Baseline Characteristics of Patients Enrolled in Clinical Trials of Biologics for Severe Asthma as Potential Predictors of Outcomes
Abstract
:1. Introduction
2. Asthma Phenotyping for Correct Patient Selection
3. Results of Major Clinical Trials
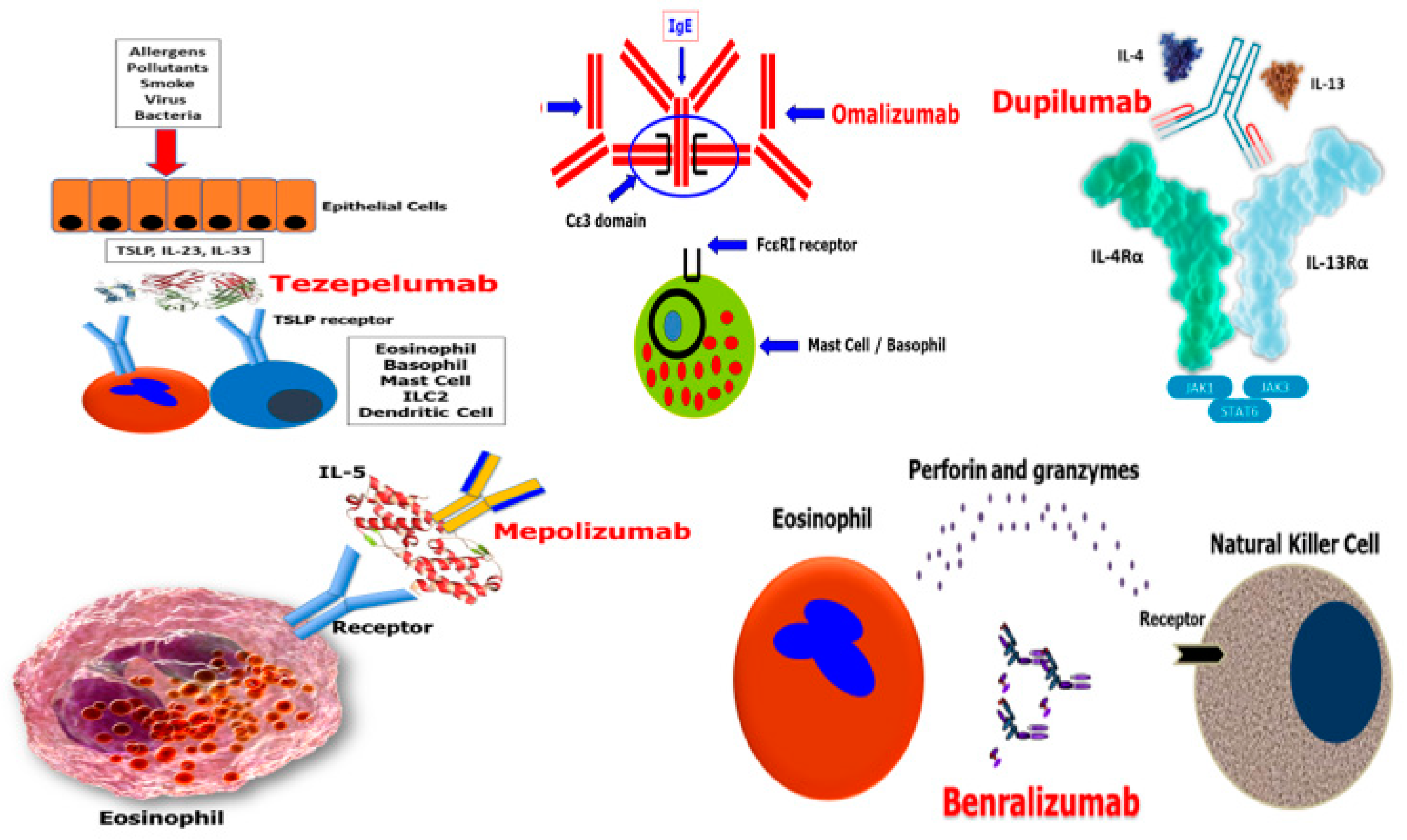
3.1. Omalizumab
3.2. Mepolizumab
3.3. Benralizumab
3.4. Dupilumab
3.5. Tezepelumab
4. Overview of Clinical Trials According to Patient Characteristics
4.1. Biomarkers
4.2. Disease Severity, OCS Use, and Respiratory Function
4.3. Comorbidities
5. Conclusions
6. Future Directions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wenzel, S.E. Asthma phenotypes: The evolution from clinical to molecular approaches. Nat. Med. 2012, 18, 716–725. [Google Scholar] [CrossRef] [PubMed]
- Woodruff, P.G.; Modrek, B.; Choy, D.F.; Jia, G.; Abbas, A.R.; Ellwanger, A.; Koth, L.L.; Arron, J.R.; Fahy, J.V. Thelper type 2-driven inflammation defines major subphenotypes of asthma. Am. J. Respir. Crit. Care. Med. 2009, 180, 388–395. [Google Scholar] [CrossRef] [Green Version]
- Menzella, F.; Ballarin, A.; Sartor, M.; Floriani, A.F.; Corsi, L.; Dartora, C.; Tonin, S.; Romagnoli, M. Comparison between clinical trials and real-world evidence studies on biologics for severe asthma. J. Int. Med. Res. 2022, 50, 3000605221133689. [Google Scholar] [CrossRef] [PubMed]
- Feist, J.; Lipari, M.; Kale-Pradhan, P. Tezepelumab in the Treatment of Uncontrolled Severe Asthma. Ann. Pharmacother. 2023, 57, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Akenroye, A.; Lassiter, G.; Jackson, J.W.; Keet, C.; Segal, J.; Alexander, G.C.; Hong, H. Comparative efficacy of mepolizumab, benralizumab, and dupilumab in eosinophilic asthma: A Bayesian network meta-analysis. J. Allergy Clin. Immunol. 2022, 150, 1097–1105.e12. [Google Scholar] [CrossRef]
- Bourdin, A.; Husereau, D.; Molinari, N.; Golam, S.; Siddiqui, M.K.; Lindner, L.; Xu, X. Matching-adjusted indirect comparison of benralizumab versus interleukin-5 inhibitors for the treatment of severe asthma: A systematic review. Eur. Respir. J. 2018, 52, 1801393. [Google Scholar] [CrossRef] [Green Version]
- Nopsopon, T.; Lassiter, G.; Chen, M.L.; Alexander, G.C.; Keet, C.; Hong, H.; Akenroye, A. Comparative efficacy of tezepelumab to mepolizumab, benralizumab, and dupilumab in eosinophilic asthma: A Bayesian network meta-analysis. J. Allergy Clin. Immunol. 2022, in press. [Google Scholar] [CrossRef]
- Tran, T.N.; Zeiger, R.S.; Peters, S.P.; Colice, G.; Newbold, P.; Goldman, M.; Chipps, B.E. Overlap of atopic, eosinophilic, and TH2-high asthma phenotypes in a general population with current asthma. Ann. Allergy Asthma Immunol. 2016, 116, 37–42. [Google Scholar] [CrossRef]
- Albers, F.C.; Müllerová, H.; Gunsoy, N.B.; Shin, J.Y.; Nelsen, L.M.; Bradford, E.S.; Cockle, S.M.; Suruki, R.Y. Biologic treatment eligibility for real-world patients with severe asthma: The IDEAL study. J. Asthma 2018, 55, 152–160. [Google Scholar] [CrossRef] [Green Version]
- Kavanagh, J.E.; Hearn, A.P.; Jackson, D.J. A pragmatic guide to choosing biologic therapies in severe asthma. Breathe 2021, 17, 210144. [Google Scholar] [CrossRef]
