Novel 3D Echocardiographic Technique for Mitral Calcium Mapping
Abstract
:1. Introduction
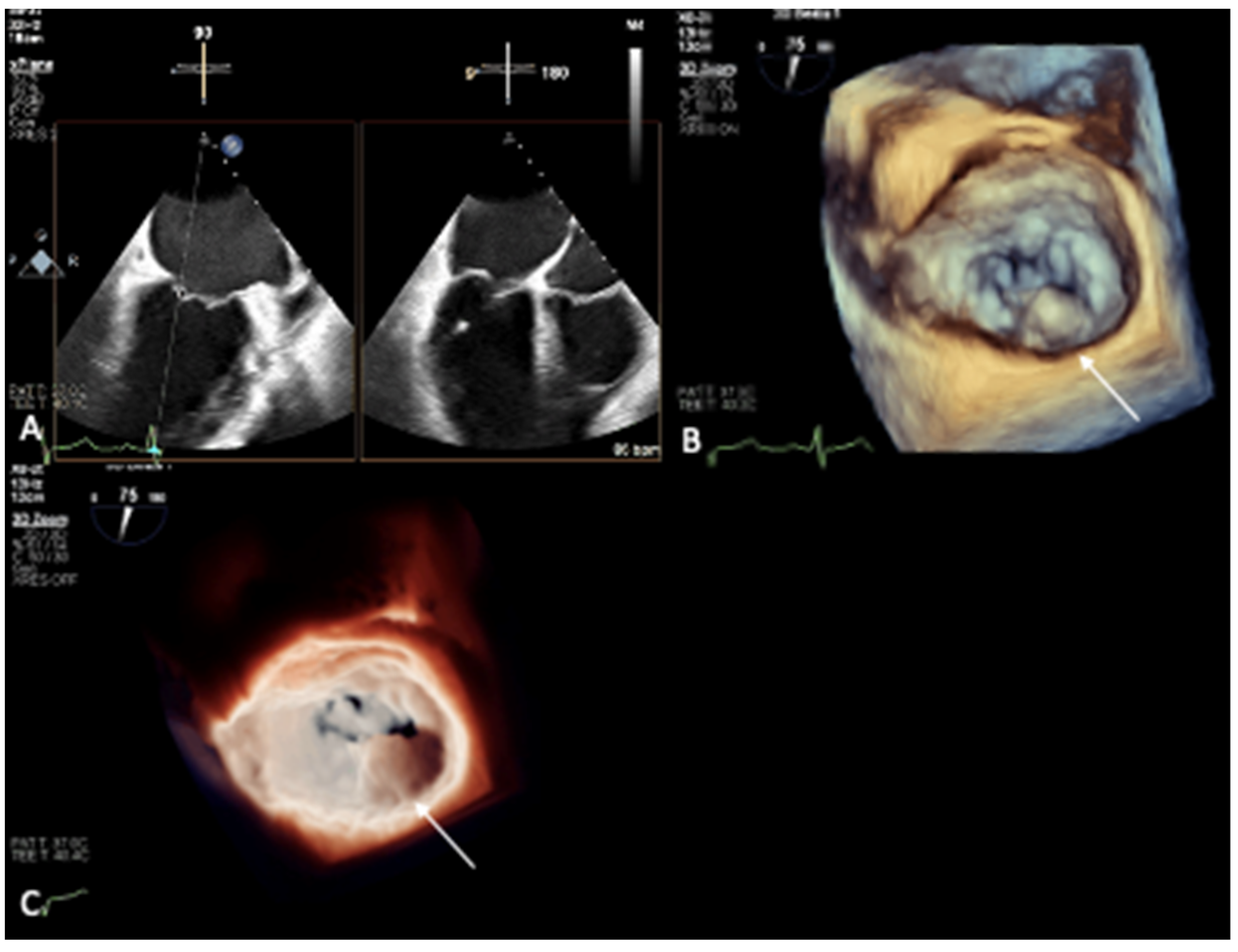
2. Mitral Annular Calcification Imaging
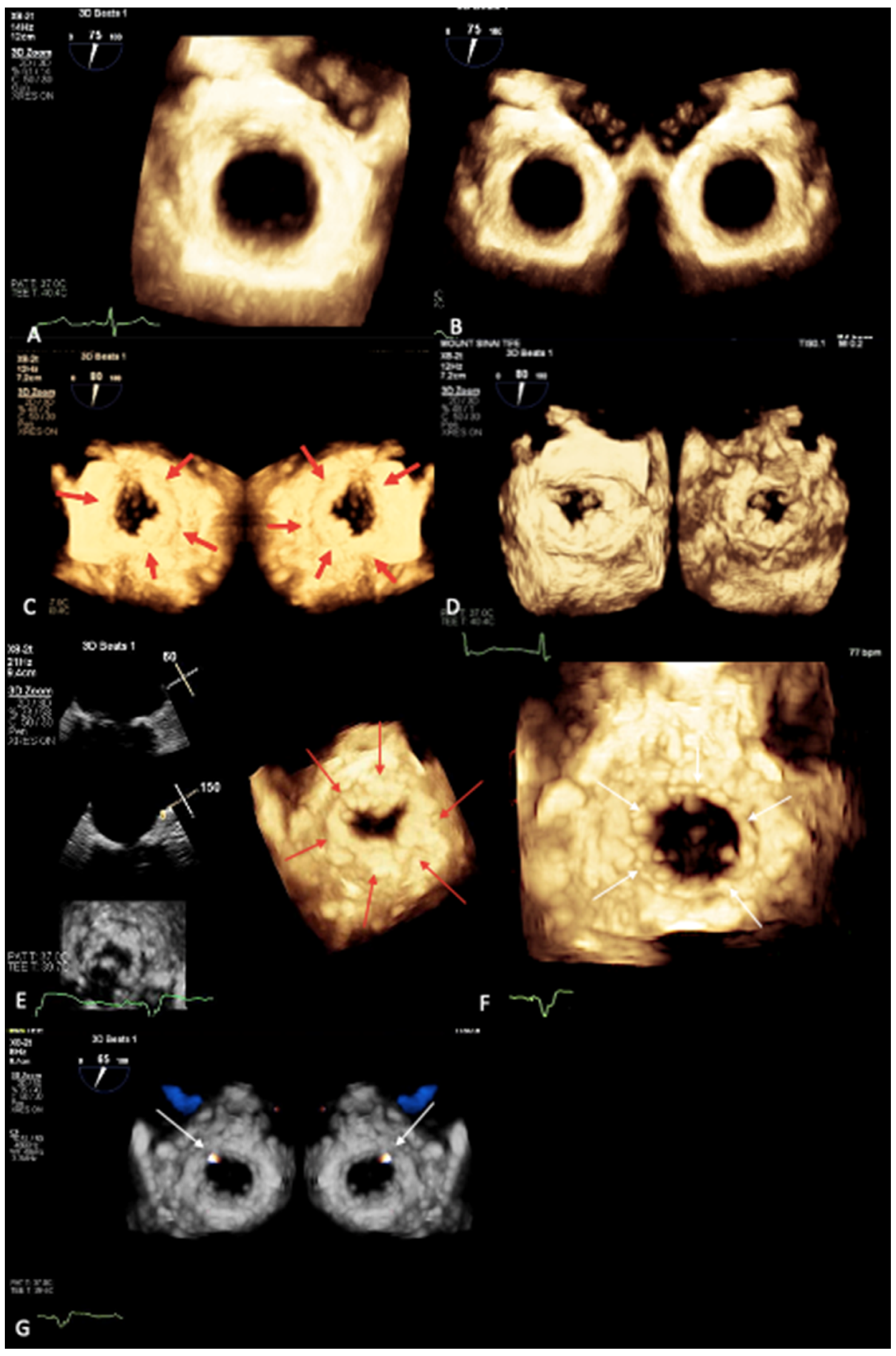
3. Novel 3D Echocardiographic Technique for MAC
4. Maximal Intensity Projection Map Applications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kanjanauthai, S.; Nasir, K.; Katz, R.; Rivera, J.J.; Takasu, J.; Blumenthal, R.S.; Eng, J.; Budoff, M.J. Relationships of mitral annular calcification to cardiovascular risk factors: The Multi-Ethnic Study of Atherosclerosis (MESA). Atherosclerosis 2010, 213, 558–562. [Google Scholar] [CrossRef] [PubMed]
- Foley, P.W.; Hamaad, A.; El-Gendi, H.; Leyva, F. Incidental cardiac findings on computed tomography imaging of the thorax. BMC Res. Notes 2010, 3, 326. [Google Scholar] [CrossRef] [PubMed]
- Simon, M.A.; Liu, S.F. Calcification of the mitral valve annulus and its relation to functional valvular disturbance. Am. Heart J. 1954, 48, 497. [Google Scholar] [CrossRef] [PubMed]
- Barasch, E.; Gottdiener, J.S.; Larsen, E.K.; Chaves, P.H.; Newman, A.B.; Manolio, T.A. Clinical significance of calcification of the fibrous skeleton of the heart and aortosclerosis in community dwelling elderly. The Cardiovascular Health Study (CHS). Am. Heart J. 2006, 151, 39–47. [Google Scholar] [CrossRef]
- Eleid, M.F.; Foley, T.A.; Said, S.M.; Pislaru, S.V.; Rihal, C.S. Severe Mitral Annular Calcification: Multimodality Imaging for Therapeutic Strategies and Interventions. JACC Cardiovasc. Imaging 2016, 9, 1318–1337. [Google Scholar] [CrossRef] [PubMed]
- Massera, D.; Kizer, J.R.; Dweck, M.R. Mechanisms of mitral annular calcification. Trends Cardiovasc. Med. 2020, 30, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Abramowitz, H.Y.; Jilaihawi, T.; Chakravarty, M.J.; Mack, R.; Makkar, R. Mitral annulus calcification. J. Am. Coll. Cardiol. 2015, 66, 1934–1941. [Google Scholar] [CrossRef] [PubMed]
