Polycystic Ovary Syndrome: Etiology, Current Management, and Future Therapeutics
Abstract
1. Introduction
2. Phenotypes of PCOS
3. Disease Pathophysiology
3.1. Hyperandrogenism
3.2. Hyperinsulinemia
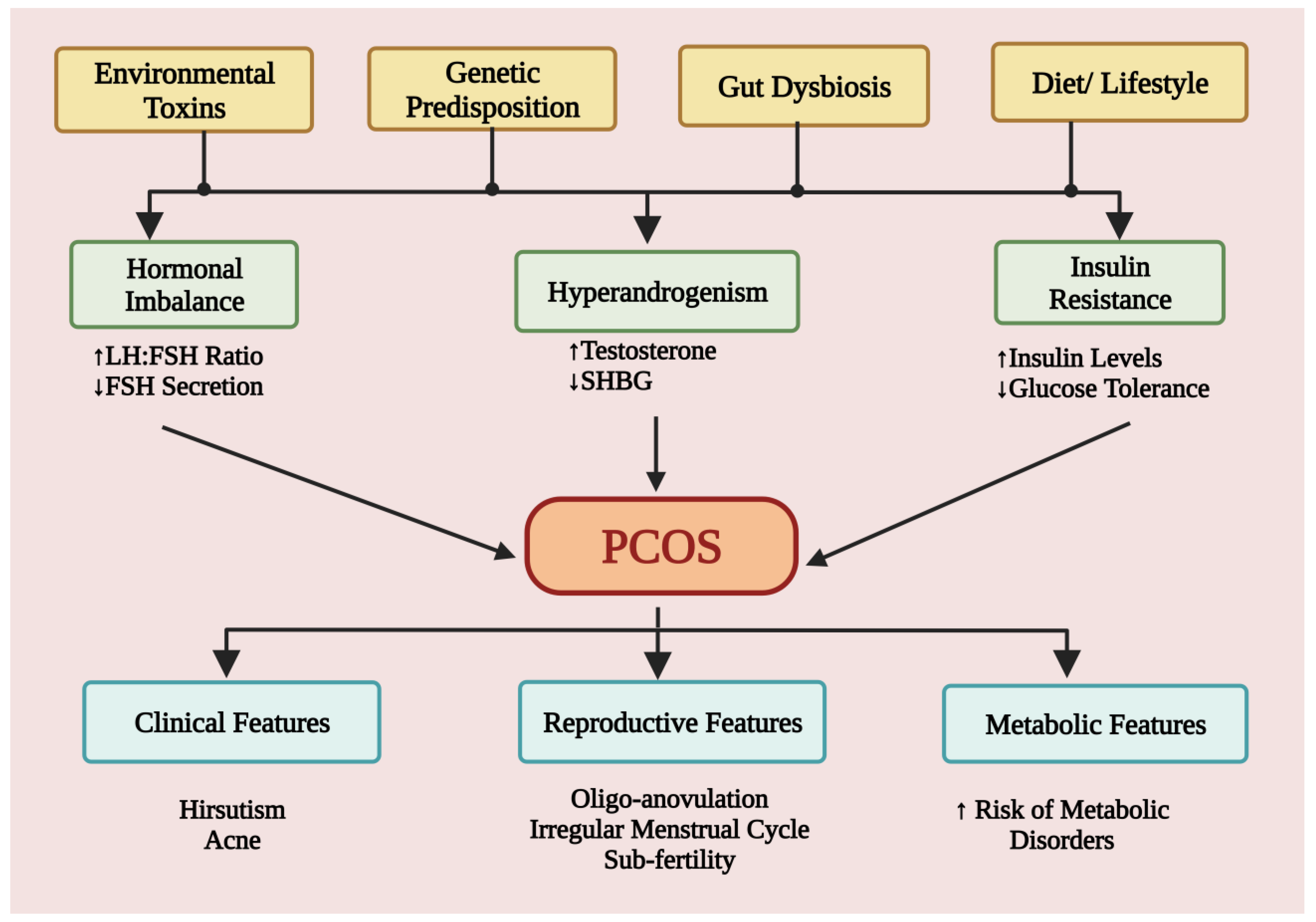
4. Causes and Risk Factors
4.1. Etiological Role of Environmental Pollutants
4.2. Role of Diet and Lifestyle
4.3. Role of Genes and Genetics
4.4. Gut Microbiota Dysbiosis: Critical Correlation
5. Treatment and Management
5.1. Oral Contraceptives and Anti-Androgens
5.2. Insulin Sensitizers
5.3. Ovulation Inducers
5.4. Calcium and Vitamin D Supplements
6. Emerging Therapeutics
6.1. Statins
6.2. Glucagon-Like Peptide-1 (GLP-1) Agonist
6.3. Inositols
6.4. MicroRNA (miRNA) Therapy
6.5. Interleukin (IL)-22 Therapy
6.6. Restoration of the Gut Microbiome: Towards Treatment
6.6.1. Probiotics and Prebiotics
6.6.2. Fecal Microbiota Transplantations (FMTs)
7. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- El Hayek, S.; Bitar, L.; Hamdar, L.H.; Mirza, F.G.; Daoud, G. Poly Cystic Ovarian Syndrome: An Updated Overview. Front. Physiol. 2016, 7, 124. [Google Scholar] [CrossRef]
- Motlagh Asghari, K.; Nejadghaderi, S.A.; Alizadeh, M.; Sanaie, S.; Sullman, M.J.M.; Kolahi, A.-A.; Avery, J.; Safiri, S. Burden of polycystic ovary syndrome in the Middle East and North Africa region, 1990–2019. Sci. Rep. 2022, 12, 7039. [Google Scholar] [CrossRef] [PubMed]
- Balen, A.H.; Tan, S.L.; MacDougall, J.; Jacobs, H.S. Miscarriage rates following in-vitro fertilization are increased in women with polycystic ovaries and reduced by pituitary desensitization with buserelin. Hum. Reprod. 1993, 8, 959–964. [Google Scholar] [CrossRef]
- Azziz, R.; Woods, K.S.; Reyna, R.; Key, T.J.; Knochenhauer, E.S.; Yildiz, B.O. The prevalence and features of the polycystic ovary syndrome in an unselected population. J. Clin. Endocrinol. Metab. 2004, 89, 2745–2749. [Google Scholar] [CrossRef] [PubMed]
- Vidya Bharathi, R.; Swetha, S.; Neerajaa, J.; Varsha Madhavica, J.; Janani, D.M.; Rekha, S.N.; Ramya, S.; Usha, B. An epidemiological survey: Effect of predisposing factors for PCOS in Indian urban and rural population. Middle East Fertil. Soc. J. 2017, 22, 313–316. [Google Scholar] [CrossRef]
- Azziz, R.; Marin, C.; Hoq, L.; Badamgarav, E.; Song, P. Health care-related economic burden of the polycystic ovary syndrome during the reproductive life span. J. Clin. Endocrinol. Metab. 2005, 90, 4650–4658. [Google Scholar] [CrossRef]
- Bremer, A.A. Polycystic ovary syndrome in the pediatric population. Metab. Syndr. Relat. Disorders 2010, 8, 375–394. [Google Scholar] [CrossRef]
- Liu, J.; Wu, Q.; Hao, Y.; Jiao, M.; Wang, X.; Jiang, S.; Han, L. Measuring the global disease burden of polycystic ovary syndrome in 194 countries: Global Burden of Disease Study 2017. Hum. Reprod. 2021, 36, 1108–1119. [Google Scholar] [CrossRef]
- Hashemipour, M.; Amini, M.; Iranpour, R.; Sadri, G.H.; Javaheri, N.; Haghighi, S.; Hovsepian, S.; Javadi, A.A.; Nematbakhsh, M.; Sattari, G. Prevalence of congenital hypothyroidism in Isfahan, Iran: Results of a survey on 20,000 neonates. Horm. Res. 2004, 62, 79–83. [Google Scholar] [CrossRef]
- Lujan, M.E.; Chizen, D.R.; Pierson, R.A. Diagnostic criteria for polycystic ovary syndrome: Pitfalls and controversies. J. Obstet. Gynaecol. Can. 2008, 30, 671–679. [Google Scholar] [CrossRef] [PubMed]
- Merkin, S.S.; Phy, J.L.; Sites, C.K.; Yang, D. Environmental determinants of polycystic ovary syndrome. Fertil. Steril. 2016, 106, 16–24. [Google Scholar] [CrossRef]
- Batra, M.; Bhatnager, R.; Kumar, A.; Suneja, P. Interplay between PCOS and microbiome: The road less travelled. Am. J. Reprod. Immunol. 2022, 88, e13580. [Google Scholar] [CrossRef] [PubMed]
- Mumusoglu, S.; Yildiz, B.O. Polycystic ovary syndrome phenotypes and prevalence: Differential impact of diagnostic criteria and clinical versus unselected population. Curr. Opin. Endocr. Metab. Res. 2020, 12, 66–71. [Google Scholar] [CrossRef]
- Witchel, S.F.; Oberfield, S.E.; Peña, A.S. Polycystic Ovary Syndrome: Pathophysiology, Presentation, and Treatment With Emphasis on Adolescent Girls. J. Endocr. Soc. 2019, 3, 1545–1573. [Google Scholar] [CrossRef]
- Ibáñez, L.; Oberfield, S.E.; Witchel, S.; Auchus, R.J.; Chang, R.J.; Codner, E.; Dabadghao, P.; Darendeliler, F.; Elbarbary, N.S.; Gambineri, A.; et al. An International Consortium Update: Pathophysiology, Diagnosis, and Treatment of Polycystic Ovarian Syndrome in Adolescence. Horm. Res. Paediatr. 2017, 88, 371–395. [Google Scholar] [CrossRef] [PubMed]
- Barrea, L.; Marzullo, P.; Muscogiuri, G.; Di Somma, C.; Scacchi, M.; Orio, F.; Aimaretti, G.; Colao, A.; Savastano, S. Source and amount of carbohydrate in the diet and inflammation in women with polycystic ovary syndrome. Nutr. Res. Rev. 2018, 31, 291–301. [Google Scholar] [CrossRef] [PubMed]
- Kanbour, S.A.; Dobs, A.S. Hyperandrogenism in Women with Polycystic Ovarian Syndrome: Pathophysiology and Controversies. Androg. Clin. Res. Ther. 2022, 3, 22–30. [Google Scholar] [CrossRef]
- Bulsara, J.; Patel, P.; Soni, A.; Acharya, S. A review: Brief insight into Polycystic Ovarian syndrome. Endocr. Metab. Sci. 2021, 3, 100085. [Google Scholar] [CrossRef]