- Oishi, K.; Matsunaga, K. Three-step algorithm for biological therapy targeted IgE and IL-5 in severe asthma. Immun. Inflamm. Dis. 2018, 6, 374–376. [Google Scholar] [CrossRef] [PubMed]
- Pérez de Llano, L.; Dávila, I.; Martínez-Moragón, E.; Domínguez-Ortega, J.; Almonacid, C.; Colás, C.; García-Rivero, J.L.; Carmona, L.; García de Yébenes, M.J.; Cosío, B.G. Development of a Tool to Measure the Clinical Response to Biologic Therapy in Uncontrolled Severe Asthma: The FEV1, Exacerbations, Oral Corticosteroids, Symptoms Score. J. Allergy Clin. Immunol. Pract. 2021, 9, 2725–2731. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Quoc, Q.L.; Park, H.S. Biomarkers for severe asthma: Lessons from longitudinal cohort studies. Allergy Asthma Immunol. Res. 2021, 13, 375–389. [Google Scholar] [CrossRef] [PubMed]
- Porsbjerg, C.M.; Menzies-Gow, A.N.; Tran, T.N.; Murray, R.B.; Unni, B.; Audrey Ang, S.L.; Alacqua, M.; Al-Ahmad, M.; Al-Lehebi, R.; Altraja, A.; et al. Global Variability in Administrative Approval Prescription Criteria for Biologic Therapy in Severe Asthma. J. Allergy Clin. Immunol. Pract. 2022, 10, 1202–1216.e23. [Google Scholar] [CrossRef] [PubMed]
- Belliveau, P.P. Omalizumab: A monoclonal anti-IgE antibody. MedGenMed 2005, 7, 27. [Google Scholar] [PubMed]
- Incorvaia, C.; Mauro, M.; Makri, E.; Leo, G.; Ridolo, E. Two decades with omalizumab: What we still have to learn. Biologics 2018, 12, 135–142. [Google Scholar] [CrossRef] [Green Version]
- He, L.; Yi, W.; Huang, X.; Lu, Q. Chronic Urticaria: Advances in Understanding of the Disease and Clinical Management. Clin. Rev. Allergy Immunol. 2021, 61, 424–448. [Google Scholar] [CrossRef]
- Gevaert, P.; Omachi, T.A.; Corren, J.; Mullol, J.; Han, J.; Lee, S.E.; Kaufman, D.; Ligueros-Saylan, M.; Howard, M.; Zhu, R.; et al. Efficacy and safety of omalizumab in nasal polyposis: 2 randomized phase 3 trials. J. Allergy Clin. Immunol. 2020, 146, 595–605. [Google Scholar] [CrossRef]
- Nagase, H.; Suzukawa, M.; Oishi, K.; Matsunaga, K. Biologics for severe asthma: The real-world evidence, effectiveness of switching, and prediction factors for the efficacy. Allergol. Int. 2022, 72, 11–23. [Google Scholar] [CrossRef]
- Solèr, M.; Matz, J.; Townley, R.; Buhl, R.; O’Brien, J.; Fox, H.; Thirlwell, J.; Gupta, N.; Della Cioppa, G. The anti-IgE antibody omalizumab reduces exacerbations and steroid requirement in allergic asthmatics. Eur. Respir. J. 2001, 18, 254–261. [Google Scholar] [CrossRef]
- Busse, W.; Corren, J.; Lanier, B.Q.; McAlary, M.; Fowler-Taylor, A.; Cioppa, G.D.; van As, A.; Gupta, N. Omalizumab, anti-IgE recombinant humanized monoclonal antibody, for the treatment of severe allergic asthma. J. Allergy Clin. Immunol. 2001, 108, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Humbert, M.; Beasley, R.; Ayres, J.; Slavin, R.; Hébert, J.; Bousquet, J.; Beeh, K.M.; Ramos, S.; Canonica, G.W.; Hedgecock, S.; et al. Benefits of omalizumab as add-on therapy in patients with severe persistent asthma who are inadequately controlled despite best available therapy (GINA 2002 step 4 treatment): INNOVATE. Allergy 2005, 60, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Iribarren, C.; Rahmaoui, A.; Long, A.A.; Szefler, S.J.; Bradley, M.S.; Carrigan, G.; Eisner, M.D.; Chen, H.; Omachi, T.A.; Farkouh, M.E.; et al. Cardiovascular and cerebrovascular events among patients receiving omalizumab: Results from EXCELS, a prospective cohort study in moderate to severe asthma. J. Allergy Clin. Immunol. 