Severe and Very Severe Myalgic Encephalopathy/Chronic Fatigue Syndrome ME/CFS in Norway: Symptom Burden and Access to Care
Abstract
:1. Introduction
2. Materials and Methods
3. Results
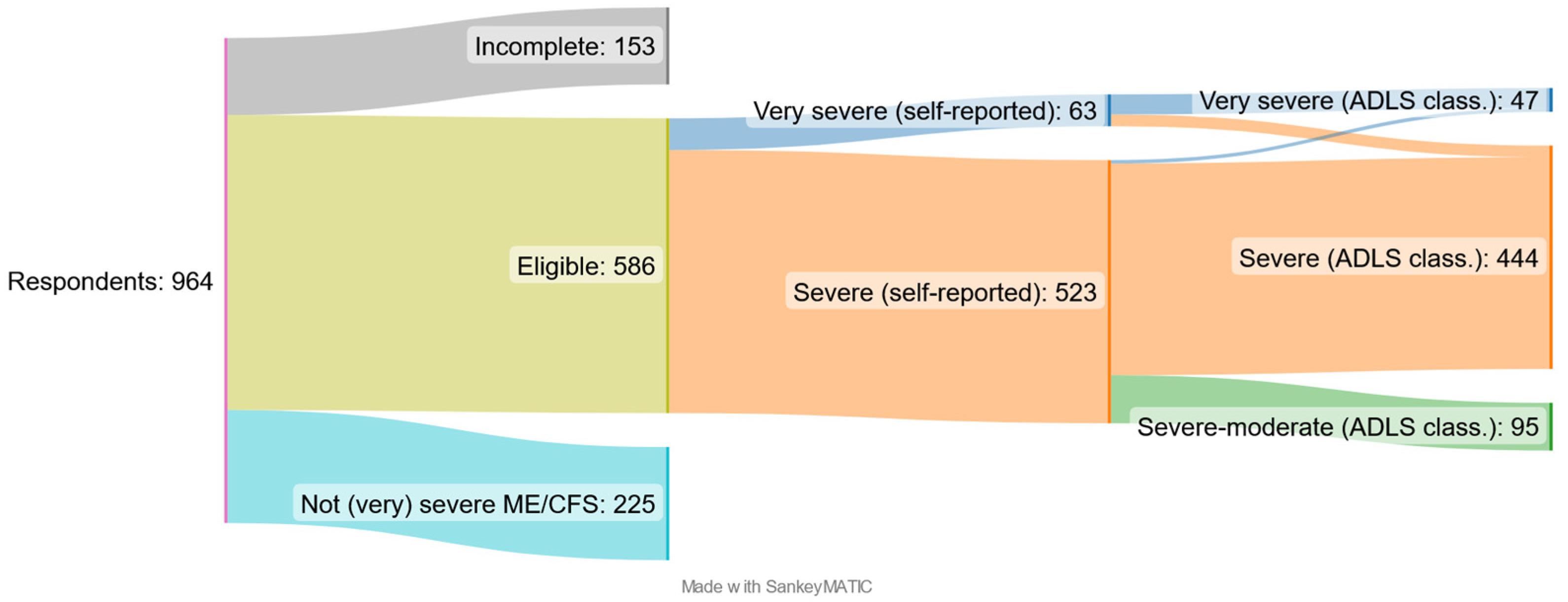
3.1. Description of the Sample
3.2. Symptom Burden
3.3. Healthcare, Carers, and Family Situation
4. Discussion
4.1. The Patients and Their Disease Burden
4.2. Support from Healthcare System and Family Carers
4.3. Strengths and Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Committee on the Diagnostic Criteria for Myalgic Encephalomyelitis/Chronic Fatigue Syndrome; Board on the Health of Select Populations; Institute of Medicine. The National Academies Collection: Reports funded by National Institutes of Health. In Beyond Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: Redefining an Illness; National Academies Press (US): Washington, DC, USA, 2015. [Google Scholar]
- Lim, E.J.; Ahn, Y.C.; Jang, E.S.; Lee, S.W.; Lee, S.H.; Son, C.G. Systematic review and meta-analysis of the prevalence of chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME). J. Transl. Med. 2020, 18, 100. [Google Scholar] [CrossRef] [PubMed]
- Valdez, A.R.; Hancock, E.E.; Adebayo, S.; Kiernicki, D.J.; Proskauer, D.; Attewell, J.R.; Bateman, L.; DeMaria, A., Jr.; Lapp, C.W.; Rowe, P.C.; et al. Estimating Prevalence, Demographics, and Costs of ME/CFS Using Large Scale Medical Claims Data and Machine Learning. Front Pediatr. 2018, 6, 412. [Google Scholar] [CrossRef] [PubMed]
- Carruthers, B.M.; Jain, A.K.; De Meirleir, K.L.; Peterson, D.L.; Klimas, N.G.; Lerner, A.M.; Bested, A.C.; Flor-Henry, P.; Joshi, P.; Powles, A.C.P.; et al. Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. J. Chronic Fatigue Syndr. 2003, 11, 7–115. [Google Scholar] [CrossRef]
- Fukuda, K.; Straus, S.E.; Hickie, I.; Sharpe, M.C.; Dobbins, J.G.; Komaroff, A. The chronic fatigue syndrome: A comprehensive approach to its definition and study. International Chronic Fatigue Syndrome Study Group. Ann. Intern Med. 1994, 121, 953–959. [Google Scholar] [CrossRef] [PubMed]
- Jason, L.A.; Jordan, K.; Miike, T.; Bell, D.S.; Lapp, C.; Torres-Harding, S.; Rowe, K.; Gurwitt, A.; De Meirleir, K.; Van Hoof, E.L.S. A Pediatric Case Definition for Myalgic Encephalomyelitis and Chronic Fatigue Syndrome. J. Chronic Fatigue Syndr. 2006, 13, 1–44. [Google Scholar] [CrossRef]
- Crowhurst, G. Supporting people with severe myalgic encephalomyelitis. Nurs. Stand. 2005, 19, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Harris, K.; Band, R.J.; Cooper, H.; Macintyre, V.G.; Mejia, A.; Wearden, A.J. Distress in significant others of patients with chronic fatigue syndrome: A systematic review of the literature. Br. J. Health Psychol. 2016, 21, 881–893. [Google Scholar] [CrossRef] [PubMed]
- Carruthers, B.M.; van de Sande, M.I.; De Meirleir, K.L.; Klimas, N.G.; Broderick, G.; Mitchell, T.; Staines, D.; Powles, A.C.; Speight, N.; Vallings, R.; et al. Myalgic encephalomyelitis: International Consensus Criteria. J. Intern. Med. 2011, 270, 327–338. [Google Scholar] [CrossRef]
- Ghali, A.; Lacout, C.; Fortrat, J.O.; Depres, K.; Ghali, M.; Lavigne, C. Factors Influencing the Prognosis of Patients with Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. Diagnostics 2022, 12, 2540. [Google Scholar] [CrossRef]