- Carpentier, A.F.; Pellerin, M.; Fuzellier, J.F.; Relland, J.Y. Extensive calcification of the mitral valve anulus: Pathology and surgical management. J. Thorac. Cardiovasc. Surg. 1996, 111, 718, discussion 729–730. [Google Scholar] [CrossRef]
- Karan, A.; Feghaly, J.; Guo, H.J.; Akinjogbin, T.O.; Sattiraju, S. A Case of Multiple Calcific Embolic Strokes in a Patient With a Porcelain Left Atrium. Cureus 2021, 13, e18585. [Google Scholar] [CrossRef]
- Kim, C.Y.D.; Shim, G.; Hong, H.; Jeong, J.H. Morphological and functional characteristics of mitral annular calcification and their relationship to stroke. PLoS ONE 2020, 15, e0227753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burnside, J.W.; Desanctis, R.W. Bacterial endocarditis on calcification of the mitral anulus fibrosus. Ann. Intern. Med. 1972, 76, 615–618. [Google Scholar] [CrossRef]
- Fox, C.S.; Vasan, R.S.; Parise, H.; Levy, D.; O’Donnell, C.J.; D’Agostino, R.B.; Benjamin, E.J.; Framingham Heart Study. Mitral annular calcification predicts cardiovascular morbidity and mortality: The Framingham Heart Study. Circulation 2003, 107, 1492–1496. [Google Scholar] [CrossRef] [PubMed]
- Adler, Y.; Herz, I.; Vaturi, M.; Fusman, R.; Shohat-Zabarski, R.; Fink, N.; Porter, A.; Shapira, Y.; Assali, A.; Sagie, A. Mitral annular calcium detected by transthoracic echocardiography is a marker for high prevalence and severity of coronary artery disease in patients undergoing coronary angiography. Am. J. Cardiol. 1998, 82, 1183–1186. [Google Scholar] [CrossRef]
- Gardin, J.M.; McClelland, R.; Kitzman, D.; Lima, J.A.; Bommer, W.; Klopfenstein, H.; Wong, N.; Smith, V.-E.; Gottdiener, J. M-mode echocardiographic predictors of six- to seven-year incidence of coronary heart disease, stroke, congestive heart failure, and mortality in an elderly cohort (the Cardiovascular Health Study). Am. J. Cardiol. 2001, 87, 1051–1057. [Google Scholar] [CrossRef]
- Benjamin, E.J.; Plehn, J.F.; D’Agostino, R.B.; Belanger, A.J.; Comai, K.; Fuller, D.L.; Wolf, A.; Levy, D. Mitral annular calcification and the risk of stroke in an elderly cohort. N. Engl. J. Med. 1992, 327, 374–379. [Google Scholar] [CrossRef] [PubMed]
