Current Surgical Treatment for Neurogenic Lower Urinary Tract Dysfunction in Patients with Chronic Spinal Cord Injury
Abstract
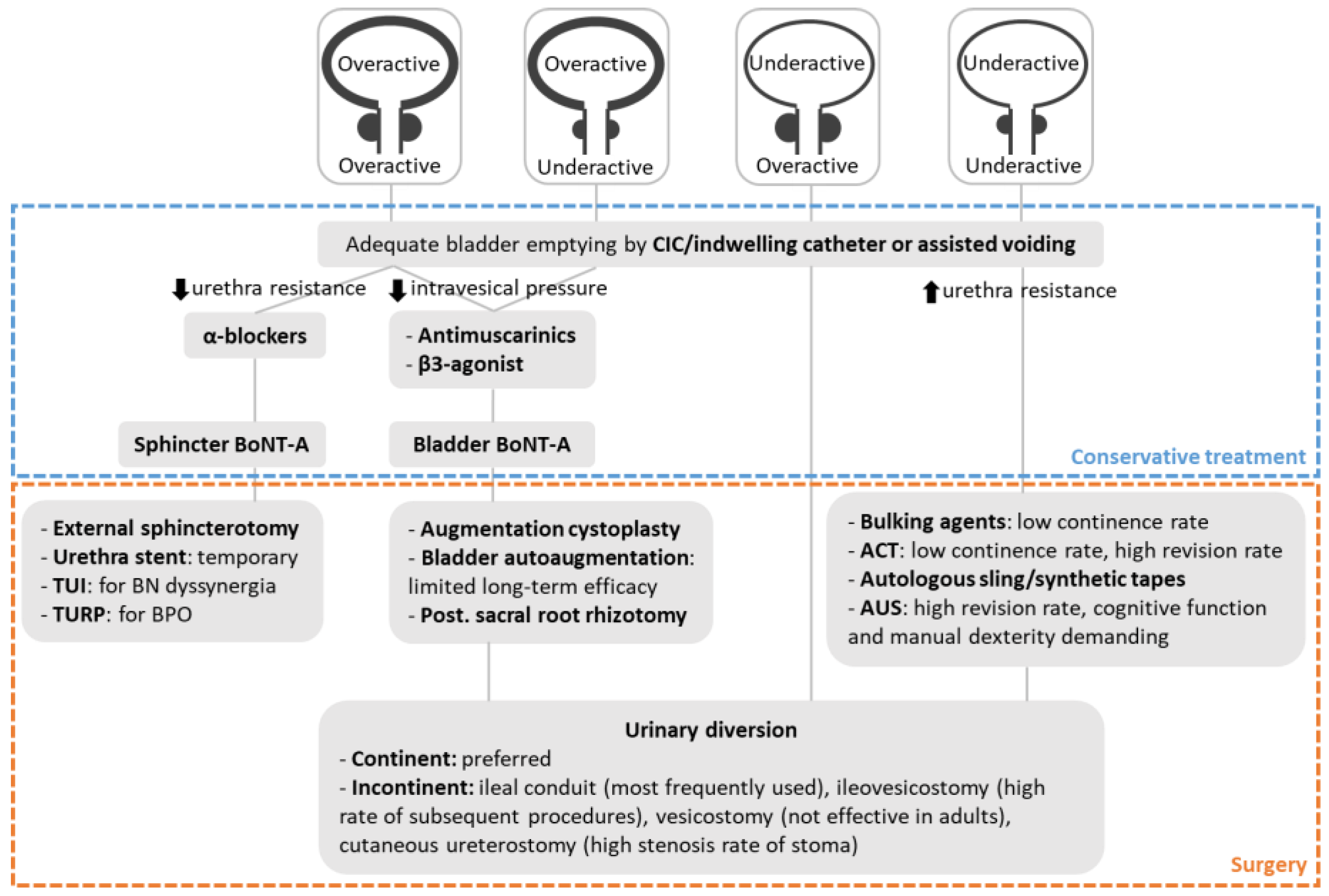
:1. Introduction
2. Reducing Intravesical Pressures
2.1. Augmentation Cystoplasty
2.2. Bladder Auto-Augmentation
2.3. Sacral Anterior Root Stimulation, Posterior Sacral Root Rhizotomy (SARS)
2.4. Sacral Neuromodulation
3. Reducing Urethra Resistance
3.1. External Sphincterotomy
3.2. Urethral Stent
3.3. Transurethral Incision of Bladder Neck
3.4. Transurethral Resection of Prostate
4. Increasing Urethra Resistance
4.1. Artificial Urinary Sphincter
4.2. Sling Surgery
4.2.1. Autologous Sling
4.2.2. Synthetic Tapes
4.3. Adjustable Continence Therapy
4.4. Bulking Agents
5. Urinary Diversion
5.1. Continent Diversion
5.1.1. Tunneled Channel
5.1.2. Nipple Valve
5.2. Incontinent Diversion
5.2.1. Ileal Conduit
5.2.2. Ileovesicostomy
5.2.3. Vesicostomy
5.2.4. Cutaneous Ureterostomy
6. Discussion
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- van den Berg, M.E.; Castellote, J.M.; Mahillo-Fernandez, I.; de Pedro-Cuesta, J. Incidence of spinal cord injury worldwide: A systematic review. Neuroepidemiology 2010, 34, 184–192, discussion 192. [Google Scholar] [CrossRef] [PubMed]
- Lai, E.C.; Kao Yang, Y.H.; Kuo, H.C. Complication rate of neurogenic lower urinary tract dysfunction after spinal cord injury in Taiwan. Int. Urol. Nephrol. 2014, 46, 1063–1071. [Google Scholar] [CrossRef] [PubMed]
- Manack, A.; Motsko, S.P.; Haag-Molkenteller, C.; Dmochowski, R.R.; Goehring, E.L., Jr.; Nguyen-Khoa, B.A.; Jones, J.K. Epidemiology and healthcare utilization of neurogenic bladder patients in a US claims database. Neurourol. Urodyn. 2011, 30, 395–401. [Google Scholar] [CrossRef]
- Kaplan, S.A.; Chancellor, M.B.; Blaivas, J.G. Bladder and sphincter behavior in patients with spinal cord lesions. J. Urol. 1991, 146, 113–117. [Google Scholar] [CrossRef]
- Panicker, J.N.; Fowler, C.J.; Kessler, T.M. Lower urinary tract dysfunction in the neurological patient: Clinical assessment and management. Lancet Neurol. 2015, 14, 720–732. [Google Scholar] [CrossRef]
- Weld, K.J.; Dmochowski, R.R. Association of level of injury and bladder behavior in patients with post-traumatic spinal cord injury. Urology 2000, 55, 490–494. [Google Scholar] [CrossRef] [PubMed]
- Wada, N.; Karnup, S.; Kadekawa, K.; Shimizu, N.; Kwon, J.; Shimizu, T.; Gotoh, D.; Kakizaki, H.; de Groat, W.C.; Yoshimura, N. Current Knowledge and Novel Frontiers in Lower Urinary Tract Dysfunction after Spinal Cord Injury: Basic Research Perspectives. Urol. Sci. 2022, 33, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Ku, J.H. The management of neurogenic bladder and quality of life in spinal cord injury. BJU Int. 2006, 98, 739–745. [Google Scholar] [CrossRef]
- Chen, S.-F.; Jiang, Y.-H.; Jhang, J.-F.; Lee, C.-L.; Kuo, H.-C. Bladder management and urological complications in patients with chronic spinal cord injuries in Taiwan. Tzu Chi Med. J. 2014, 26, 25–28. [Google Scholar] [CrossRef]
- Gor, R.A.; Elliott, S.P. Surgical Management of Neurogenic Lower Urinary Tract Dysfunction. Urol. Clin. N. Am. 2017, 44, 475–490. [Google Scholar] [CrossRef]
- Biers, S.M.; Venn, S.N.; Greenwell, T.J. The past, present and future of augmentation cystoplasty. BJU Int. 2012, 109, 1280–1293. [Google Scholar] [CrossRef] [PubMed]
- Mundy, A.R.; Stephenson, T.P. “Clam” ileocystoplasty for the treatment of refractory urge incontinence. Br. J. Urol. 1985, 57, 641–646. [Google Scholar] [CrossRef]
- Gurung, P.M.; Attar, K.H.; Abdul-Rahman, A.; Morris, T.; Hamid, R.; Shah, P.J. Long-term outcomes of augmentation ileocystoplasty in patients with spinal cord injury: A minimum of 10 years of follow-up. BJU Int. 2012, 109, 1236–1242. [Google Scholar] [CrossRef] [PubMed]
- Kilic, N.; Celayir, S.; Elicevik, M.; Sarimurat, N.; Soylet, Y.; Buyukunal, C.; Danismend, N. Bladder augmentation: Urodynamic findings and clinical outcome in different augmentation techniques. Eur. J. Pediatr. Surg. 1999, 9, 29–32. [Google Scholar] [CrossRef] [PubMed]
- Chiba, H.; Kitta, T.; Higuchi, M.; Kusakabe, N.; Kon, M.; Nakamura, M.; Shinohara, N. Ureteral reimplantation during augmentation cystoplasty is not needed for vesicoureteral reflux in patients with neurogenic bladder: A long-term retrospective study. BMC Urol. 2022, 22, 48. [Google Scholar] [CrossRef] [PubMed]