2017, 139, 1489–1495.e5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bousquet, J.; Siergiejko, Z.; Swiebocka, E.; Humbert, M.; Rabe, K.F.; Smith, N.; Leo, J.; Peckitt, C.; Maykut, R.; Peachey, G. Persistency of response to omalizumab therapy in severe allergic (IgE-mediated) asthma. Allergy 2011, 66, 671–678. [Google Scholar] [CrossRef]
- Hanania, N.A.; Wenzel, S.; Rosén, K.; Hsieh, H.J.; Mosesova, S.; Choy, D.F.; Lal, P.; Arron, J.R.; Harris, J.M.; Busse, W. Exploring the effects of omalizumab in allergic asthma: An analysis of biomarkers in the EXTRA study. Am. J. Respir. Crit. Care Med. 2013, 187, 804–811. [Google Scholar] [CrossRef]
- Busse, W.; Spector, S.; Rosén, K.; Wang, Y.; Alpan, O. High eosinophil count: A potential biomarker for assessing successful omalizumab treatment effects. J. Allergy Clin. Immunol. 2013, 132, 485–486. [Google Scholar] [CrossRef] [Green Version]
- Pavord, I.D.; Korn, S.; Howarth, P.; Bleecker, E.R.; Buhl, R.; Keene, O.N.; Ortega, H.; Chanez, P. Mepolizumab for severe eosinophilic asthma (DREAM): A multicentre, doubleblind, placebo-controlled trial. Lancet 2012, 380, 651–659. [Google Scholar] [CrossRef]
- Ortega, H.G.; Liu, M.C.; Pavord, I.D.; Brusselle, G.G.; FitzGerald, J.M.; Chetta, A.; Humbert, M.; Katz, L.E.; Keene, O.N.; Yancey, S.W.; et al. Mepolizumab treatment in patients with severe eosinophilic asthma. N. Engl. J. Med. 2014, 371, 1198–1207. [Google Scholar] [CrossRef] [Green Version]
- Bel, E.H.; Wenzel, S.E.; Thompson, P.J.; Prazma, C.M.; Keene, O.N.; Yancey, S.W.; Ortega, H.G.; Pavord, I.D.; SIRIUS Investigators. Oral glucocorticoid-sparing effect of mepolizumab in eosinophilic asthma. N. Engl. J. Med. 2014, 371, 1189–1197. [Google Scholar] [CrossRef]
- Katz, L.E.; Gleich, G.J.; Hartley, B.F.; Yancey, S.W.; Ortega, H.G. Blood eosinophil count is a useful biomarker to identify patients with severe eosinophilic asthma. Ann. Am. Thorac. Soc. 2014, 11, 531–536. [Google Scholar] [CrossRef]
- Ortega, H.G.; Yancey, S.W.; Mayer, B.; Gunsoy, N.B.; Keene, O.N.; Bleecker, E.R.; Brightling, C.E.; Pavord, I.D. Severe eosinophilic asthma treated with mepolizumab stratified by baseline eosinophil thresholds: A secondary analysis of the DREAM and studies. Lancet Respir. Med. 2016, 4, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Chupp, G.L.; Bradford, E.S.; Albers, F.C.; Bratton, D.J.; Wang-Jairaj, J.; Nelsen, L.M.; Trevor, J.L.; Magnan, A.; Ten Brinke, A. Efficacy of mepolizumab add-on therapy on health-related quality of life and markers of asthma control in severe eosinophilic asthma (MUSCA): A randomised, double-blind, placebo-controlled, parallel-group, multicentre, phase 3b trial. Lancet Respir. Med. 2017, 5, 390–400. [Google Scholar] [CrossRef] [PubMed]
- Chapman, K.R.; Albers, F.C.; Chipps, B.; Muñoz, X.; Devouassoux, G.; Bergna, M.; Galkin, D.; Azmi, J.; Mouneimne, D.; Price, R.G.; et al. The clinical benefit of mepolizumab replacing omalizumab in uncontrolled severe eosinophilic asthma. Allergy 2019, 74, 1716–1726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lugogo, N.; Domingo, C.; Chanez, P.; Leigh, R.; Gilson, M.J.; Price, R.G.; Yancey, S.W.; Ortega, H.G. Long-term efficacy and safety of mepolizumab in patients with severe eosinophilic asthma: A multi-center, open-label, phase IIIb study. Clin. Ther. 2016, 38, 2058–2070.e1. [Google Scholar] [CrossRef] [Green Version]