- Takamoto, T.; Popp, R.L. Conduction disturbances related to the site and severity of mitral anular calcification: A 2-dimensional echocardiographic and electrocardiographic correlative study. Am. J. Cardiol. 1983, 51, 1644. [Google Scholar] [CrossRef] [PubMed]
- O’Neal, W.T.; Efird, J.T.; Nazarian, S.; Alonso, A.; Michos, E.D.; Szklo, M.; Heckbert, S.R.; Soliman, E.Z. Mitral annular calcification progression and the risk of atrial fibrillation: Results from MESA. Eur. Heart J. Cardiovasc. Imaging 2018, 19, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Fusini, L.; Ali, S.G.; Tamborini, G.; Muratori, M.; Gripari, P.; Maffessanti, F.; Celeste, F.; Guglielmo, M.; Cefalù, C.; Alamanni, F.; et al. Prevalence of calcification of the mitral valve annulus in patients undergoing surgical repair of mitral valve prolapse. Am. J. Cardiol. 2014, 113, 1867–1873. [Google Scholar] [CrossRef] [PubMed]
- Sud, K.; Agarwal, S.; Parashar, A.; Raza, M.Q.; Patel, K.; Min, D.; Rodriguez, L.L.; Krishnaswamy, A.; Mick, S.L.; Gillinov, A.M.; et al. Degenerative mitral stenosis: Unmet need for percutaneous interventions. Circulation 2016, 133, 1594–1604. [Google Scholar] [CrossRef]
- Okada, Y. Surgical management of mitral annular calcification. Gen. Thorac. Cardiovasc. Surg. 2013, 61, 619. [Google Scholar] [CrossRef] [PubMed]
- Fukui, M.; Cavalcante, J.L.; Ahmed, A.; Bae, R.; Bapat, V.N.; Gössl, M.; Garcia, S.; Enriquez-Sarano, M.; Sorajja, P. Clinical Outcomes of Mitral Valve Disease With Mitral Annular Calcification. Am. J. Cardiol. 2022, 174, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Alexis, S.L.; Malik, A.H.; El-Eshmawi, A.; George, I.; Sengupta, A.; Kodali, S.K.; Hahn, R.T.; Khalique, O.K.; Zaid, S.; Guerrero, M.; et al. Surgical and Transcatheter Mitral Valve Replacement in Mitral Annular Calcification: A Systematic Review. J. Am. Heart Assoc. 2021, 10, e018514. [Google Scholar] [CrossRef]