- Walters, K.A.; Gilchrist, R.B.; Ledger, W.L.; Teede, H.J.; Handelsman, D.J.; Campbell, R.E. Metabolism. New perspectives on the pathogenesis of PCOS: Neuroendocrine origins. Trends Endocrinol. Metab. 2018, 29, 841–852. [Google Scholar] [CrossRef]
- Ashraf, S.; Nabi, M.; Rasool, S.u.A.; Rashid, F.; Amin, S. Hyperandrogenism in polycystic ovarian syndrome and role of CYP gene variants: A review. Egypt. J. Med. Hum. Genet. 2019, 20, 25. [Google Scholar] [CrossRef]
- Tsilchorozidou, T.; Honour, J.W.; Conway, G.S. Altered cortisol metabolism in polycystic ovary syndrome: Insulin enhances 5alpha-reduction but not the elevated adrenal steroid production rates. J. Clin. Endocrinol. Metab. 2003, 88, 5907–5913. [Google Scholar] [CrossRef]
- Ding, H.; Zhang, J.; Zhang, F.; Zhang, S.; Chen, X.; Liang, W.; Xie, Q. Resistance to the Insulin and Elevated Level of Androgen: A Major Cause of Polycystic Ovary Syndrome. Front. Endocrinol. 2021, 12, 741764. [Google Scholar] [CrossRef]
- Marshall, J.C.; Dunaif, A. Should all women with PCOS be treated for insulin resistance? Fertil Steril 2012, 97, 18–22. [Google Scholar] [CrossRef]
- De Leo, V.; la Marca, A.; Petraglia, F. Insulin-Lowering Agents in the Management of Polycystic Ovary Syndrome. Endocr. Rev. 2003, 24, 633–667. [Google Scholar] [CrossRef]
- Kandaraki, E.; Chatzigeorgiou, A.; Livadas, S.; Palioura, E.; Economou, F.; Koutsilieris, M.; Palimeri, S.; Panidis, D.; Diamanti-Kandarakis, E. Endocrine disruptors and polycystic ovary syndrome (PCOS): Elevated serum levels of bisphenol A in women with PCOS. J. Clin. Endocrinol. Metab. 2011, 96, E480–E484. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, T.; Tsutsumi, O.; Ikezuki, Y.; Takai, Y.; Taketani, Y. Positive relationship between androgen and the endocrine disruptor, bisphenol A, in normal women and women with ovarian dysfunction. Endocr. J. 2004, 51, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Konieczna, A.; Rachoń, D.; Owczarek, K.; Kubica, P.; Kowalewska, A.; Kudłak, B.; Wasik, A.; Namieśnik, J. Serum bisphenol A concentrations correlate with serum testosterone levels in women with polycystic ovary syndrome. Reprod. Toxicol. 2018, 82, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Vagi, S.J.; Azziz-Baumgartner, E.; Sjödin, A.; Calafat, A.M.; Dumesic, D.; Gonzalez, L.; Kato, K.; Silva, M.J.; Ye, X.; Azziz, R. Exploring the potential association between brominated diphenyl ethers, polychlorinated biphenyls, organochlorine pesticides, perfluorinated compounds, phthalates, and bisphenol a in polycystic ovary syndrome: A case–control study. BMC Endocr. Disord. 2014, 14, 86. [Google Scholar] [CrossRef]
- Priya, K.; Setty, M.; Babu, U.V.; Pai, K.S.R. Implications of environmental toxicants on ovarian follicles: How it can adversely affect the female fertility? Environ. Sci. Pollut. Res. 2021, 28, 67925–67939. [Google Scholar] [CrossRef]
- Ananthasubramanian, P.; Ananth, S.; Kumaraguru, S.; Barathi, S.; Santosh, W.; Vasantharekha, R. Associated Effects of Endocrine Disrupting Chemicals (EDCs) on Neuroendocrine Axes and Neurotransmitter Profile in Polycystic Ovarian Syndrome Condition. Proc. Zool. Soc. 2021, 74, 378–386. [Google Scholar] [CrossRef]
- Palioura, E.; Diamanti-Kandarakis, E. Polycystic ovary syndrome (PCOS) and endocrine disrupting chemicals (EDCs). Rev. Endocr. Metab. Disord. 2015, 16, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Rutkowska, A.Z.; Diamanti-Kandarakis, E.J. Polycystic ovary syndrome and environmental toxins. Fertil. Steril. 2016, 106, 948–958. [Google Scholar] [CrossRef]
- Zhang, B.; Zhou, W.; Shi, Y.; Zhang, J.; Cui, L.; Chen, Z.-J. Lifestyle and environmental contributions to ovulatory dysfunction in women of polycystic ovary syndrome. BMC Endocr. Disord. 2020, 20, 19. [Google Scholar] [CrossRef] [PubMed]
- Victor, V.M.; Rocha, M.; Banuls, C.; Alvarez, A.; de Pablo, C.; Sanchez-Serrano, M.; Gomez, M.; Hernandez-Mijares, A. Induction of oxidative stress and human leukocyte/endothelial cell interactions in polycystic ovary syndrome patients with insulin resistance. J. Clin. Endocrinol. Metab. 2011, 96, 3115–3122. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Zhao, Y.; Qiu, X.; Zhang, C.; Li, R.; Qiao, J. Association of serum levels of typical organic pollutants with polycystic ovary syndrome (PCOS): A case–control study. Hum. Reprod. 2015, 30, 1964–1973. [Google Scholar] [CrossRef]
- Lin, S.Y.; Yang, Y.C.; Chang, C.Y.; Lin, C.C.; Hsu, W.H.; Ju, S.W.; Hsu, C.Y.; Kao, C.H. Risk of Polycystic Ovary Syndrome in Women Exposed to Fine Air Pollutants and Acidic Gases: A Nationwide Cohort Analysis. Int. J. Env. Res. Public Health 2019, 16, 4816. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, E.; Klukovich, R.; Sadler-Riggleman, I.; Beck, D.; Xie, Y.; Yan, W.; Skinner, M.K. Environmental toxicant induced epigenetic transgenerational inheritance of ovarian pathology and granulosa cell epigenome and transcriptome alterations: Ancestral origins of polycystic ovarian syndrome and primary ovarian insufficiency. Epigenetics 2018, 13, 875–895. [Google Scholar] [CrossRef]