- Hoen, L.; Ecclestone, H.; Blok, B.F.M.; Karsenty, G.; Phe, V.; Bossier, R.; Groen, J.; Castro-Diaz, D.; Padilla Fernandez, B.; Del Popolo, G.; et al. Long-term effectiveness and complication rates of bladder augmentation in patients with neurogenic bladder dysfunction: A systematic review. Neurourol. Urodyn. 2017, 36, 1685–1702. [Google Scholar] [CrossRef]
- Zhang, H.C.; Yang, J.; Ye, X.; Hu, H.F. Augmentation enterocystoplasty without reimplantation for patients with neurogenic bladder and vesicoureteral reflux. Kaohsiung J. Med. Sci. 2016, 32, 323–326. [Google Scholar] [CrossRef]
- Husmann, D.A.; Cain, M.P. Fecal and urinary continence after ileal cecal cystoplasty for the neurogenic bladder. J. Urol. 2001, 165, 922–925. [Google Scholar] [CrossRef]
- Quek, M.L.; Ginsberg, D.A. Long-term urodynamics followup of bladder augmentation for neurogenic bladder. J. Urol. 2003, 169, 195–198. [Google Scholar] [CrossRef]
- Shekarriz, B.; Upadhyay, J.; Demirbilek, S.; Barthold, J.S.; Gonzalez, R. Surgical complications of bladder augmentation: Comparison between various enterocystoplasties in 133 patients. Urology 2000, 55, 123–128. [Google Scholar] [CrossRef]
- Veenboer, P.W.; Nadorp, S.; de Jong, T.P.; Dik, P.; van Asbeck, F.W.; Bosch, J.L.; de Kort, L.M. Enterocystoplasty vs detrusorectomy: Outcome in the adult with spina bifida. J. Urol. 2013, 189, 1066–1070. [Google Scholar] [CrossRef]
- Mast, P.; Hoebeke, P.; Wyndaele, J.J.; Oosterlinck, W.; Everaert, K. Experience with augmentation cystoplasty. A review. Paraplegia 1995, 33, 560–564. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.L.; Kuo, H.C. Long-term outcomes of augmentation enterocystoplasty with an ileal segment in patients with spinal cord injury. J. Med. Assoc. 2009, 108, 475–480. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.Y.; Kuo, H.C. A real-world experience with augmentation enterocystoplasty-High patient satisfaction with high complication rates. Neurourol. Urodyn. 2018, 37, 744–750. [Google Scholar] [CrossRef]
- Chartier-Kastler, E.J.; Mongiat-Artus, P.; Bitker, M.O.; Chancellor, M.B.; Richard, F.; Denys, P. Long-term results of augmentation cystoplasty in spinal cord injury patients. Spinal Cord 2000, 38, 490–494. [Google Scholar] [CrossRef]
- Herschorn, S.; Hewitt, R.J. Patient perspective of long-term outcome of augmentation cystoplasty for neurogenic bladder. Urology 1998, 52, 672–678. [Google Scholar] [CrossRef]
- Luangkhot, R.; Peng, B.C.; Blaivas, J.G. Ileocecocystoplasty for the management of refractory neurogenic bladder: Surgical technique and urodynamic findings. J. Urol. 1991, 146, 1340–1344. [Google Scholar] [CrossRef]
- Lapides, J.; Diokno, A.C.; Silber, S.J.; Lowe, B.S. Clean, intermittent self-catheterization in the treatment of urinary tract disease. J. Urol. 1972, 107, 458–461. [Google Scholar] [CrossRef]
- De la Torre, G.G.; Martin, A.; Cervantes, E.; Guil, R.; Mestre, J.M. Attention lapses in children with spina bifida and hydrocephalus and children with attention-deficit/hyperactivity disorder. J. Clin. Exp. Neuropsychol. 2017, 39, 563–573. [Google Scholar] [CrossRef] [PubMed]
- Welk, B.; Herschorn, S.; Law, C.; Nam, R. Population based assessment of enterocystoplasty complications in adults. J. Urol. 2012, 188, 464–469. [Google Scholar] [CrossRef]
- Blaivas, J.G.; Weiss, J.P.; Desai, P.; Flisser, A.J.; Stember, D.S.; Stahl, P.J. Long-term followup of augmentation enterocystoplasty and continent diversion in patients with benign disease. J. Urol. 2005, 173, 1631–1634. [Google Scholar] [CrossRef] [PubMed]
- DeFoor, W.; Minevich, E.; Reddy, P.; Sekhon, D.; Polsky, E.; Wacksman, J.; Sheldon, C. Bladder calculi after augmentation cystoplasty: Risk factors and prevention strategies. J. Urol. 2004, 172, 1964–1966. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, T.T.; Granberg, C.F.; Fox, J.A.; Husmann, D.A. Augmentation cystoplasty and risk of neoplasia: Fact, fiction and controversy. J. Urol. 2010, 184, 2492–2496. [Google Scholar] [CrossRef] [PubMed]
- Somani, B.K.; Kumar, V.; Wong, S.; Pickard, R.; Ramsay, C.; Nabi, G.; Grant, A.; N’Dow, J.; Group, A.R. Bowel dysfunction after transposition of intestinal segments into the urinary tract: 8-year prospective cohort study. J. Urol. 2007, 177, 1793–1798. [Google Scholar] [CrossRef]
- Schlomer, B.J.; Copp, H.L. Cumulative incidence of outcomes and urologic procedures after augmentation cystoplasty. J.Pediatr. Urol. 2014, 10, 1043–1050. [Google Scholar] [CrossRef]