- Khatri, S.; Moore, W.; Gibson, P.G.; Leigh, R.; Bourdin, A.; Maspero, J.; Barros, M.; Buhl, R.; Howarth, P.; Albers, F.C.; et al. Assessment of the long-term safety of mepolizumab and durability of clinical response in patients with severe eosinophilic asthma. J. Allergy Clin. Immunol. 2019, 143, 1742–1751.e1747. [Google Scholar] [CrossRef] [Green Version]
- Khurana, S.; Brusselle, G.G.; Bel, E.H.; FitzGerald, J.M.; Masoli, M.; Korn, S.; Kato, M.; Albers, F.C.; Bradford, E.S.; Gilson, M.J.; et al. Long-term safety and clinical benefit of mepolizumab in patients with the most severe eosinophilic asthma: The COSMEX study. Clin. Ther. 2019, 41, 2041–2056. [Google Scholar] [CrossRef] [Green Version]
- Menzella, F.; Lusuardi, M.; Galeone, C.; Facciolongo, N.; Zucchi, L. The clinical profile of benralizumab in the management of severe eosinophilic asthma. Ther. Adv. Respir. Dis. 2016, 10, 534–548. [Google Scholar] [CrossRef] [Green Version]
- FitzGerald, J.M.; Bleecker, E.R.; Nair, P.; Korn, S.; Ohta, K.; Lommatzsch, M.; Ferguson, G.T.; Busse, W.W.; Barker, P.; Sproule, S.; et al. Benralizumab, an anti-interleukin-5 receptor α monoclonal antibody, as add-on treatment for patients with severe, uncontrolled, eosinophilic asthma (CALIMA): A randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2016, 388, 2128–2141. [Google Scholar] [CrossRef]
- Bleecker, E.R.; FitzGerald, J.M.; Chanez, P.; Papi, A.; Weinstein, S.F.; Barker, P.; Sproule, S.; Gilmartin, G.; Aurivillius, M.; Werkström, V.; et al. Efficacy and safety of benralizumab for patients with severe asthma uncontrolled with high-dosage inhaled corticosteroids and long-acting β2-agonists (SIROCCO): A randomised, multicentre, placebo-controlled phase 3 trial. Lancet 2016, 388, 2115–2127. [Google Scholar] [CrossRef]
- FitzGerald, J.M.; Bleecker, E.R.; Menzies-Gow, A.; Zangrilli, J.G.; Hirsch, I.; Metcalfe, P.; Newbold, P.; Goldman, M. Predictors of enhanced response with benralizumab for patients with severe asthma: Pooled analysis of the SIROCCO and CALIMA studies. Lancet Respir. Med. 2018, 6, 51–64. [Google Scholar] [CrossRef]
- Nair, P.; Wenzel, S.; Rabe, K.F.; Bourdin, A.; Lugogo, N.L.; Kuna, P.; Barker, P.; Sproule, S.; Ponnarambil, S.; Goldman, M. Oral Glucocorticoid-Sparing Effect of Benralizumab in Severe Asthma. N. Engl. J. Med. 2017, 376, 2448–2458. [Google Scholar] [CrossRef] [PubMed]
- Menzies-Gow, A.; Hoyte, F.L.; Price, D.B.; Cohen, D.; Barker, P.; Kreindler, J.; Jison, M.; Brooks, C.L.; Papeleu, P.; Katial, R. Clinical Remission in Severe Asthma: A Pooled Post Hoc Analysis of the Patient Journey with Benralizumab. Adv. Ther. 2022, 39, 2065–2084. [Google Scholar] [CrossRef] [PubMed]
- Menzies-Gow, A.; Gurnell, M.; Heaney, L.G.; Corren, J.; Bel, E.H.; Maspero, J.; Harrison, T.; Jackson, D.J.; Price, D.; Lugogo, N.; et al. Oral corticosteroid elimination via a personalised reduction algorithm in adults with severe, eosinophilic asthma treated with benralizumab (PONENTE): A multicentre, open-label, single-arm study. Lancet Respir. Med. 2022, 10, 47–58. [Google Scholar] [CrossRef]