- Mimouni, N.E.H.; Paiva, I.; Barbotin, A.-L.; Timzoura, F.E.; Plassard, D.; Le Gras, S.; Ternier, G.; Pigny, P.; Catteau-Jonard, S.; Simon, V. Polycystic ovary syndrome is transmitted via a transgenerational epigenetic process. Cell Metab. 2021, 33, 513–530.e518. [Google Scholar] [CrossRef]
- Kazemi, M.; Hadi, A.; Pierson, R.A.; Lujan, M.E.; Zello, G.A.; Chilibeck, P.D. Effects of dietary glycemic index and glycemic load on cardiometabolic and reproductive profiles in women with polycystic ovary syndrome: A systematic review and meta-analysis of randomized controlled trials. Adv. Nutrit. 2021, 12, 161–178. [Google Scholar] [CrossRef]
- Szczuko, M.; Skowronek, M.; Zapalowska-Chwyc, M.; Starczewski, A. Quantitative assessment of nutrition in patients with polycystic ovary syndrome (PCOS). Roczniki Państwowego Zakładu Higieny 2016, 67, 4. [Google Scholar]
- González, F.; Considine, R.V.; Abdelhadi, O.A.; Acton, A.J. Saturated fat ingestion promotes lipopolysaccharide-mediated inflammation and insulin resistance in polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 2019, 104, 934–946. [Google Scholar] [CrossRef]
- Wang, T.; Sha, L.; Li, Y.; Zhu, L.; Wang, Z.; Li, K.; Lu, H.; Bao, T.; Guo, L.; Zhang, X.; et al. Dietary α-Linolenic acid-rich flaxseed oil exerts beneficial effects on polycystic ovary syndrome through sex steroid hormones—Microbiota—Inflammation axis in rats. Front. Endocrinol. 2020, 11, 284. [Google Scholar] [CrossRef] [PubMed]
- Barber, T.M.; Hanson, P.; Weickert, M.O.; Franks, S. Obesity and Polycystic Ovary Syndrome: Implications for Pathogenesis and Novel Management Strategies. Clin. Med. Insights. Reprod. Health 2019, 13, 1179558119874042. [Google Scholar] [CrossRef] [PubMed]
- Glueck, C.J.; Goldenberg, N. Characteristics of obesity in polycystic ovary syndrome: Etiology, treatment, and genetics. Metabolism 2019, 92, 108–120. [Google Scholar] [CrossRef] [PubMed]
- Barber, T.M.; Kabisch, S.; Pfeiffer, A.F.; Weickert, M.O. The health benefits of dietary fibre. Nutrients 2020, 12, 3209. [Google Scholar] [CrossRef]
- Szczuko, M.; Zapalowska-Chwyć, M.; Drozd, R. A low glycemic index decreases inflammation by increasing the concentration of uric acid and the activity of glutathione peroxidase (GPx3) in patients with polycystic ovary syndrome (PCOS). Molecules 2019, 24, 1508. [Google Scholar] [CrossRef]
- Hoover, S.E.; Gower, B.A.; Cedillo, Y.E.; Chandler-Laney, P.C.; Deemer, S.E.; Goss, A.M. Changes in Ghrelin and Glucagon following a Low Glycemic Load Diet in Women with PCOS. J. Clin. Endocrinol. Metab. 2021, 106, e2151–e2161. [Google Scholar] [CrossRef]
- Akintayo, C.O.; Johnson, A.D.; Badejogbin, O.C.; Olaniyi, K.S.; Oniyide, A.A.; Ajadi, I.O.; Ojewale, A.O.; Adeyomoye, O.I.; Kayode, A.B. High fructose-enriched diet synergistically exacerbates endocrine but not metabolic changes in letrozole-induced polycystic ovarian syndrome in Wistar rats. Heliyon 2021, 7, e05890. [Google Scholar] [CrossRef]
- Porchia, L.M.; Hernandez-Garcia, S.C.; Gonzalez-Mejia, M.E.; López-Bayghen, E. Diets with lower carbohydrate concentrations improve insulin sensitivity in women with polycystic ovary syndrome: A meta-analysis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 248, 110–117. [Google Scholar] [CrossRef]
- Shang, Y.; Zhou, H.; Hu, M.; Feng, H.J. Effect of diet on insulin resistance in polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 2020, 105, 3346–3360. [Google Scholar] [CrossRef]
- Shishehgar, F.; Mirmiran, P.; Rahmati, M.; Tohidi, M.; Ramezani Tehrani, F. Does a restricted energy low glycemic index diet have a different effect on overweight women with or without polycystic ovary syndrome? BMC Endocr. Disord. 2019, 19, 93. [Google Scholar] [CrossRef]
- Paoli, A.; Mancin, L.; Giacona, M.C.; Bianco, A.; Caprio, M. Effects of a ketogenic diet in overweight women with polycystic ovary syndrome. J. Transl. Med. 2020, 18, 104. [Google Scholar] [CrossRef]
- Kshetrimayum, C.; Sharma, A.; Mishra, V.V.; Kumar, S. Polycystic ovarian syndrome: Environmental/occupational, lifestyle factors; an overview. J. Turk. Ger. Gynecol. Assoc. 2019, 20, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Szczuko, M.; Kikut, J.; Szczuko, U.; Szydłowska, I.; Nawrocka-Rutkowska, J.; Ziętek, M.; Verbanac, D.; Saso, L. Nutrition Strategy and Life Style in Polycystic Ovary Syndrome-Narrative Review. Nutrients 2021, 13, 2452. [Google Scholar] [CrossRef] [PubMed]
- Patten, R.K.; Boyle, R.A.; Moholdt, T.; Kiel, I.; Hopkins, W.G.; Harrison, C.L.; Stepto, N.K.J.F.i.p. Exercise interventions in polycystic ovary syndrome: A systematic review and meta-analysis. Front. Physiol. 2020, 11, 606. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Minerva, S.; Shah, R.; Bhat, A.; Verma, S.; Chander, G.; Bhat, G.R.; Thapa, N.; Bhat, A.; Wakhloo, A.; et al. Role of genetic, environmental, and hormonal factors in the progression of PCOS: A review. J. Reprod. Healthc. Med. 2022, 3, 3. [Google Scholar] [CrossRef]
- Khan, M.J.; Ullah, A.; Basit, S. Genetic Basis of Polycystic Ovary Syndrome (PCOS): Current Perspectives. Appl. Clin. Genet. 2019, 12, 249–260. [Google Scholar] [CrossRef]
- Ajmal, N.; Khan, S.Z.; Shaikh, R. Polycystic ovary syndrome (PCOS) and genetic predisposition: A review article. Eur. J. Obstet. Gynecol. Reprod. Biol. X 2019, 3, 100060. [Google Scholar] [CrossRef]