- Scott, F.B.; Bradley, W.E.; Timm, G.W. Treatment of urinary incontinence by an implantable prosthetic urinary sphincter. J. Urol. 1974, 112, 75–80. [Google Scholar] [CrossRef]
- Biardeau, X.; Rizk, J.; Marcelli, F.; Flamand, V. Robot-assisted laparoscopic approach for artificial urinary sphincter implantation in 11 women with urinary stress incontinence: Surgical technique and initial experience. Eur. Urol. 2015, 67, 937–942. [Google Scholar] [CrossRef]
- Duel, B.P.; Gonzalez, R.; Barthold, J.S. Alternative techniques for augmentation cystoplasty. J. Urol. 1998, 159, 998–1005. [Google Scholar] [CrossRef]
- Snow, B.W.; Cartwright, P.C. Bladder autoaugmentation. Urol. Clin. N. Am. 1996, 23, 323–331. [Google Scholar] [CrossRef]
- Stohrer, M.; Kramer, A.; Goepel, M.; Lochner-Ernst, D.; Kruse, D.; Rubben, H. Bladder auto-augmentation—An alternative for enterocystoplasty: Preliminary results. Neurourol. Urodyn. 1995, 14, 11–23. [Google Scholar] [CrossRef]
- Stohrer, M.; Kramer, G.; Goepel, M.; Lochner-Ernst, D.; Kruse, D.; Rubben, H. Bladder autoaugmentation in adult patients with neurogenic voiding dysfunction. Spinal Cord 1997, 35, 456–462. [Google Scholar] [CrossRef]
- Sun, X.G.; Wang, R.Y.; Xu, J.L.; Li, D.G.; Chen, W.X.; Li, J.L.; Wang, J.; Li, A.W. Surgical outcomes of bladder augmentation: A comparison of three different augmentation procedures. World J. Clin. Cases 2020, 8, 3240–3248. [Google Scholar] [CrossRef] [PubMed]
- Hansen, E.L.; Hvistendahl, G.M.; Rawashdeh, Y.F.; Olsen, L.H. Promising long-term outcome of bladder autoaugmentation in children with neurogenic bladder dysfunction. J. Urol. 2013, 190, 1869–1875. [Google Scholar] [CrossRef] [PubMed]
- MacNeily, A.E.; Afshar, K.; Coleman, G.U.; Johnson, H.W. Autoaugmentation by detrusor myotomy: Its lack of effectiveness in the management of congenital neuropathic bladder. J. Urol. 2003, 170, 1643–1646, discussion 1646. [Google Scholar] [CrossRef] [PubMed]
- Marte, A.; Di Meglio, D.; Cotrufo, A.M.; Di Iorio, G.; De Pasquale, M.; Vessella, A. A long-term follow-up of autoaugmentation in myelodysplastic children. BJU Int. 2002, 89, 928–931. [Google Scholar] [CrossRef]
- Brindley, G.S.; Polkey, C.E.; Rushton, D.N.; Cardozo, L. Sacral anterior root stimulators for bladder control in paraplegia: The first 50 cases. J. Neurol. Neurosurg. Psychiatry 1986, 49, 1104–1114. [Google Scholar] [CrossRef]
- Sauerwein, D. Surgical treatment of spastic bladder paralysis in paraplegic patients. Sacral deafferentation with implantation of a sacral anterior root stimulator. Urol. A 1990, 29, 196–203. [Google Scholar]
- Martens, F.M.; Heesakkers, J.P. Clinical results of a brindley procedure: Sacral anterior root stimulation in combination with a rhizotomy of the dorsal roots. Adv. Urol. 2011, 2011, 709708. [Google Scholar] [CrossRef]
- Krasmik, D.; Krebs, J.; van Ophoven, A.; Pannek, J. Urodynamic results, clinical efficacy, and complication rates of sacral intradural deafferentation and sacral anterior root stimulation in patients with neurogenic lower urinary tract dysfunction resulting from complete spinal cord injury. Neurourol. Urodyn. 2014, 33, 1202–1206. [Google Scholar] [CrossRef]
- Van Kerrebroeck, P.E.; Koldewijn, E.L.; Debruyne, F.M. Worldwide experience with the Finetech-Brindley sacral anterior root stimulator. Neurourol. Urodyn. 1993, 12, 497–503. [Google Scholar] [CrossRef]
- Kutzenberger, J. Surgical therapy of neurogenic detrusor overactivity (hyperreflexia) in paraplegic patients by sacral deafferentation and implant driven micturition by sacral anterior root stimulation: Methods, indications, results, complications, and future prospects. Acta Neurochir. Suppl. 2007, 97, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Egon, G.; Barat, M.; Colombel, P.; Visentin, C.; Isambert, J.L.; Guerin, J. Implantation of anterior sacral root stimulators combined with posterior sacral rhizotomy in spinal injury patients. World J. Urol. 1998, 16, 342–349. [Google Scholar] [CrossRef]
- Ginsberg, D.A.; Boone, T.B.; Cameron, A.P.; Gousse, A.; Kaufman, M.R.; Keays, E.; Kennelly, M.J.; Lemack, G.E.; Rovner, E.S.; Souter, L.H.; et al. The AUA/SUFU Guideline on Adult Neurogenic Lower Urinary Tract Dysfunction: Treatment and Follow-up. J. Urol. 2021, 206, 1106–1113. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, G.; Musco, S.; Celso, M.; Del Corso, F.; Del Popolo, G. Sacral neuromodulation for neurogenic non-obstructive urinary retention in incomplete spinal cord patients: A ten-year follow-up single-centre experience. Spinal Cord 2014, 52, 241–245. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, G.; Nelli, F.; Mencarini, M.; Del Popolo, G. Clinical concomitant benefits on pelvic floor dysfunctions after sacral neuromodulation in patients with incomplete spinal cord injury. Spinal Cord 2011, 49, 629–636. [Google Scholar] [CrossRef] [PubMed]