- Korn, S.; Bourdin, A.; Chupp, G.; Cosio, B.G.; Arbetter, D.; Shah, M.; Gil, E.G. Integrated safety and efficacy among patients receiving benralizumab for up to 5 years. J. Allergy Clin. Immunol. Pract. 2021, 9, 4381–4392.e4. [Google Scholar] [CrossRef] [PubMed]
- Bakakos, A.; Schleich, F.; Bakakos, P. Biological therapy of severe asthma and nasal polyps. J. Pers. Med. 2022, 12, 976. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, N.A.; Pirozzi, G.; Graham, N.M.H. Commonality of the IL-4/IL-13 pathway in atopic diseases. Expert Rev. Clin. Immunol. 2017, 13, 425–437. [Google Scholar] [CrossRef]
- Castro, M.; Corren, J.; Pavord, I.D.; Maspero, J.; Wenzel, S.; Rabe, K.F.; Busse, W.W.; Ford, L.; Sher, L.; Fitzgerald, J.M.; et al. Dupilumab efficacy and safety in moderate-to-severe uncontrolled asthma. N. Engl. J. Med. 2018, 378, 2486–2496. [Google Scholar] [CrossRef]
- Rabe, K.F.; Nair, P.; Brusselle, G.; Maspero, J.F.; Castro, M.; Sher, L.; Zhu, H.; Hamilton, J.D.; Swanson, B.N.; Khan, A.; et al. Efficacy and safety of dupilumab in glucocorticoid-dependent severe asthma. N. Engl. J. Med. 2018, 378, 2475–2485. [Google Scholar] [CrossRef]
- Brusselle, G.; Quirce, S.; Papi, A.; Kuna, P.; Chipps, B.E.; Hanania, N.A.; Blaiss, M.; Msihid, J.; Jacob-Nara, J.A.; Deniz, Y.; et al. Dupilumab Efficacy in Patients with Uncontrolled or Oral Corticosteroid-Dependent Allergic and Non-allergic Asthma. J. Allergy Clin. Immunol. Pract. 2022, in press. [Google Scholar] [CrossRef]
- Wechsler, M.E.; Ford, L.B.; Maspero, J.F.; Pavord, I.D.; Papi, A.; Bourdin, A.; Watz, H.; Castro, M.; Nenasheva, N.M.; Tohda, Y.; et al. Long-term safety and efficacy of dupilumab in patients with moderate-to-severe asthma (TRAVERSE): An open-label extension study. Lancet Respir. Med. 2022, 10, 11–25. [Google Scholar] [CrossRef]
- Sher, L.D.; Wechsler, M.E.; Rabe, K.F.; Maspero, J.F.; Daizadeh, N.; Mao, X.; Ortiz, B.; Mannent, L.P.; Laws, E.; Ruddy, M.; et al. Dupilumab Reduces Oral Corticosteroid Use in Patients With Corticosteroid-Dependent Severe Asthma: An Analysis of the Phase 3, Open-Label Extension TRAVERSE Trial. Chest 2022, 162, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Berger, P.; Menzies-Gow, A.; Peters, A.T.; Kuna, P.; Rabe, K.F.; Altincatal, A.; Soler, X.; Pandit-Abid, N.; Siddiqui, S.; Jacob-Nara, J.A.; et al. Long-term efficacy of dupilumab in asthma with or without chronic rhinosinusitis and nasal polyps. Ann. Allergy Asthma Immunol. 2022, in press. [Google Scholar] [CrossRef] [PubMed]
- Wechsler, M.E.; Klion, A.D.; Paggiaro, P.L.; Nair, P.; Staumont-Salle, D.; Radwan, A.; Johnson, R.R.; Kapoor, U.; Khokhar, F.A.; Daizadeh, N.; et al. Effect of Dupilumab on Blood Eosinophil Counts in Patients With Asthma, Chronic Rhinosinusitis With Nasal Polyps, Atopic Dermatitis, or Eosinophilic Esophagitis. J. Allergy Clin. Immunol. Pract. 2022, 10, 2695–2709. [Google Scholar] [CrossRef]
- Available online: https://www.fdanews.com/articles/188355-fda-awards-astrazeneca-and-amgens-tezepelumab-breakthrough-designation (accessed on 11 January 2023).