- Singh, S.; Sharma, P.; Pal, N.; Kumawat, M.; Shubham, S.; Sarma, D.K.; Tiwari, R.R.; Kumar, M.; Nagpal, R. Impact of Environmental Pollutants on Gut Microbiome and Mental Health via the Gut–Brain Axis. Microorganisms 2022, 10, 1457. [Google Scholar] [CrossRef]
- Yurtdaş, G.; Akdevelioğlu, Y. A New Approach to Polycystic Ovary Syndrome: The Gut Microbiota. J. Am. Coll. Nutr. 2020, 39, 371–382. [Google Scholar] [CrossRef]
- Liu, R.; Zhang, C.; Shi, Y.; Zhang, F.; Li, L.; Wang, X.; Ling, Y.; Fu, H.; Dong, W.; Shen, J.; et al. Dysbiosis of gut microbiota associated with clinical parameters in polycystic ovary syndrome. Front. Microbiol. 2017, 8, 324. [Google Scholar] [CrossRef]
- Rizk, M.G.; Thackray, V.G. Intersection of Polycystic Ovary Syndrome and the Gut Microbiome. J. Endocr. Soc. 2020, 5, bvaa177. [Google Scholar] [CrossRef]
- Tremellen, K.; Pearce, K. Dysbiosis of Gut Microbiota (DOGMA)—A novel theory for the development of Polycystic Ovarian Syndrome. Med. Hypotheses 2012, 79, 104–112. [Google Scholar] [CrossRef]
- Martin, K.A.; Chang, R.J.; Ehrmann, D.A.; Ibanez, L.; Lobo, R.A.; Rosenfield, R.L.; Shapiro, J.; Montori, V.M.; Swiglo, B.A. Evaluation and treatment of hirsutism in premenopausal women: An endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab. 2008, 93, 1105–1120. [Google Scholar] [CrossRef]
- Rotterdam, E.S.; ASRM Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil. Steril. 2004, 81, 19–25. [Google Scholar]
- Rashid, R.; Mir, S.A.; Kareem, O.; Ali, T.; Ara, R.; Malik, A.; Amin, F.; Bader, G.N. Polycystic ovarian syndrome-current pharmacotherapy and clinical implications. Taiwan. J. Obstet. Gynecol. 2022, 61, 40–50. [Google Scholar] [CrossRef]
- Zimmerman, Y.; Eijkemans, M.; Coelingh Bennink, H.; Blankenstein, M.; Fauser, B. The effect of combined oral contraception on testosterone levels in healthy women: A systematic review and meta-analysis. Hum. Reprod. Update 2014, 20, 76–105. [Google Scholar] [CrossRef]
- Mendoza, N.; Simoncini, T.; Genazzani, A.D. Hormonal contraceptive choice for women with PCOS: A systematic review of randomized trials and observational studies. Gynecol. Endocrinol. 2014, 30, 850–860. [Google Scholar] [CrossRef]
- Rodriguez Paris, V.; Bertoldo, M.J. The Mechanism of Androgen Actions in PCOS Etiology. Med. Sci. 2019, 7, 89. [Google Scholar] [CrossRef] [PubMed]
- Moghetti, P.; Tosi, F.; Tosti, A.; Negri, C.; Misciali, C.; Perrone, F.; Caputo, M.; Muggeo, M.; Castello, R. Comparison of spironolactone, flutamide, and finasteride efficacy in the treatment of hirsutism: A randomized, double blind, placebo-controlled trial. J. Clin. Endocrinol. Metab. 2000, 85, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Venturoli, S.; Marescalchi, O.; Colombo, F.; Macrelli, S.; Ravaioli, B.; Bagnoli, A.; Paradisi, R.; Flamigni, C. A prospective randomized trial comparing low dose flutamide, finasteride, ketoconazole, and cyproterone acetate-estrogen regimens in the treatment of hirsutism. J. Clin. Endocrinol. Metab. 1999, 84, 1304–1310. [Google Scholar] [CrossRef] [PubMed]
- Calaf, J.; Lopez, E.; Millet, A.; Alcañiz, J.; Fortuny, A.; Vidal, O.; Callejo, J.; Escobar-Jimenez, F.; Torres, E.; Espinos, J.J.; et al. Long-term efficacy and tolerability of flutamide combined with oral contraception in moderate to severe hirsutism: A 12-month, double-blind, parallel clinical trial. J. Clin. Endocrinol. Metab. 2007, 92, 3446–3452. [Google Scholar] [CrossRef]
- Paradisi, R.; Fabbri, R.; Battaglia, C.; Venturoli, S. Ovulatory effects of flutamide in the polycystic ovary syndrome. Gynecol. Endocrinol. 2013, 29, 391–395. [Google Scholar] [CrossRef]
- De Leo, V.; Lanzetta, D.; D’Antona, D.; La Marca, A.; Morgante, G. Hormonal effects of flutamide in young women with polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 1998, 83, 99–102. [Google Scholar] [CrossRef] [PubMed]
- Diamanti-Kandarakis, E.; Mitrakou, A.; Raptis, S.; Tolis, G.; Duleba, A.J. The effect of a pure antiandrogen receptor blocker, flutamide, on the lipid profile in the polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 1998, 83, 2699–2705. [Google Scholar] [CrossRef][Green Version]
- Badawy, A.; Elnashar, A.J. Treatment options for polycystic ovary syndrome. Int. J. Women’s Health. 2011, 3, 25. [Google Scholar] [CrossRef]
- Zulian, E.; Sartorato, P.; Benedini, S.; Baro, G.; Armanini, D.; Mantero, F.; Scaroni, C.J. Spironolactone in the treatment of polycystic ovary syndrome: Effects on clinical features, insulin sensitivity and lipid profile. J. Endocrinol. Investig. 2005, 28, 49–53. [Google Scholar] [CrossRef]
- Tartagni, M.V.; Alrasheed, H.; Damiani, G.R.; Montagnani, M.; Maria, A.; De Pergola, G.; Tartagni, M.; Loverro, G.J. Intermittent low-dose finasteride administration is effective for treatment of hirsutism in adolescent girls: A pilot study. J. Pediatr. Adolesc. Gynecol. 2014, 27, 161–165. [Google Scholar] [CrossRef]