- Cheng, R.; Tat, E.; Siegel, R.J.; Arsanjani, R.; Hussaini, A.; Makar, M.; Mizutani, Y.; Trento, A.; Kar, S. Mitral annular calcification is not associated with decreased procedural success, durability of repair, or left ventricular remodelling in percutaneous edge-to-edge repair of mitral regurgitation. EuroIntervention 2016, 12, 1176–1184. [Google Scholar] [CrossRef] [PubMed]
- Mauri, L.; Garg, P.; Massaro, J.M.; Foster, E.; Glower, D.; Mehoudar, P.; Powell, F.; Komtebedde, J.; McDermott, E.; Feldman, T. The EVEREST II Trial: Design and rationale for a randomized study of the evalve mitraclip system compared with mitral valve surgery for mitral regurgitation. Am. Heart J. 2010, 160, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Thaden, J.J.; Malouf, J.F.; Nkomo, V.T.; Pislaru, S.V.; Holmes, D.R.; Reeder, G.S.; Rihal, C.S.; Eleid, M.F. Mitral Valve Anatomic Predictors of Hemodynamic Success With Transcatheter Mitral Valve Repair. J. Am. Heart Assoc. 2018, 7, e007315. [Google Scholar] [CrossRef] [PubMed]
- Abramowitz, Y.; Kazuno, Y.; Chakravarty, T.; Kawamori, H.; Maeno, Y.; Anderson, D.; Allison, Z.; Mangat, G.; Cheng, W.; Gopal, A.; et al. Concomitant mitral annular calcification and severe aortic stenosis: Prevalence, characteristics and outcome following transcatheter aortic valve replacement. Eur. Heart J. 2017, 38, 1194–1203. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Kocyigit, D.; Wang, T.K.M.; Tan, C.; Rodriguez, R.; Pettersson, G.; Unai, S.; Griffin, B. Mitral annular calcification and valvular dysfunction: Multimodality imaging evaluation, grading, and management. Eur. Heart J. -Cardiovasc. Imaging 2021, 1, jeab185. [Google Scholar] [CrossRef] [PubMed]
- Kohsaka, S.; Jin, Z.; Rundek, T.; Boden-Albala, B.; Monna, S.; Sacco, R.; Di Tullio, M.R. Impact of mitral annular calcification on cardiovascular events in a multiethnic community: The Northern Manhattan Study. JACC Cardiovasc. Imaging 2008, 1, 617–623. [Google Scholar] [CrossRef] [PubMed]