- Parnes, J.R.; Molfino, N.A.; Colice, G.; Martin, U.; Corren, J.; Menzies-Gow, A. Targeting TSLP in Asthma. J. Asthma Allergy 2022, 15, 749–765. [Google Scholar] [CrossRef] [PubMed]
- Menzies-Gow, A.; Corren, J.; Bourdin, A.; Chupp, G.; Israel, E.; Wechsler, M.E.; Brightling, C.E.; Griffiths, J.M.; Hellqvist, Å.; Bowen, K.; et al. Tezepelumab in Adults and Adolescents with Severe, Uncontrolled Asthma. N. Engl. J. Med. 2021, 384, 1800–1809. [Google Scholar] [CrossRef]
- Wechsler, M.E.; Colice, G.; Griffiths, J.M.; Almqvist, G.; Skärby, T.; Piechowiak, T.; Kaur, P.; Bowen, K.; Hellqvist, Å.; Mo, M.; et al. SOURCE: A phase 3, multicentre, randomized, double-blind, placebo-controlled, parallel group trial to evaluate the efficacy and safety of tezepelumab in reducing oral corticosteroid use in adults with oral corticosteroid dependent asthma. Respir. Res. 2020, 21, 264. [Google Scholar] [CrossRef]
- Available online: https://clinicaltrials.gov/ct2/show/NCT05274815 (accessed on 11 January 2023).
- Sverrild, A.; Hansen, S.; Hvidtfeldt, M.; Clausson, C.M.; Cozzolino, O.; Cerps, S.; Uller, L.; Backer, V.; Erjefält, J.; Porsbjerg, C.; et al. The effect of tezepelumab on airway hyperresponsiveness to mannitol in asthma (UPSTREAM). Eur. Respir. J. 2021, 59, 2101296. [Google Scholar] [CrossRef]
- Adatia, A.; Wahab, M.; Satia, I. Is tezepelumab more than just an anti-eosinophil drug? Eur. Respir. J. 2021, 59, 2101700. [Google Scholar] [CrossRef]
- Corren, J.; Ambrose, C.S.; Griffiths, J.M.; Hellqvist, Å.; Lindsley, A.W.; Llanos, J.P.; Colice, G.; Menzies-Gow, A. Efficacy of tezepelumab in patients with evidence of severe allergic asthma: Results from the phase 3 NAVIGATOR study. Clin. Exp. Allergy 2022, in press. [Google Scholar] [CrossRef]
- Menzies-Gow, A.; Ponnarambil, S.; Downie, J.; Bowen, K.; Hellqvist, Å.; Colice, G. DESTINATION: A phase 3, multicentre, randomized, double-blind, placebo-controlled, parallel-group trial to evaluate the long-term safety and tolerability of tezepelumab in adults and adolescents with severe, uncontrolled asthma. Respir. Res. 2020, 21, 279. [Google Scholar] [CrossRef]
- Menzies-Gow, A.; Moore, W.C.; Wechsler, M.E. Difficult-to-control asthma management in adults. J. Allergy Clin. Immunol. Pract. 2022, 10, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Massanari, M.; Holgate, S.T.; Busse, W.W.; Jimenez, P.; Kianifard, F.; Zeldin, R. Effect of omalizumab on peripheral blood eosinophilia in allergic asthma. Respir. Med. 2010, 104, 188–196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Humbert, M.; Taillé, C.; Mala, L.; Le Gros, V.; Just, J.; Molimard, M. Omalizumab effectiveness in patients with severe allergic asthma according to blood eosinophil count: The STELLAIR study. Eur. Respir. J. 2018, 51, 1702523. [Google Scholar] [CrossRef] [PubMed]
- Casale, T.B.; Luskin, A.T.; Busse, W.; Zeiger, R.S.; Trzaskoma, B.; Yang, M.; Griffin, N.M.; Chipps, B.E. Omalizumab Effectiveness by Biomarker Status in Patients with Asthma: Evidence From PROSPERO, A Prospective Real-World Study. J. Allergy Clin. Immunol. Pract. 2019, 7, 156–164.e1. [Google Scholar] [CrossRef]
- Domingo Ribas, C.; Carrillo Díaz, T.; Martínez Moragón, E.; Banas Conejero, D.; Sánchez Herrero, M.G. REal worlD Effectiveness and Safety of Mepolizumab in a Multicentric Spanish Cohort of Asthma Patients Stratified by Eosinophils: The REDES Study. Drugs 2021, 81, 1763–1774. [Google Scholar] [CrossRef]
- Bousquet, J.; Rabe, K.; Humbert, M.; Chung, K.F.; Berger, W.; Fox, H.; Ayre, G.; Chen, H.; Thomas, K.; Blogg, M.; et al. Predicting and evaluating response to omalizumab in patients with severe allergic asthma. Respir. Med. 2007, 101, 1483–1492. [Google Scholar] [CrossRef] [Green Version]