- Lakryc, E.; Motta, E.; Soares, J.; Haidar, M.A.; Rodrigues de Lima, G.; Baracat, E. The benefits of finasteride for hirsute women with polycystic ovary syndrome or idiopathic hirsutism. Gynecol. Endocrinol. 2003, 17, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Abdalla, M.A.; Deshmukh, H.; Atkin, S.; Sathyapalan, T. A review of therapeutic options for managing the metabolic aspects of polycystic ovary syndrome. Ther. Adv. Endocrinol. Metab. 2020, 11, 2042018820938305. [Google Scholar] [CrossRef]
- Grundy, S.M. Obesity, metabolic syndrome, and coronary atherosclerosis. Circulation 2002, 105, 2696–2698. [Google Scholar] [CrossRef] [PubMed]
- Curi, D.D.; Fonseca, A.M.; Marcondes, J.A.M.; Almeida, J.A.M.; Bagnoli, V.R.; Soares, J.M., Jr.; Baracat, E.C. Metformin versus lifestyle changes in treating women with polycystic ovary syndrome. Gynecol. Endocrinol. 2012, 28, 182–185. [Google Scholar] [CrossRef]
- Harborne, L.R.; Sattar, N.; Norman, J.E.; Fleming, R. Metformin and weight loss in obese women with polycystic ovary syndrome: Comparison of doses. J. Clin. Endocrinol. Metab. 2005, 90, 4593–4598. [Google Scholar] [CrossRef]
- Fleming, R.; Hopkinson, Z.E.; Wallace, A.M.; Greer, I.A.; Sattar, N. Ovarian function and metabolic factors in women with oligomenorrhea treated with metformin in a randomized double blind placebo-controlled trial. J. Clin. Endocrinol. Metab. 2002, 87, 569–574. [Google Scholar] [CrossRef]
- Ng, E.H.Y.; Wat, N.M.S.; Ho, P.C. Effects of metformin on ovulation rate, hormonal and metabolic profiles in women with clomiphene-resistant polycystic ovaries: A randomized, double-blinded placebo-controlled trial. Hum. Reprod. 2001, 16, 1625–1631. [Google Scholar] [CrossRef] [PubMed]
- Sin, H.Y.; Kim, J.Y.; Jung, K.H. Total cholesterol, high density lipoprotein and triglyceride for cardiovascular disease in elderly patients treated with metformin. Arch. Pharmacal Res. 2011, 34, 99–107. [Google Scholar] [CrossRef]
- Madnani, N.; Khan, K.; Chauhan, P.; Parmar, G. Polycystic ovarian syndrome. Indian J. Dermatol. Venereol. Leprol. 2013, 79, 310–321. [Google Scholar] [CrossRef]
- Brettenthaler, N.; De Geyter, C.; Huber, P.R.; Keller, U. Effect of the insulin sensitizer pioglitazone on insulin resistance, hyperandrogenism, and ovulatory dysfunction in women with polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 2004, 89, 3835–3840. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wu, Y.; Huang, Q. Comparison of the effect between pioglitazone and metformin in treating patients with PCOS: A meta-analysis. Arch. Gynecol. Obstet. 2017, 296, 661–677. [Google Scholar] [CrossRef]
- Zhao, H.; Xing, C.; Zhang, J.; He, B. Comparative efficacy of oral insulin sensitizers metformin, thiazolidinediones, inositol, and berberine in improving endocrine and metabolic profiles in women with PCOS: A network meta-analysis. Reprod. Health 2021, 18, 171. [Google Scholar] [CrossRef]
- Thessaloniki ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Consensus on infertility treatment related to polycystic ovary syndrome. Hum. Reprod. 2008, 23, 462–477. [Google Scholar] [CrossRef] [PubMed]
- Christin-Maitre, S.; Hugues, J.N. A comparative randomized multicentric study comparing the step-up versus step-down protocol in polycystic ovary syndrome. Hum. Reprod. 2003, 18, 1626–1631. [Google Scholar] [CrossRef] [PubMed]
- Gysler, M.; March, C.M.; Mishell, D.R., Jr.; Bailey, E.J. A decade’s experience with an individualized clomiphene treatment regimen including its effect on the postcoital test. Fertil. Steril. 1982, 37, 161–167. [Google Scholar] [CrossRef]
- Casper, R.F.; Mitwally, M.F. Use of the aromatase inhibitor letrozole for ovulation induction in women with polycystic ovarian syndrome. Clin. Obstet. Gynecol. 2011, 54, 685–695. [Google Scholar] [CrossRef] [PubMed]
- Carroll, N.; Palmer, J.R. A comparison of intrauterine versus intracervical insemination in fertile single women. Fertil. Steril. 2001, 75, 656–660. [Google Scholar] [CrossRef]
- Lin, M.W.; Wu, M.H. The role of vitamin D in polycystic ovary syndrome. Indian J. Med. Res. 2015, 142, 238–240. [Google Scholar] [CrossRef]
- dehghani Firouzabadi, R.; Aflatoonian, A.; Modarresi, S.; Sekhavat, L.; MohammadTaheri, S. Therapeutic effects of calcium & vitamin D supplementation in women with PCOS. Complement. Ther. Clin. Pract. 2012, 18, 85–88. [Google Scholar]
- Rashidi, B.; Haghollahi, F.; Shariat, M.; Zayerii, F.J. The effects of calcium-vitamin D and metformin on polycystic ovary syndrome: A pilot study. Eur. J. Obstet. Gynecol. Reprod. Biol. 2009, 48, 142–147. [Google Scholar] [CrossRef]
- Irani, M.; Minkoff, H.; Seifer, D.B.; Merhi, Z. Vitamin D increases serum levels of the soluble receptor for advanced glycation end products in women with PCOS. J. Clin. Endocrinol. Metab. 2014, 99, E886–E890. [Google Scholar] [CrossRef] [PubMed]
- Shojaeian, Z.; Sadeghi, R.; Latifnejad Roudsari, R. Calcium and vitamin D supplementation effects on metabolic factors, menstrual cycles and follicular responses in women with polycystic ocvary syndrome: A systematic review and meta-analysis. Casp. J. Intern. Med. 2019, 10, 359–369. [Google Scholar] [CrossRef]