- Sievert, K.D.; Amend, B.; Gakis, G.; Toomey, P.; Badke, A.; Kaps, H.P.; Stenzl, A. Early sacral neuromodulation prevents urinary incontinence after complete spinal cord injury. Ann. Neurol. 2010, 67, 74–84. [Google Scholar] [CrossRef]
- Perkash, I. Transurethral sphincterotomy provides significant relief in autonomic dysreflexia in spinal cord injured male patients: Long-term followup results. J. Urol. 2007, 177, 1026–1029. [Google Scholar] [CrossRef]
- Vainrib, M.; Reyblat, P.; Ginsberg, D.A. Long-term efficacy of repeat incisions of bladder neck/external sphincter in patients with spinal cord injury. Urology 2014, 84, 940–945. [Google Scholar] [CrossRef]
- Madersbacher. Urinary flow and flow pattern in paralegics. Paraplegia 1975, 13, 95–100. [Google Scholar] [CrossRef]
- Perkash, I. Laser sphincterotomy and ablation of the prostate using a sapphire chisel contact tip firing neodymium:YAG laser. J. Urol. 1994, 152, 2020–2024. [Google Scholar] [CrossRef]
- Noll, F.; Sauerwein, D.; Stohrer, M. Transurethral sphincterotomy in quadriplegic patients: Long-term-follow-up. Neurourol. Urodyn. 1995, 14, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Chancellor, M.B.; Bennett, C.; Simoneau, A.R.; Finocchiaro, M.V.; Kline, C.; Bennett, J.K.; Foote, J.E.; Green, B.G.; Martin, S.H.; Killoran, R.W.; et al. Sphincteric stent versus external sphincterotomy in spinal cord injured men: Prospective randomized multicenter trial. J. Urol. 1999, 161, 1893–1898. [Google Scholar] [CrossRef] [PubMed]
- Seoane-Rodriguez, S.; Sanchez, R.L.J.; Montoto-Marques, A.; Salvador-de la Barrera, S.; Ferreiro-Velasco, M.E.; Alvarez-Castelo, L.; Balsa-Mosquera, B.; Rodriguez-Sotillo, A. Long-term follow-up study of intraurethral stents in spinal cord injured patients with detrusor-sphincter dyssynergia. Spinal Cord 2007, 45, 621–626. [Google Scholar] [CrossRef]
- Peyton, C.C.; Badlani, G.H. The management of prostatic obstruction with urethral stents. Can. J. Urol. 2015, 22 (Suppl. 1), 75–81. [Google Scholar] [PubMed]
- Stöhrer, M.; Kramer, G.; Löchner-Ernst, D.; Goepel, M.; Noll, F.; Rübben, H. Diagnosis and treatment of bladder dysfunction in spinal cord injury patients. Eur. Urol. Update Ser. 1994, 3, 170–175. [Google Scholar]
- Ke, Q.S.; Kuo, H.C. Transurethral incision of the bladder neck to treat bladder neck dysfunction and voiding dysfunction in patients with high-level spinal cord injuries. Neurourol. Urodyn. 2010, 29, 748–752. [Google Scholar] [CrossRef]
- Elsaesser, E.; Stoephasius, E. Urological operations for improvement of bladder voiding in paraplegic patients. Paraplegia 1972, 10, 68–77. [Google Scholar] [CrossRef]
- Lai, H.H.; Hsu, E.I.; Teh, B.S.; Butler, E.B.; Boone, T.B. 13 years of experience with artificial urinary sphincter implantation at Baylor College of Medicine. J. Urol. 2007, 177, 1021–1025. [Google Scholar] [CrossRef]
- Phe, V.; Leon, P.; Granger, B.; Denys, P.; Bitker, M.O.; Mozer, P.; Chartier-Kastler, E. Stress urinary incontinence in female neurological patients: Long-term functional outcomes after artificial urinary sphincter (AMS 800(TM) ) implantation. Neurourol. Urodyn. 2017, 36, 764–769. [Google Scholar] [CrossRef]
- Mor, Y.; Leibovitch, I.; Golomb, J.; Ben-Chaim, J.; Nadu, A.; Pinthus, J.H.; Ramon, J. Lower urinary tract reconstruction by augmentation cystoplasty and insertion of artificial urinary sphincter cuff only: Long term follow-up. Prog. Urol. 2004, 14, 310–314. [Google Scholar]
- Fulford, S.C.; Sutton, C.; Bales, G.; Hickling, M.; Stephenson, T.P. The fate of the ‘modern’ artificial urinary sphincter with a follow-up of more than 10 years. Br. J. Urol. 1997, 79, 713–716. [Google Scholar] [CrossRef] [PubMed]
- Light, J.K.; Scott, F.B. Use of the artificial urinary sphincter in spinal cord injury patients. J. Urol. 1983, 130, 1127–1129. [Google Scholar] [CrossRef] [PubMed]
- Ramsay, A.K.; Granitsiotis, P.; Conn, I.G. The use of the artificial urinary sphincter in the West of Scotland: A single centre 10-year experience. Scott. Med. J. 2007, 52, 14–17. [Google Scholar] [CrossRef] [PubMed]