- Ortega, H.; Chupp, G.; Bardin, P.; Bourdin, A.; Garcia, G.; Hartley, B.; Yancey, S.; Humbert, M. The role of mepolizumab in atopic and nonatopic severe asthma with persistent eosinophilia. Eur. Respir. J. 2014, 44, 239–241. [Google Scholar] [CrossRef] [Green Version]
- Chipps, B.E.; Newbold, P.; Hirsch, I.; Trudo, F.; Goldman, M. Benralizumab efficacy by atopy status and serum immunoglobulin E for patients with severe, uncontrolled asthma. Ann. Allergy Asthma Immunol. 2018, 120, 504–511.e4. [Google Scholar] [CrossRef] [Green Version]
- Corren, J.; Castro, M.; O’Riordan, T.; Hanania, N.A.; Pavord, I.D.; Quirce, S.; Chipps, B.E.; Wenzel, S.E.; Thangavelu, K.; Rice, M.S.; et al. Dupilumab Efficacy in Patients with Uncontrolled, Moderate-to-Severe Allergic Asthma. J. Allergy Clin. Immunol. Pract. 2020, 8, 516–526. [Google Scholar] [CrossRef]
- Marcos, M.C.; Cisneros Serrano, C. What is the added value of FeNO as T2 biomarker? Front. Allergy 2022, 3, 957106. [Google Scholar] [CrossRef]
- Ledford, D.; Busse, W.; Trzaskoma, B.; Omachi, T.A.; Rosén, K.; Chipps, B.E.; Luskin, A.T.; Solari, P.G. A randomized multicenter study evaluating Xolair persistence of response after long-term therapy. J. Allergy Clin. Immunol. 2017, 140, 162–169.e2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hearn, A.P.; Kavanagh, J.; d’Ancona, G.; Roxas, C.; Green, L.; Thomson, L.; Fernandes, M.; Kent, B.D.; Dhariwal, J.; Nanzer, A.M.; et al. The relationship between Feno and effectiveness of mepolizumab and benralizumab in severe eosinophilic asthma. J. Allergy Clin. Immunol. Pract. 2021, 9, 2093–2096.e1. [Google Scholar] [CrossRef]
- Pavord, I.D.; Deniz, Y.; Corren, J.; Casale, T.B.; FitzGerald, J.M.; Izuhara, K.; Daizadeh, N.; Ortiz, B.; Johnson, R.R.; Harel, S.; et al. Baseline FeNO Independently Predicts the Dupilumab Response in Patients with Moderate-To-Severe Asthma. J. Allergy Clin. Immunol. Pract. 2022, in press. [Google Scholar] [CrossRef] [PubMed]
- Corren, J.; Pham, T.H.; Garcia Gil, E.; Sałapa, K.; Ren, P.; Parnes, J.R.; Colice, G.; Griffiths, J.M. Baseline type 2 biomarker levels and response to tezepelumab in severe asthma. Allergy 2022, 77, 1786–1796. [Google Scholar] [CrossRef] [PubMed]
- Diver, S.; Khalfaoui, L.; Emson, C.; Wenzel, S.E.; Menzies-Gow, A.; Wechsler, M.E.; Johnston, J.; Molfino, N.; Parnes, J.R.; Megally, A.; et al. Effect of tezepelumab on airway inflammatory cells, remodelling, and hyperresponsiveness in patients with moderate-to-severe uncontrolled asthma (CASCADE): A double-blind, randomised, placebo-controlled, phase 2 trial. Lancet Respir. Med. 2021, 9, 1299–1312. [Google Scholar] [CrossRef] [PubMed]
- Siergiejko, Z.; Świebocka, E.; Smith, N.; Peckitt, C.; Leo, J.; Peachey, G.; Maykut, R. Oral corticosteroid sparing with omalizumab in severe allergic (IgE-mediated) asthma patients. Curr. Med. Res. Opin. 2011, 27, 2223–2228. [Google Scholar] [CrossRef] [PubMed]
- Menzella, F.; Latorre, M.; Ruggiero, P.; Bagnasco, D.; Heffler, E. Reduction of oral corticosteroids in patients with severe eosinophilic asthma treated with Benralizumab: Could it represent a marker of treatment efficacy? Expert. Opin. Biol. Ther. 2019, 19, 601–606. [Google Scholar] [CrossRef] [PubMed]
- Laidlaw, T.M.; Mullol, J.; Woessner, K.M.; Amin, N.; Mannent, L.P. Chronic Rhinosinusitis with Nasal Polyps and Asthma. J. Allergy Clin. Immunol. Pract. 2021, 9, 1133–1141. [Google Scholar] [CrossRef]
- Wu, Q.; Zhang, Y.; Kong, W.; Wang, X.; Wang, X.; Yuan, L.; Zheng, L.; Qiu, L.; Huang, X.; Yang, Q. Which Is the Best Biologic for Nasal Polyps: Dupilumab, Omalizumab, or Mepolizumab? A Network Meta-Analysis. Int. Arch. Allergy Immunol. 2022, 183, 279–288. [Google Scholar] [CrossRef]