- Kodaman, P.H.; Duleba, A.J. HMG-CoA Reductase Inhibitors: Do they have potential in the treatment of polycystic ovary syndrome? Drugs 2008, 68, 1771–1785. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo, D.; Foyouzi, N.; Kwintkiewicz, J.; Duleba, A.J. Mevastatin inhibits ovarian theca–interstitial cell proliferation and steroidogenesis. Fertil. Steril. 2004, 82, 1193–1197. [Google Scholar] [CrossRef] [PubMed]
- Kodaman, P.H.; Duleba, A.J. Statins in the treatment of polycystic ovary syndrome. Semin. Reprod. Med. 2008, 26, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Sathyapalan, T.; Kilpatrick, E.S.; Coady, A.M.; Atkin, S.L. Atorvastatin pretreatment augments the effect of metformin in patients with polycystic ovary syndrome (PCOS). Clin. Endocrinol. 2010, 72, 566–568. [Google Scholar] [CrossRef]
- Sathyapalan, T.; Hobkirk, J.P.; Javed, Z.; Carroll, S.; Coady, A.-M.; Pemberton, P.; Smith, A.; Cianflone, K.; Atkin, S.L. The effect of atorvastatin (and subsequent metformin) on adipose tissue acylation-stimulatory-protein concentration and inflammatory biomarkers in overweight/obese women with polycystic ovary syndrome. Front. Endocrinol. 2019, 10, 394. [Google Scholar] [CrossRef]
- Sathyapalan, T.; Kilpatrick, E.S.; Coady, A.-M.; Atkin, S.L. The effect of atorvastatin in patients with polycystic ovary syndrome: A randomized double-blind placebo-controlled study. J. Clin. Endocrinol. Metab. 2009, 94, 103–108. [Google Scholar] [CrossRef]
- Chen, L.-L.; Zheng, J.-H. Effects of atorvastatin on the insulin resistance in women of polycystic ovary syndrome: A systematic review and meta-analysis. Medicine 2021, 100, e26289. [Google Scholar] [CrossRef]
- Sathyapalan, T.; Shepherd, J.; Coady, A.-M.; Kilpatrick, E.S.; Atkin, S.L. Atorvastatin reduces malondialdehyde concentrations in patients with polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 2012, 97, 3951–3955. [Google Scholar] [CrossRef]
- Sathyapalan, T.; Smith, K.A.; Coady, A.-M.; Kilpatrick, E.S.; Atkin, S.L. Atorvastatin therapy decreases androstenedione and dehydroepiandrosterone sulphate concentrations in patients with polycystic ovary syndrome: Randomized controlled study. Ann. Clin. Biochem. 2012, 49, 80–85. [Google Scholar] [CrossRef]
- Chen, J.; Huang, C.; Zhang, T.; Gong, W.; Deng, X.; Liu, H.; Liu, J.; Guo, Y. The effects of statins on hyperandrogenism in women with polycystic ovary syndrome: A systematic review and meta-analysis of randomized controlled trials. Reprod. Biol. Endocrinol. 2021, 19, 189. [Google Scholar] [CrossRef]
- Miao, K.; Zhou, H. Effect of statins combined or not combined with metformin on polycystic ovary syndrome: A systematic review and meta-analysis. J. Obstet. Gynaecol. Res. 2022, 48, 1806–1815. [Google Scholar] [CrossRef]
- Cefalu, W.T. The physiologic role of incretin hormones: Clinical applications. J. Osteopath. Med. 2010, 110, 8–14. [Google Scholar]
- Yaribeygi, H.; Sathyapalan, T.; Sahebkar, A.J. Molecular mechanisms by which GLP-1 RA and DPP-4i induce insulin sensitivity. Life Sci. 2019, 234, 116776. [Google Scholar] [CrossRef]
- Han, Y.; Li, Y.; He, B.J.R.b.o. GLP-1 receptor agonists versus metformin in PCOS: A systematic review and meta-analysis. Reprod. Biomed. Online 2019, 39, 332–342. [Google Scholar] [CrossRef] [PubMed]
- Tzotzas, T.; N Karras, S.; Katsiki, N. Glucagon-like peptide-1 (GLP-1) receptor agonists in the treatment of obese women with polycystic ovary syndrome. Curr. Vasc. Pharmacol. 2017, 15, 218–229. [Google Scholar] [CrossRef] [PubMed]
- Kahal, H.; Kilpatrick, E.; Rigby, A.; Coady, A.; Atkin, S. The effects of treatment with liraglutide on quality of life and depression in young obese women with PCOS and controls. Gynecol. Endocrinol. 2019, 35, 142–145. [Google Scholar] [CrossRef]
- Unfer, V.; Facchinetti, F.; Orrù, B.; Giordani, B.; Nestler, J. Myo-inositol effects in women with PCOS: A meta-analysis of randomized controlled trials. Endocr. Connect. 2017, 6, 647–658. [Google Scholar] [CrossRef]
- Kalra, B.; Kalra, S.; Sharma, J.B. The inositols and polycystic ovary syndrome. Indian J. Endocrinol. Metab. 2016, 20, 720–724. [Google Scholar] [CrossRef] [PubMed]
- Papaleo, E.; Unfer, V.; Baillargeon, J.-P.; De Santis, L.; Fusi, F.; Brigante, C.; Marelli, G.; Cino, I.; Redaelli, A.; Ferrari, A. Myo-inositol in patients with polycystic ovary syndrome: A novel method for ovulation induction. Gynecol. Endocrinol. 2007, 23, 700–703. [Google Scholar] [CrossRef]
- Artini, P.G.; Di Berardino, O.M.; Papini, F.; Genazzani, A.D.; Simi, G.; Ruggiero, M.; Cela, V. Endocrine and clinical effects of myo-inositol administration in polycystic ovary syndrome. A randomized study. Gynecol. Endocrinol. Off. J. Int. Soc. Gynecol. Endocrinol. 2013, 29, 375–379. [Google Scholar] [CrossRef]
- Piomboni, P.; Focarelli, R.; Capaldo, A.; Stendardi, A.; Cappelli, V.; Cianci, A.; La Marca, A.; Luddi, A.; De Leo, V. Protein modification as oxidative stress marker in follicular fluid from women with polycystic ovary syndrome: The effect of inositol and metformin. J. Assist. Reprod. Genet. 2014, 31, 1269–1276. [Google Scholar] [CrossRef] [PubMed]
- Genazzani, A.D.; Santagni, S.; Rattighieri, E.; Chierchia, E.; Despini, G.; Marini, G.; Prati, A.; Simoncini, T. Modulatory role of D-chiro-inositol (DCI) on LH and insulin secretion in obese PCOS patients. Gynecol. Endocrinol. 2014, 30, 438–443. [Google Scholar] [CrossRef] [PubMed]