- Bersch, U.; Gocking, K.; Pannek, J. The artificial urinary sphincter in patients with spinal cord lesion: Description of a modified technique and clinical results. Eur. Urol. 2009, 55, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Castro Diaz, D.; Ravina Pisaca, M.; Diaz Garcia, J.L.; Concepcion Masip, T.; Rodriguez Hernandez, P.; Banares Baudet, F. Artificial urinary sphincter in the treatment of urinary incontinence caused by sphincter insufficiency. Arch. Esp. Urol. 1997, 50, 595–601. [Google Scholar]
- Costa, P.; Poinas, G.; Ben Naoum, K.; Bouzoubaa, K.; Wagner, L.; Soustelle, L.; Boukaram, M.; Droupy, S. Long-term results of artificial urinary sphincter for women with type III stress urinary incontinence. Eur. Urol. 2013, 63, 753–758. [Google Scholar] [CrossRef]
- Guillot-Tantay, C.; Chartier-Kastler, E.; Mozer, P.; Bitker, M.O.; Richard, F.; Ambrogi, V.; Denys, P.; Leon, P.; Phe, V. Male neurogenic stress urinary incontinence treated by artificial urinary sphincter AMS 800 (Boston Scientific, Boston, USA): Very long-term results (>25 years). Prog. Urol. 2018, 28, 39–47. [Google Scholar] [CrossRef]
- Chartier Kastler, E.; Genevois, S.; Game, X.; Denys, P.; Richard, F.; Leriche, A.; Saramon, J.P.; Ruffion, A. Treatment of neurogenic male urinary incontinence related to intrinsic sphincter insufficiency with an artificial urinary sphincter: A French retrospective multicentre study. BJU Int. 2011, 107, 426–432. [Google Scholar] [CrossRef]
- Khene, Z.E.; Paret, F.; Perrouin-Verbe, M.A.; Prudhomme, T.; Hascoet, J.; Nedelec, M.; Kerdraon, J.; Menard, H.; Jezequel, M.; Le Normand, L.; et al. Artificial Urinary Sphincter in Male Patients with Spina Bifida: Comparison of Perioperative and Functional Outcomes between Bulbar Urethra and Bladder Neck Cuff Placement. J. Urol. 2018, 199, 791–797. [Google Scholar] [CrossRef] [PubMed]
- Tricard, T.; Schirmann, A.; Munier, P.; Schroeder, A.; Saussine, C. Outcomes of artificial urinary sphincter in female with neurological stress urinary incontinence: A long-term follow-up. World J. Urol. 2021, 39, 157–162. [Google Scholar] [CrossRef]
- Chung, E.; Liao, L.; Kim, J.H.; Wang, Z.; Kitta, T.; Lin, A.T.; Lee, K.S.; Ye, L.; Chu, P.; Kaiho, Y.; et al. The Asia-Pacific AMS800 artificial urinary sphincter consensus statement. Int. J. Urol. 2022. [Google Scholar] [CrossRef] [PubMed]
- El-Azab, A.S.; El-Nashar, S.A. Midurethral slings versus the standard pubovaginal slings for women with neurogenic stress urinary incontinence. Int. Urogynecol. J. 2015, 26, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Fontaine, E.; Bendaya, S.; Desert, J.F.; Fakacs, C.; Le Mouel, M.A.; Beurton, D. Combined modified rectus fascial sling and augmentation ileocystoplasty for neurogenic incontinence in women. J. Urol. 1997, 157, 109–112. [Google Scholar] [CrossRef] [PubMed]
- Athanasopoulos, A.; Gyftopoulos, K.; McGuire, E.J. Treating stress urinary incontinence in female patients with neuropathic bladder: The value of the autologous fascia rectus sling. Int. Urol. Nephrol. 2012, 44, 1363–1367. [Google Scholar] [CrossRef]
- Chancellor, M.B.; Erhard, M.J.; Kiilholma, P.J.; Karasick, S.; Rivas, D.A. Functional urethral closure with pubovaginal sling for destroyed female urethra after long-term urethral catheterization. Urology 1994, 43, 499–505. [Google Scholar] [CrossRef]
- Schmidbauer, C.P.; Chiang, H.; Raz, S. Surgical treatment possibilities of severe urinary incontinence in women. Urol. A 1988, 27, 291–296. [Google Scholar]
- Daneshmand, S.; Ginsberg, D.A.; Bennet, J.K.; Foote, J.; Killorin, W.; Rozas, K.P.; Green, B.G. Puboprostatic sling repair for treatment of urethral incompetence in adult neurogenic incontinence. J. Urol. 2003, 169, 199–202. [Google Scholar] [CrossRef]
- Herschorn, S.; Radomski, S.B. Fascial slings and bladder neck tapering in the treatment of male neurogenic incontinence. J. Urol. 1992, 147, 1073–1075. [Google Scholar] [CrossRef]
- Groen, L.A.; Spinoit, A.F.; Hoebeke, P.; Van Laecke, E.; De Troyer, B.; Everaert, K. The AdVance male sling as a minimally invasive treatment for intrinsic sphincter deficiency in patients with neurogenic bladder sphincter dysfunction: A pilot study. Neurourol. Urodyn. 2012, 31, 1284–1287. [Google Scholar] [CrossRef]
- Pannek, J.; Wollner, J. Treatment of stress urinary incontinence in men with spinal cord injury: Minimally invasive=minimally effective? Spinal Cord 2017, 55, 739–742. [Google Scholar] [CrossRef]