- Conroy, K.; Bhatia, S.; Islam, M.; Jason, L.A. Homebound versus Bedridden Status among Those with Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. Healthcare 2021, 9, 106. [Google Scholar] [CrossRef]
- Bakken, I.J.; Tveito, K.; Gunnes, N.; Ghaderi, S.; Stoltenberg, C.; Trogstad, L.; Håberg, S.E.; Magnus, P. Two age peaks in the incidence of chronic fatigue syndrome/myalgic encephalomyelitis: A population-based registry study from Norway 2008–2012. BMC Med. 2014, 12, 167. [Google Scholar] [CrossRef]
- Kingdon, C.; Giotas, D.; Nacul, L.; Lacerda, E. Health Care Responsibility and Compassion-Visiting the Housebound Patient Severely Affected by ME/CFS. Healthcare 2020, 8, 197. [Google Scholar] [CrossRef]
- Boulazreg, S.; Rokach, A. The Lonely, Isolating, and Alienating Implications of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. Healthcare 2020, 8, 413. [Google Scholar] [CrossRef]
- Strassheim, V.; Newton, J.L.; Collins, T. Experiences of Living with Severe Chronic Fatigue Syndrome/Myalgic Encephalomyelitis. Healthcare 2021, 9, 168. [Google Scholar] [CrossRef]
- Dafoe, W. Extremely Severe ME/CFS-A Personal Account. Healthcare 2021, 9, 504. [Google Scholar] [CrossRef] [PubMed]
- Fennell, P.A.; Dorr, N.; George, S.S. Elements of Suffering in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: The Experience of Loss, Grief, Stigma, and Trauma in the Severely and Very Severely Affected. Healthcare 2021, 9, 553. [Google Scholar] [CrossRef] [PubMed]
- Chu, L.; Elliott, M.; Stein, E.; Jason, L.A. Identifying and Managing Suicidality in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. Healthcare 2021, 9, 629. [Google Scholar] [CrossRef]
- Komaroff, A.L. Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: When Suffering Is Multiplied. Healthcare 2021, 9, 919. [Google Scholar] [CrossRef]
- Chang, C.J.; Hung, L.Y.; Kogelnik, A.M.; Kaufman, D.; Aiyar, R.S.; Chu, A.M.; Wilhelmy, J.; Li, P.; Tannenbaum, L.; Xiao, W.; et al. A Comprehensive Examination of Severely Ill ME/CFS Patients. Healthcare 2021, 9, 1290. [Google Scholar] [CrossRef] [PubMed]
- Jason, L.A.; Katz, B.Z.; Sunnquist, M.; Torres, C.; Cotler, J.; Bhatia, S. The Prevalence of Pediatric Myalgic Encephalomyelitis/Chronic Fatigue Syndrome in a Community-Based Sample. Child Youth Care Forum 2020, 49, 563–579. [Google Scholar] [CrossRef]
- Montoya, J.G.; Dowell, T.G.; Mooney, A.E.; Dimmock, M.E.; Chu, L. Caring for the Patient with Severe or Very Severe Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. Healthcare 2021, 9, 1331. [Google Scholar] [CrossRef] [PubMed]








| Severity-ME/CFS | International ConsensusCriteria (ICC) | Norwegian National Guidelines for CFS/ME (Based on the ICC Criteria) |
|---|---|---|
| Mild | An approximate 50% reduction in pre-illness activity level | Activity level reduced by at least 50% compared to before illness onset, i.e., one is self-reliant, can for example manage light housework, and some may be able have a job, but this often results in a lack of capacity for leisure and social activities, and need of days of rest and weekends to recuperate |
| Moderate | Mostly housebound | Mostly housebound, i.e., all activities are strongly reduced, and it is often necessary with some hours of daytime sleep |