- Nestler, J.E.; Jakubowicz, D.J.; Reamer, P.; Gunn, R.D.; Allan, G. Ovulatory and metabolic effects of D-chiro-inositol in the polycystic ovary syndrome. N. Engl. J. Med. 1999, 340, 1314–1320. [Google Scholar] [CrossRef]
- Iuorno, M.J.; Jakubowicz, D.J.; Baillargeon, J.-P.; Dillon, P.; Gunn, R.D.; Allan, G.; Nestler, J.E. Effects of d-chiro-inositol in lean women with the polycystic ovary syndrome. Endocr. Pr. 2002, 8, 417–423. [Google Scholar] [CrossRef]
- Luo, Y.; Cui, C.; Han, X.; Wang, Q.; Zhang, C. The role of miRNAs in polycystic ovary syndrome with insulin resistance. J. Assist. Reprod. Genet. 2021, 38, 289–304. [Google Scholar] [CrossRef]
- Vitale, S.G.; Fulghesu, A.M.; Mikuš, M.; Watrowski, R.; D’Alterio, M.N.; Lin, L.T.; Shah, M.; Reyes-Muñoz, E.; Sathyapalan, T.; Angioni, S. The Translational Role of miRNA in Polycystic Ovary Syndrome: From Bench to Bedside-A Systematic Literature Review. Biomedicines 2022, 10, 1816. [Google Scholar] [CrossRef]
- Abdalla, M.; Deshmukh, H.; Atkin, S.L.; Sathyapalan, T. miRNAs as a novel clinical biomarker and therapeutic targets in polycystic ovary syndrome (PCOS): A review. Life Sci. 2020, 259, 118174. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Kim, M.; Han, J.; Yeom, K.H.; Lee, S.; Baek, S.H.; Kim, V.N. MicroRNA genes are transcribed by RNA polymerase II. EMBO J. 2004, 23, 4051–4060. [Google Scholar] [CrossRef] [PubMed]
- Krol, J.; Loedige, I.; Filipowicz, W. The widespread regulation of microRNA biogenesis, function and decay. Nat. Rev. Genet. 2010, 11, 597–610. [Google Scholar] [CrossRef] [PubMed]
- Guay, C.; Regazzi, R. Circulating microRNAs as novel biomarkers for diabetes mellitus. Nat. Rev. Endocrinol. 2013, 9, 513–521. [Google Scholar] [CrossRef]
- Sørensen, A.E.; Udesen, P.B.; Wissing, M.L.; Englund, A.L.M.; Dalgaard, L.T. MicroRNAs related to androgen metabolism and polycystic ovary syndrome. Chem. Interact. 2016, 259, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.-L.; Heneidi, S.; Chuang, T.-Y.; Diamond, M.P.; Layman, L.C.; Azziz, R.; Chen, Y.-H. The expression of the miR-25/93/106b family of micro-RNAs in the adipose tissue of women with polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 2014, 99, E2754–E2761. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Zhang, Y.-W.; Tong, X.-H.; Liu, Y.-S. Characterization of microRNA profile in human cumulus granulosa cells: Identification of microRNAs that regulate Notch signaling and are associated with PCOS. Mol. Cell. Endocrinol. 2015, 404, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Naji, M.; Aleyasin, A.; Nekoonam, S.; Arefian, E.; Mahdian, R.; Amidi, F. Differential expression of miR-93 and miR-21 in granulosa cells and follicular fluid of polycystic ovary syndrome associating with different phenotypes. Sci. Rep. 2017, 7, 14671. [Google Scholar] [CrossRef]
- Long, W.; Zhao, C.; Ji, C.; Ding, H.; Cui, Y.; Guo, X.; Shen, R.; Liu, J. Characterization of serum microRNAs profile of PCOS and identification of novel non-invasive biomarkers. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2014, 33, 1304–1315. [Google Scholar] [CrossRef]
- Sirotkin, A.V.; Kisová, G.; Brenaut, P.; Ovcharenko, D.; Grossmann, R.; Mlyncek, M. Involvement of microRNA Mir15a in control of human ovarian granulosa cell proliferation, apoptosis, steroidogenesis, and response to FSH. MicroRNA 2014, 3, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Yun, C.; Sun, L.; Xia, J.; Wu, Q.; Wang, Y.; Wang, L.; Zhang, Y.; Liang, X.; Wang, L. Gut microbiota–bile acid–interleukin-22 axis orchestrates polycystic ovary syndrome. J. Endocrinol. 2019, 25, 1225–1233. [Google Scholar]
- Qi, X.; Yun, C.; Liao, B.; Qiao, J.; Pang, Y. The therapeutic effect of interleukin-22 in high androgen-induced polycystic ovary syndrome. J. Endocrinol. 2020, 245, 281–289. [Google Scholar] [CrossRef]
- Wang, X.; Ota, N.; Manzanillo, P.; Kates, L.; Zavala-Solorio, J.; Eidenschenk, C.; Zhang, J.; Lesch, J.; Lee, W.; Ross, J. Interleukin-22 alleviates metabolic disorders and restores mucosal immunity in diabetes. Nature 2014, 514, 237–241. [Google Scholar] [CrossRef]
- Giampaolino, P.; Foreste, V.; Di Filippo, C.; Gallo, A.; Mercorio, A.; Serafino, P.; Improda, F.P.; Verrazzo, P.; Zara, G.; Buonfantino, C.J. Microbiome and PCOS: State-of-art and future aspects. Int. J. Mol. Sci. 2021, 22, 2048. [Google Scholar] [CrossRef]
- Guarner, F.; Khan, A.G.; Garisch, J.; Eliakim, R.; Gangl, A.; Thomson, A.; Krabshuis, J.; Lemair, T.; Kaufmann, P.; De Paula, J.A. World gastroenterology organisation global guidelines: Probiotics and prebiotics october 2011. J. Clin. Gastroenterol. 2012, 46, 468–481. [Google Scholar] [CrossRef]
- Bhalla, P.; Rengaswamy, R.; Karunagaran, D.; Suraishkumar, G.; Sahoo, S. Metabolic modeling of host–microbe interactions for therapeutics in colorectal cancer. NPJ Syst. Biol. Applicat. 2022, 8, 1. [Google Scholar] [CrossRef]