- Vainrib, M.; Reyblat, P.; Ginsberg, D. Outcomes of Male Sling Mesh Kit Placement in Patients with Neuropathic Stress Urinary Incontinence: A Single Institution Experience. Urol. Int. 2015, 95, 406–410. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.Y.; Jiang, Y.H.; Kuo, H.C. Therapeutic Efficacy of a New Procedure for Male Urinary Incontinence Combining a Suburethral Polypropylene Mesh and Cardiovascular Patch. Int. Neurourol. J. 2017, 21, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Wyndaele, J.J.; Birch, B.; Borau, A.; Burks, F.; Castro-Diaz, D.; Chartier-Kastler, E.; Drake, M.; Ishizuka, O.; Minigawa, T.; Opisso, E.; et al. Surgical management of the neurogenic bladder after spinal cord injury. World J. Urol. 2018, 36, 1569–1576. [Google Scholar] [CrossRef] [PubMed]
- Losco, G.S.; Burki, J.R.; Omar, Y.A.; Shah, P.J.; Hamid, R. Long-term outcome of transobturator tape (TOT) for treatment of stress urinary incontinence in females with neuropathic bladders. Spinal Cord 2015, 53, 544–546. [Google Scholar] [CrossRef]
- Abdul-Rahman, A.; Attar, K.H.; Hamid, R.; Shah, P.J. Long-term outcome of tension-free vaginal tape for treating stress incontinence in women with neuropathic bladders. BJU Int. 2010, 106, 827–830. [Google Scholar] [CrossRef]
- Sakalis, V.I.; Floyd, M.S., Jr.; Caygill, P.; Price, C.; Hartwell, B.; Guy, P.J.; Davies, M.C. Management of stress urinary incontinence in spinal cord injured female patients with a mid-urethral tape—A single center experience. J. Spinal Cord Med. 2018, 41, 703–709. [Google Scholar] [CrossRef]
- Kocjancic, E.; Crivellaro, S.; Smith, J.J., 3rd; Ranzoni, S.; Bonvini, D.; Frea, B. Adjustable continence therapy for treatment of recurrent female urinary incontinence. J. Endourol. 2008, 22, 1403–1407. [Google Scholar] [CrossRef]
- Ronzi, Y.; Le Normand, L.; Chartier-Kastler, E.; Game, X.; Grise, P.; Denys, P.; Perrouin-Verbe, B. Neurogenic stress urinary incontinence: Is there a place for Adjustable Continence Therapy (ACT and ProACT, Uromedica, Plymouth, MN, USA)? A retrospective multicenter study. Spinal Cord 2019, 57, 388–395. [Google Scholar] [CrossRef]
- Kabore, F.A.; Gaillet, S.; Blanc, J.; Boissier, R.; Aurier, K.L.; Delaporte, V.; Coulange, C.; Lechevallier, E.; Karsenty, G. Initial results of adjustable periurethral balloons (ACT and pro ACT) in the treatment of adult stress urinary incontinence with intrinsic sphincter deficiency. Prog. Urol. 2014, 24, 1132–1138. [Google Scholar] [CrossRef]
- Ammirati, E.; Manassero, A.; Giammo, A.; Carone, R. Management of male and female neurogenic stress urinary incontinence in spinal cord injured (SCI) patients using adjustable continence therapy. Urologia 2017, 84, 165–168. [Google Scholar] [CrossRef]
- Mehnert, U.; Bastien, L.; Denys, P.; Cardot, V.; Even-Schneider, A.; Kocer, S.; Chartier-Kastler, E. Treatment of neurogenic stress urinary incontinence using an adjustable continence device: 4-year followup. J. Urol. 2012, 188, 2274–2280. [Google Scholar] [CrossRef]
- Bennett, J.K.; Green, B.G.; Foote, J.E.; Gray, M. Collagen injections for intrinsic sphincter deficiency in the neuropathic urethra. Paraplegia 1995, 33, 697–700. [Google Scholar] [CrossRef] [PubMed]
- Hamid, R.; Arya, M.; Khastgir, J.; Patel, H.R.; Shah, P.J. The treatment of male stress urinary incontinence with polydimethylsiloxane in compliant bladders following spinal cord injury. Spinal Cord 2003, 41, 286–289. [Google Scholar] [CrossRef] [PubMed]
- Peterson, A.C.; Curtis, L.H.; Shea, A.M.; Borawski, K.M.; Schulman, K.A.; Scales, C.D., Jr. Urinary diversion in patients with spinal cord injury in the United States. Urology 2012, 80, 1247–1251. [Google Scholar] [CrossRef] [PubMed]
- Mitrofanoff, P. Trans-appendicular continent cystostomy in the management of the neurogenic bladder. Chir. Pediatr. 1980, 21, 297–305. [Google Scholar] [PubMed]
- Monti, P.R.; Lara, R.C.; Dutra, M.A.; de Carvalho, J.R. New techniques for construction of efferent conduits based on the Mitrofanoff principle. Urology 1997, 49, 112–115. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.H. Yang needle tunneling technique in creating antireflux and continent mechanisms. J. Urol. 1993, 150, 830–834. [Google Scholar] [CrossRef] [PubMed]
- Dodat, H.; Denis, E.; Pelizzo, G.; Dubois, R.; Carlioz, P.; Chavrier, Y. Continent urinary diversion using a tubulized sigmoid segment. An alternative to trans-appendicular diversion. Prog. Urol. 1998, 8, 58–61. [Google Scholar] [PubMed]