- Elgendy, I.; Conti, C.R. Caseous calcification of the mitral annulus: A review. Clin. Cardiol. 2013, 36, E27–E31. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.M.; Addetia, K.; Narang, A.; Mor-Avi, V. 3-Dimensional Echocardiography: Latest Developments and Future Directions. JACC Cardiovasc. Imaging 2018, 11, 1854–1878. [Google Scholar] [CrossRef]
- Yang, H.S.; Bansal, R.C.; Mookadam, F.; Khandheria, B.K.; Tajik, A.J.; Chandrasekaran, K.; American Society of Echocardiography. Practical guide for three-dimensional transthoracic echocardiography using a fully sampled matrix array transducer. J. Am. Soc. Echocardiogr. 2008, 21, 979–989, quiz 1081–1082. [Google Scholar] [CrossRef] [PubMed]
- Prandi, F.R.; Anastasius, M.O.; Zhang, L.; Tang, G.H.; Moreno, P.R.; Romeo, F.; Barillà, F.; Sharma, S.; Kini, A.; Lerakis, S. Novel Three-Dimensional Transesophageal Echocardiographic Method for Mapping Mitral Annular Calcifications. J. Am. Soc. Echocardiogr. 2022, 35, 1004–1005. [Google Scholar] [CrossRef] [PubMed]
- Prandi, F.R.; Dangas, G.D.; Kini, A.; Romeo, F.; Suleman, S.; Khera, S.; Tang, G.H.; Sharma, S.; Lerakis, S. Intraprocedural Mapping of the Mitral Calcium for Positioning and Deployment of Transcatheter Valve-in-Mitral Annular Calcification. JACC Cardiovasc. Interv. 2022, 15, 2341–2343. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prandi, F.R.; Romeo, F.; Barillà, F.; Sharma, S.; Kini, A.; Lerakis, S. Novel 3D Echocardiographic Technique for Mitral Calcium Mapping. J. Clin. Med. 2023, 12, 1470. https://doi.org/10.3390/jcm12041470
Prandi FR, Romeo F, Barillà F, Sharma S, Kini A, Lerakis S. Novel 3D Echocardiographic Technique for Mitral Calcium Mapping. Journal of Clinical Medicine. 2023; 12(4):1470. https://doi.org/10.3390/jcm12041470
Chicago/Turabian StylePrandi, Francesca Romana, Francesco Romeo, Francesco Barillà, Samin Sharma, Annapoorna Kini, and Stamatios Lerakis. 2023. "Novel 3D Echocardiographic Technique for Mitral Calcium Mapping" Journal of Clinical Medicine 12, no. 4: 1470. https://doi.org/10.3390/jcm12041470




