS-CoV-2? Front. Cell. Infect. Microbiol. 2020, 10, 605334. [Google Scholar] [CrossRef]
- Lipsitch, M.; Grad, Y.H.; Sette, A.; Crotty, S. Cross-reactive memory T cells and herd immunity to SARS-CoV-2. Nat. Rev. Immunol. 2020, 20, 709–713. [Google Scholar] [CrossRef]
- Stepanova, M.; Lam, B.; Younossi, E.; Felix, S.; Ziayee, M.; Price, J.; Pham, H.; de Avila, L.; Terra, K.; Austin, P.; et al. The impact of variants and vaccination on the mortality and resource utilization of hospitalized patients with COVID-19. BMC Infect. Dis. 2022, 22, 702. [Google Scholar] [CrossRef]
- Ward, I.L.; Bermingham, C.; Ayoubkhani, D.; Gethings, O.J.; Pouwels, K.B.; Yates, T.; Khunti, K.; Hippisley-Cox, J.; Banerjee, A.; Walker, A.S.; et al. Risk of covid-19 related deaths for SARS-CoV-2 omicron (B.1.1.529) compared with delta (B.1.617.2): Retrospective cohort study. BMJ 2022, 378, e070695. [Google Scholar] [CrossRef]
- Twohig, K.A.; Nyberg, T.; Zaidi, A.; Thelwall, S.; Sinnathamby, M.A.; Aliabadi, S.; Seaman, S.R.; Harris, R.J.; Hope, R.; Lopez-Bernal, J.; et al. Hospital admission and emergency care attendance risk for SARS-CoV-2 delta (B.1.617.2) compared with alpha (B.1.1.7) variants of concern: A cohort study. Lancet Infect. Dis. 2022, 22, 35–42. [Google Scholar] [CrossRef]
- Nyberg, T.; Ferguson, N.M.; Nash, S.G.; Webster, H.H.; Flaxman, S.; Andrews, N.; Hinsley, W.; Bernal, J.L.; Kall, M.; Bhatt, S.; et al. Comparative analysis of the risks of hospitalisation and death associated with SARS-CoV-2 omicron (B.1.1.529) and delta (B.1.617.2) variants in England: A cohort study. Lancet 2022, 399, 1303–1312. [Google Scholar] [CrossRef] [PubMed]
- Ito, N.; Kitahara, Y.; Miwata, K.; Okimoto, M.; Takafuta, T. Comparison of COVID-19 pneumonia during the SARS-CoV-2 Omicron wave and the previous non-Omicron wave in a single facility. Respir. Investig. 2022, 60, 772–778. [Google Scholar] [CrossRef] [PubMed]
- Ong, S.W.X.; Chiew, C.J.; Ang, L.W.; Mak, T.M.; Cui, L.; Toh, M.P.H.S.; Lim, Y.D.; Lee, P.H.; Lee, T.H.; Chia, P.Y.; et al. Clinical and Virological Features of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Variants of Concern: A Retrospective Cohort Study Comparing B.1.1.7 (Alpha), B.1.351 (Beta), and B.1.617.2 (Delta). Clin. Infect. Dis. 2022, 75, e1128–e1136. [Google Scholar] [CrossRef] [PubMed]
- Esper, F.P.; Adhikari, T.M.; Tu, Z.J.; Cheng, Y.-W.; El-Haddad, K.; Farkas, D.H.; Bosler, D.; Rhoads, D.; Procop, G.W.; Ko, J.S.; et al. Alpha to Omicron: Disease Severity and Clinical Outcomes of Major SARS-CoV-2 Variants. J. Infect. Dis. 2022, 227, 344–352. [Google Scholar] [CrossRef]
- Magazine, N.; Zhang, T.; Wu, Y.; McGee, M.C.; Veggiani, G.; Huang, W. Mutations and Evolution of the SARS-CoV-2 Spike Protein. Viruses 2022, 14, 640. [Google Scholar] [CrossRef]
- Tian, F.; Tong, B.; Sun, L.; Shi, S.; Zheng, B.; Wang, Z.; Dong, X.; Zheng, P. N501Y mutation of spike protein in SARS-CoV-2 strengthens its binding to receptor ACE2. Elife 2021, 10, e69091. [Google Scholar] [CrossRef]
- Chakraborty, C.; Saha, A.; Sharma, A.R.; Bhattacharya, M.; Lee, S.-S.; Agoramoorthy, G. D614G mutation eventuates in all VOI and VOC in SARS-CoV-2: Is it part of the positive selection pioneered by Darwin? Mol. Ther. Nucleic Acids 2021, 26, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, M.; Chatterjee, S.; Sharma, A.R.; Lee, S.-S.; Chakraborty, C. Delta variant (B.1.617.2) of SARS-CoV-2: Current understanding of infection, transmission, immune escape, and mutational landscape. Folia Microbiol. 2022, 12, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, J.; Johnson, B.A.; Xia, H.; Ku, Z.; Schindewolf, C.; Widen, S.G.; An, Z.; Weaver, S.C.; Menachery, V.D.; et al. Delta spike P681R mutation enhances SARS-CoV-2 fitness over Alpha variant. Cell Rep. 2021, 39, 110829. [Google Scholar] [CrossRef] [PubMed]
- Dimopoulos, G.; Almyroudi, M.-P.; Myrianthefs, P.; Rello, J. COVID-19-Associated Pulmonary Aspergillosis (CAPA). J. Intensiv. Med. 2021, 1, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Wagner, C.; Griesel, M.; Mikolajewska, A.; Mueller, A.; Nothacker, M.; Kley, K.; Metzendorf, M.-I.; Fischer, A.-L.; Kopp, M.; Stegemann, M.; et al. Systemic corticosteroids for the treatment of COVID-19. Cochrane Database Syst. Rev. 2021, 8, CD014963. [Google Scholar] [CrossRef]
- Beigel, J.H.; Tomashek, K.M.; Dodd, L.E.; Mehta, A.K.; Zingman, B.S.; Kalil, A.C.; Hohmann, E.; Chu, H.Y.; Luetkemeyer, A.; Kline, S.; et al. Remdesivir for the Treatment of COVID-19—Preliminary report. N. Engl. J. Med. 2020, 383, 1813–1826. [Google Scholar] [CrossRef]
- Ali, K.; Azher, T.; Baqi, M.; Binnie, A.; Borgia, S.; Carrier, F.M.; Cavayas, Y.A.; Chagnon, N.; Cheng, M.P.; Conly, J.; et al. Remdesivir for the treatment of patients in hospital with COVID-19 in Canada: A randomized controlled trial. Can. Med. Assoc. J. 2022, 194, E242–E251. [Google Scholar] [CrossRef]
- Ghosn, L.; Chaimani, A.; Evrenoglou, T.; Davidson, M.; Graña, C.; Schmucker, C.; Bollig, C.; Henschke, N.; Sguassero, Y.; Nejstgaard, C.H.; et al. Interleukin-6 blocking agents for treating COVID-19: A living systematic review. Cochrane Database Syst. Rev. 2021, 2021, CD013881. [Google Scholar] [CrossRef]
- Kramer, A.; Prinz, C.; Fichtner, F.; Fischer, A.-L.; Thieme, V.; Grundeis, F.; Spagl, M.; Seeber, C.; Piechotta, V.; Metzendorf, M.-I.; et al. Janus kinase inhibitors for the treatment of COVID-19. Cochrane Database Syst. Rev. 2022, 6, CD015209. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).