- Cain, M.P.; Rink, R.C.; Yerkes, E.B.; Kaefer, M.; Casale, A.J. Long-term followup and outcome of continent catheterizable vesicocstomy using the Rink modification. J. Urol. 2002, 168, 2583–2585. [Google Scholar] [CrossRef]
- Close, C.E.; Mitchell, M.E. Continent gastric tube: New techniques and long-term followup. J. Urol. 1997, 157, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Mor, Y.; Kajbafzadeh, A.M.; German, K.; Mouriquand, P.D.; Duffy, P.G.; Ransley, P.G. The role of ureter in the creation of Mitrofanoff channels in children. J. Urol. 1997, 157, 635–637. [Google Scholar] [CrossRef]
- Castellan, M.A.; Gosalbez, R.; Labbie, A.; Ibrahim, E.; Disandro, M. Outcomes of continent catheterizable stomas for urinary and fecal incontinence: Comparison among different tissue options. BJU Int. 2005, 95, 1053–1057. [Google Scholar] [CrossRef] [PubMed]
- Perovic, S. Continent urinary diversion using preputial penile or clitoral skin flap. J. Urol. 1996, 155, 1402–1406. [Google Scholar] [CrossRef]
- Mor, Y.; Quinn, F.M.; Carr, B.; Mouriquand, P.D.; Duffy, P.G.; Ransley, P.G. Combined Mitrofanoff and antegrade continence enema procedures for urinary and fecal incontinence. J. Urol. 1997, 158, 192–195. [Google Scholar] [CrossRef]
- Franc-Guimond, J.; Gonzalez, R. Simplified technique to create a concealed catheterizable stoma: The VR flap. J. Urol. 2006, 175, 1088–1091. [Google Scholar] [CrossRef] [PubMed]
- Liard, A.; Seguier-Lipszyc, E.; Mathiot, A.; Mitrofanoff, P. The Mitrofanoff procedure: 20 years later. J. Urol. 2001, 165, 2394–2398. [Google Scholar] [CrossRef]
- Thomas, J.C.; Dietrich, M.S.; Trusler, L.; DeMarco, R.T.; Pope, J.C.t.; Brock, J.W., 3rd; Adams, M.C. Continent catheterizable channels and the timing of their complications. J. Urol. 2006, 176, 1816–1820, discussion 1820. [Google Scholar] [CrossRef] [PubMed]
- Narayanaswamy, B.; Wilcox, D.T.; Cuckow, P.M.; Duffy, P.G.; Ransley, P.G. The Yang-Monti ileovesicostomy: A problematic channel? BJU Int. 2001, 87, 861–865. [Google Scholar] [CrossRef]
- deKernion, J.B.; DenBesten, L.; Kaufman, J.J.; Ehrlich, R. The Kock pouch as a urinary reservoir. Pitfalls and perspectives. Am. J. Surg. 1985, 150, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Kock, N.G.; Nilson, A.E.; Nilsson, L.O.; Norlen, L.J.; Philipson, B.M. Urinary diversion via a continent ileal reservoir: Clinical results in 12 patients. J. Urol. 1982, 128, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Akakpo, W.; Chartier-Kastler, E.; Joussain, C.; Denys, P.; Lubetzki, C.; Phe, V. Outcomes of ileal conduit urinary diversion in patients with multiple sclerosis. Neurourol. Urodyn. 2020, 39, 771–777. [Google Scholar] [CrossRef] [PubMed]
- Davis, N.F.; Burke, J.P.; McDermott, T.; Flynn, R.; Manecksha, R.P.; Thornhill, J.A. Bricker versus Wallace anastomosis: A meta-analysis of ureteroenteric stricture rates after ileal conduit urinary diversion. Can. Urol. Assoc. J. 2015, 9, E284-290. [Google Scholar] [CrossRef] [PubMed]
- Guillotreau, J.; Game, X.; Castel-Lacanal, E.; Mallet, R.; De Boissezon, X.; Malavaud, B.; Marque, P.; Rischmann, P. Laparoscopic cystectomy and transileal ureterostomy for neurogenic vesicosphincteric disorders. Evaluation of morbidity. Prog. Urol. 2007, 17, 208–212. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.; Hosaka, K.; Kobayashi, S.; Igawa, Y.; Nishizawa, O. Fate of tetraplegic patients managed by ileal conduit for urinary control: Long-term follow-up. Int. J. Urol. 2002, 9, 253–256. [Google Scholar] [CrossRef] [PubMed]
- Chartier-Kastler, E.J.; Mozer, P.; Denys, P.; Bitker, M.O.; Haertig, A.; Richard, F. Neurogenic bladder management and cutaneous non-continent ileal conduit. Spinal Cord 2002, 40, 443–448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Legrand, G.; Roupret, M.; Comperat, E.; Even-Schneider, A.; Denys, P.; Chartier-Kastler, E. Functional outcomes after management of end-stage neurological bladder dysfunction with ileal conduit in a multiple sclerosis population: A monocentric experience. Urology 2011, 78, 937–941. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Wilkinson, J.M.; Thomas, D.G. Supravesical diversion for incontinence: A long-term follow-up. Br. J. Urol. 1997, 79, 348–353. [Google Scholar] [CrossRef]
- Zimmerman, W.B.; Santucci, R.A. Ileovesicostomy: Update. Arch. Esp. Urol. 2011, 64, 207–218. [Google Scholar]