| Severe | Mostly bedridden | In bed most of the day, and most patients lie on a bed or sofa and are only able to perform light activities such as brushing their teeth and eating. Many have serious cognitive problems and are often wheelchair-dependent |
| Very severe | Totally bedridden and need help with basic functions). There may be marked fluctuation of symptom severity and hierarchy from day to day or hour to hour. Consider activity, context, and interactive effects | In bed all day and dependent on care, will need help with personal hygiene and food intake, and are very sensitive to sensory stimuli. Some patients may not be able to swallow and will need to be tube-fed |
| Activity | Weight (Energy Use) |
|---|---|
| Leave the house | 4 |
| Go for a short walk | 5 |
| Have visitors or visit | 3 |
| Communicate online | 1 |
| Have a conversation > 5 min | 1 |
| Say a few words | 1 |
| Cook a simple meal | 3 |
| Eat without assistance | 1 |
| Get out of bed, get dressed | 2 |
| Sit up in bed | 1 |
| Turn myself over in bed | 1 |
| Basic personal hygiene | 2 |
| Shower | 3 |
| Wash my hair | 2 |
| Use the toilet | 1 |
| Very Severe (n = 47) | Severe (n = 444) | Severe-Moderate (n = 95) | Total (n = 586) * | |
|---|---|---|---|---|
| Female | 41 (87) | 391 (88) | 83 (87) | 515 (88) |
| Age 0–19 years | 9 (19) | 52 (12) | 5 (5) | 66 (12) |
| Age 20–39 years | 23 (49) | 186 (42) | 42 (44) | 232 (43) |
| Age 40+ years | 15 (32) | 206 (46) | 48 (51) | 247 (45) |
| Onset 0–15 years | 20 (43) | 143 (33) | 21 (22) | 184 (32) |
| Onset 16–29 years | 12 (26) | 137 (31) | 30 (32) | 179 (31) |
| Onset 30+ years | 15 (32) | 159 (36) | 43 (46) | 217 (37) |
| Duration 0–5 years | 10 (21) | 97 (22) | 22 (23) | 129 (22) |
| Duration 6–15 years | 28 (60) | 225 (51) | 45 (48) | 298 (51) |
| Duration 16+ years | 9 (19) | 117 (27) | 27 (29) | 153 (27) |
| Live alone | 8 (17) ** | 111 (25) | 28 (29) | 147 (25) |
| Live w/parents | 21 (45) | 111 (25) | 12 (13) | 144 (25) |
| Live w/partner/spouse | 8 (17) | 177 (40) | 45 (47) | 230 (39) |
| Live other arrangements *** | 4 (8) | 45 (10) | 10 (11) | 59 (10) |
| Live in institution | 6 (13) | 0 (0) | 0 (0) | 6 (1) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sommerfelt, K.; Schei, T.; Angelsen, A. Severe and Very Severe Myalgic Encephalopathy/Chronic Fatigue Syndrome ME/CFS in Norway: Symptom Burden and Access to Care. J. Clin. Med. 2023, 12, 1487. https://doi.org/10.3390/jcm12041487
Sommerfelt K, Schei T, Angelsen A. Severe and Very Severe Myalgic Encephalopathy/Chronic Fatigue Syndrome ME/CFS in Norway: Symptom Burden and Access to Care. Journal of Clinical Medicine. 2023; 12(4):1487. https://doi.org/10.3390/jcm12041487
Chicago/Turabian StyleSommerfelt, Kristian, Trude Schei, and Arild Angelsen. 2023. "Severe and Very Severe Myalgic Encephalopathy/Chronic Fatigue Syndrome ME/CFS in Norway: Symptom Burden and Access to Care" Journal of Clinical Medicine 12, no. 4: 1487. https://doi.org/10.3390/jcm12041487
APA StyleSommerfelt, K., Schei, T., & Angelsen, A. (2023). Severe and Very Severe Myalgic Encephalopathy/Chronic Fatigue Syndrome ME/CFS in Norway: Symptom Burden and Access to Care. Journal of Clinical Medicine, 12(4), 1487. https://doi.org/10.3390/jcm12041487