- Ahmadi, S.; Jamilian, M.; Karamali, M.; Tajabadi-Ebrahimi, M.; Jafari, P.; Taghizadeh, M.; Memarzadeh, M.R.; Asemi, Z. Probiotic supplementation and the effects on weight loss, glycaemia and lipid profiles in women with polycystic ovary syndrome: A randomized, double-blind, placebo-controlled trial. Hum. Fertil. 2017, 20, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Rashad, N.M.; Amal, S.; Amin, A.I.; Soliman, M.H. Effects of probiotics supplementation on macrophage migration inhibitory factor and clinical laboratory feature of polycystic ovary syndrome. J. Funct. Foods. 2017, 36, 317–324. [Google Scholar] [CrossRef]
- Askari, G.; Shoaei, T.; Tehrani, H.G.; Heidari-Beni, M.; Feizi, A.; Esmaillzadeh, A. Effects of probiotic supplementation on pancreatic β-cell function and c-reactive protein in women with polycystic ovary syndrome: A randomized double-blind placebo-controlled clinical trial. Int. J. Prev. Med. 2015, 6, 27. [Google Scholar] [CrossRef]
- Heshmati, J.; Farsi, F.; Yosaee, S.; Razavi, M.; Rezaeinejad, M.; Karimie, E.; Sepidarkish, M. The effects of probiotics or synbiotics supplementation in women with polycystic ovarian syndrome: A systematic review and meta-analysis of randomized clinical trials. Probiotics Antimicrob. Proteins 2019, 11, 1236–1247. [Google Scholar] [CrossRef] [PubMed]
- Shamasbi, S.G.; Ghanbari-Homayi, S.; Mirghafourvand, M. The effect of probiotics, prebiotics, and synbiotics on hormonal and inflammatory indices in women with polycystic ovary syndrome: A systematic review and meta-analysis. Eur. J. Nutr. 2020, 59, 433–450. [Google Scholar] [CrossRef]
- Xu, L.-H.; Zhang, F. Meta-analysis of the endocrine and metabolic effects of probiotics on polycystic ovary syndrome. Res. Sq. 2022. [Google Scholar] [CrossRef]
- Tabrizi, R.; Ostadmohammadi, V.; Akbari, M.; Lankarani, K.B.; Vakili, S.; Peymani, P.; Karamali, M.; Kolahdooz, F.; Asemi, Z. The Effects of Probiotic Supplementation on Clinical Symptom, Weight Loss, Glycemic Control, Lipid and Hormonal Profiles, Biomarkers of Inflammation, and Oxidative Stress in Women with Polycystic Ovary Syndrome: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Probiotics Antimicrob Proteins 2022, 14, 1–14. [Google Scholar] [CrossRef]
- Fernandes, R.; do Rosario, V.A.; Mocellin, M.C.; Kuntz, M.G.; Trindade, E.B. Effects of inulin-type fructans, galacto-oligosaccharides and related synbiotics on inflammatory markers in adult patients with overweight or obesity: A systematic review. Clin. Nutr. 2017, 36, 1197–1206. [Google Scholar] [CrossRef]
- Gholizadeh Shamasbi, S.; Dehgan, P.; Mohammad-Alizadeh Charandabi, S.; Aliasgarzadeh, A.; Mirghafourvand, M. The effect of resistant dextrin as a prebiotic on metabolic parameters and androgen level in women with polycystic ovarian syndrome: A randomized, triple-blind, controlled, clinical trial. Eur. J. Nutr. 2019, 58, 629–640. [Google Scholar] [CrossRef]
- Shen, Z.-H.; Zhu, C.-X.; Quan, Y.-S.; Yang, Z.-Y.; Wu, S.; Luo, W.-W.; Tan, B.; Wang, X.-Y. Relationship between intestinal microbiota and ulcerative colitis: Mechanisms and clinical application of probiotics and fecal microbiota transplantation. World J. Gastroenterol. 2018, 24, 5. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Cai, Y. Gut microbiota and obesity: Implications for fecal microbiota transplantation therapy. Hormones 2017, 16, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Qi, Y.; Yang, X.; Zhao, L.; Wen, S.; Liu, Y.; Tang, L. Association between polycystic ovary syndrome and gut microbiota. PLoS ONE 2016, 11, e0153196. [Google Scholar] [CrossRef]
- Quaranta, G.; Sanguinetti, M.; Masucci, L. Fecal microbiota transplantation: A potential tool for treatment of human female reproductive tract diseases. Front. Immunol. 2019, 10, 2653. [Google Scholar] [CrossRef] [PubMed]
- Corrie, L.; Gulati, M.; Vishwas, S.; Kapoor, B.; Singh, S.K.; Awasthi, A.; Khursheed, R. Combination therapy of curcumin and fecal microbiota transplant: Potential treatment of polycystic ovarian syndrome. Med. Hypotheses 2021, 154, 110644. [Google Scholar] [CrossRef]


| Feature | Phenotype A | Phenotype B | Phenotype C | Phenotype D |
|---|---|---|---|---|
| Biochemical/clinical hyperandrogenism | + | + | + | − |
| Chronic anovulation | + | + | − | + |
| Polycystic ovaries | + | − | + | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, S.; Pal, N.; Shubham, S.; Sarma, D.K.; Verma, V.; Marotta, F.; Kumar, M. Polycystic Ovary Syndrome: Etiology, Current Management, and Future Therapeutics. J. Clin. Med. 2023, 12, 1454. https://doi.org/10.3390/jcm12041454
Singh S, Pal N, Shubham S, Sarma DK, Verma V, Marotta F, Kumar M. Polycystic Ovary Syndrome: Etiology, Current Management, and Future Therapeutics. Journal of Clinical Medicine. 2023; 12(4):1454. https://doi.org/10.3390/jcm12041454
Chicago/Turabian StyleSingh, Samradhi, Namrata Pal, Swasti Shubham, Devojit Kumar Sarma, Vinod Verma, Francesco Marotta, and Manoj Kumar. 2023. "Polycystic Ovary Syndrome: Etiology, Current Management, and Future Therapeutics" Journal of Clinical Medicine 12, no. 4: 1454. https://doi.org/10.3390/jcm12041454
APA StyleSingh, S., Pal, N., Shubham, S., Sarma, D. K., Verma, V., Marotta, F., & Kumar, M. (2023). Polycystic Ovary Syndrome: Etiology, Current Management, and Future Therapeutics. Journal of Clinical Medicine, 12(4), 1454. https://doi.org/10.3390/jcm12041454