- Zimmerman, W.B.; Santucci, R.A. Ileovesicostomy update: Changes for the 21st century. Adv. Urol. 2009, 2009, 801038. [Google Scholar] [CrossRef]
- Hellenthal, N.J.; Short, S.S.; O’Connor, R.C.; Eandi, J.A.; Yap, S.A.; Stone, A.R. Incontinent ileovesicostomy: Long-term outcomes and complications. Neurourol. Urodyn. 2009, 28, 483–486. [Google Scholar] [CrossRef]
- Morrisroe, S.N.; O’Connor, R.C.; Nanigian, D.K.; Kurzrock, E.A.; Stone, A.R. Vesicostomy revisited: The best treatment for the hostile bladder in myelodysplastic children? BJU Int. 2005, 96, 397–400. [Google Scholar] [CrossRef]
- Lusuardi, L.; Lodde, M.; Pycha, A. Cutaneous ureterostomy. BJU Int. 2005, 96, 1149–1159. [Google Scholar] [CrossRef] [PubMed]
- Drake, M.; Apostolidis, A.; Emmanuel, A.; Gajewski, J.; Harrison, S.; Heesakkers, J.; Lemack, G.; Madersbacher, H.; Panicker, J.; Radziszewski, P.; et al. Neurological Urinary and Faecal Incontinence. In International Consultation on Incontinence, 5th ed.; ICUD-EAU: Paris, France, 2012. [Google Scholar]
- Gomes, C.M.; Sammour, Z.M.; Bessa Junior, J.; Barbosa, E.R.; Lopes, R.I.; Sallem, F.S.; Trigo-Rocha, F.E.; Bruschini, H.; Nitti, V.W.; Srougi, M. Neurological status predicts response to alpha-blockers in men with voiding dysfunction and Parkinson’s disease. Clinics 2014, 69, 817–822. [Google Scholar] [CrossRef] [PubMed]
- Moon, K.H.; Park, C.H.; Jung, H.C.; Oh, T.H.; Kim, J.S.; Kim, D.Y. A 12-Week, Open Label, Multi-Center Study to Evaluate the Clinical Efficacy and Safety of Silodosin on Voiding Dysfunction in Patients with Neurogenic Bladder. Low Urin. Tract. Symptoms 2015, 7, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Schneider, M.P.; Tornic, J.; Sykora, R.; Abo Youssef, N.; Mordasini, L.; Krhut, J.; Chartier-Kastler, E.; Davies, M.; Gajewski, J.; Schurch, B.; et al. Alpha-blockers for treating neurogenic lower urinary tract dysfunction in patients with multiple sclerosis: A systematic review and meta-analysis. A report from the Neuro-Urology Promotion Committee of the International Continence Society (ICS). Neurourol. Urodyn. 2019, 38, 1482–1491. [Google Scholar] [CrossRef]
- Huang, M.; Chen, H.; Jiang, C.; Xie, K.; Tang, P.; Ou, R.; Zeng, J.; Liu, Q.; Li, Q.; Huang, J.; et al. Effects of botulinum toxin A injections in spinal cord injury patients with detrusor overactivity and detrusor sphincter dyssynergia. J. Rehabil. Med. 2016, 48, 683–687. [Google Scholar] [CrossRef] [Green Version]
- Madhuvrata, P.; Singh, M.; Hasafa, Z.; Abdel-Fattah, M. Anticholinergic drugs for adult neurogenic detrusor overactivity: A systematic review and meta-analysis. Eur. Urol. 2012, 62, 816–830. [Google Scholar] [CrossRef]
- El Helou, E.; Labaki, C.; Chebel, R.; El Helou, J.; Abi Tayeh, G.; Jalkh, G.; Nemr, E. The use of mirabegron in neurogenic bladder: A systematic review. World J. Urol. 2020, 38, 2435–2442. [Google Scholar] [CrossRef]
- Wu, S.J.; Xu, Y.Q.; Gao, Z.Y.; Wang, Z.P.; Zhao, F.; Liu, L.; Wang, S. Clinical outcomes of botulinum toxin A management for neurogenic detrusor overactivity: Meta-analysis. Ren. Fail. 2019, 41, 937–945. [Google Scholar] [CrossRef]
| Complication | Frequency (%) |
|---|---|
| Urinary stones | 5–52 |
| Perforation | <1–14 |
| Bowel dysfunction | <10–55 |
| Malignancy | <1.0–5.5 |
| Metabolic abnormalities | <5 |
| Symptomatic UTI | 4–43 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, Y.-H.; Shen, Y.-C.; Hsu, C.-C.; Chow, P.-M.; Chang, P.-C.; Lin, Y.-H.; Chang, S.-J.; Jiang, Y.-H.; Liao, C.-H.; Wang, C.-C.; et al. Current Surgical Treatment for Neurogenic Lower Urinary Tract Dysfunction in Patients with Chronic Spinal Cord Injury. J. Clin. Med. 2023, 12, 1400. https://doi.org/10.3390/jcm12041400
Fan Y-H, Shen Y-C, Hsu C-C, Chow P-M, Chang P-C, Lin Y-H, Chang S-J, Jiang Y-H, Liao C-H, Wang C-C, et al. Current Surgical Treatment for Neurogenic Lower Urinary Tract Dysfunction in Patients with Chronic Spinal Cord Injury. Journal of Clinical Medicine. 2023; 12(4):1400. https://doi.org/10.3390/jcm12041400
Chicago/Turabian StyleFan, Yu-Hua, Yuan-Chi Shen, Chih-Chen Hsu, Po-Ming Chow, Po-Chih Chang, Yu-Hua Lin, Shang-Jen Chang, Yuan-Hong Jiang, Chun-Hou Liao, Chung-Cheng Wang, and et al. 2023. "Current Surgical Treatment for Neurogenic Lower Urinary Tract Dysfunction in Patients with Chronic Spinal Cord Injury" Journal of Clinical Medicine 12, no. 4: 1400. https://doi.org/10.3390/jcm12041400