- Gibson, P.G.; Prazma, C.M.; Chupp, G.L.; Bradford, E.S.; Forshag, M.; Mallett, S.A.; Yancey, S.W.; Smith, S.G.; Bel, E.H. Mepolizumab improves clinical outcomes in patients with severe asthma and comorbid conditions. Respir. Res. 2021, 22, 171. [Google Scholar] [CrossRef]
- Han, J.K.; Bachert, C.; Fokkens, W.; Desrosiers, M.; Wagenmann, M.; Lee, S.E.; Smith, S.G.; Martin, N.; Mayer, B.; Yancey, S.W.; et al. Mepolizumab for chronic rhinosinusitis with nasal polyps (SYNAPSE): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Respir. Med. 2021, 9, 1141–1153. [Google Scholar] [CrossRef] [PubMed]
- Harrison, T.; Canonica, G.W.; Chupp, G.; Lee, J.; Schleich, F.; Welte, T.; Valero, A.; Gemzoe, K.; Maxwell, A.; Joksaite, S.; et al. Real-world mepolizumab in the prospective severe asthma REALITI-A study: Initial analysis. Eur. Respir. J. 2020, 56, 2000151. [Google Scholar] [CrossRef] [PubMed]
- Hoy, S.M. Dupilumab: A Review in Chronic Rhinosinusitis with Nasal Polyps. Drugs 2020, 80, 711–717. [Google Scholar] [CrossRef] [PubMed]
- Maspero, J.F.; Katelaris, C.H.; Busse, W.W.; Castro, M.; Corren, J.; Chipps, B.E.; Peters, A.T.; Pavord, I.D.; Ford, L.B.; Sher, L.; et al. Dupilumab Efficacy in Uncontrolled, Moderate-to-Severe Asthma with Self-Reported Chronic Rhinosinusitis. J. Allergy Clin. Immunol. Pract. 2020, 8, 527–539.e9. [Google Scholar] [CrossRef] [PubMed]
- Bachert, C.; Han, J.K.; Desrosiers, M.Y.; Gevaert, P.; Heffler, E.; Hopkins, C.; Tversky, J.R.; Barker, P.; Cohen, D.; Emson, C.; et al. Efficacy and safety of benralizumab in chronic rhinosinusitis with nasal polyps: A randomized, placebo-controlled trial. J. Allergy Clin. Immunol. 2022, 149, 1309–1317.e12. [Google Scholar] [CrossRef] [PubMed]
- Bleecker, E.R.; Wechsler, M.E.; FitzGerald, J.M.; Menzies-Gow, A.; Wu, Y.; Hirsch, I.; Goldman, M.; Newbold, P.; Zangrilli, J.G. Baseline patient factors impact on the clinical efficacy of benralizumab for severe asthma. Eur. Respir. J. 2018, 52, 1800936. [Google Scholar] [CrossRef] [Green Version]
- Harrison, T.W.; Chanez, P.; Menzella, F.; Canonica, G.W.; Louis, R.; Cosio, B.G.; Lugogo, N.L.; Mohan, A.; Burden, A.; McDermott, L.; et al. Onset of effect and impact on health-related quality of life, exacerbation rate, lung function, and nasal polyposis symptoms for patients with severe eosinophilic asthma treated with benralizumab (ANDHI): A randomised, controlled, phase 3b trial. Lancet Respir. Med. 2021, 9, 260–274. [Google Scholar] [CrossRef]
- Available online: https://clinicaltrials.gov/ct2/show/NCT04157335 (accessed on 25 January 2023).
- Emson, C.; Corren, J.; Sałapa, K.; Hellqvist, Å.; Parnes, J.R.; Colice, G. Efficacy of Tezepelumab in Patients with Severe, Uncontrolled Asthma with and without Nasal Polyposis: A Post Hoc Analysis of the Phase 2b PATHWAY Study. J. Asthma Allergy 2021, 14, 91–99. [Google Scholar] [CrossRef]
- Menzies Gow, A.; Corren, J.; Israel, E.; Welte, T.; Ambrose, C.; Cook, B.; Hunter, G.; Llanos-Ackert, J.-P.; Colice, G. Tezepelumab efficacy in patients with severe, uncontrolled asthma and comorbid nasal polyps in NAVIGATOR. Eur. Respir. J. 2021, 58, PA876. [Google Scholar]
- Available online: https://clinicaltrials.gov/ct2/show/NCT04851964 (accessed on 25 January 2023).
- De Prado, G.; Khan, A.H.; Peters, A.T.; Bachert, C.; Wagenmann, M.; Heffler, E.; Hopkins, C.; Hellings, P.W.; Zhang, M.; Xing, J.; et al. Efficacy and Safety of Dupilumab Versus Omalizumab in Chronic Rhinosinusitis With Nasal Polyps and Asthma: EVEREST Trial Design. Am. J. Rhinol. Allergy 2022, 36, 788–795. [Google Scholar] [CrossRef]
- Ricciardolo, F.L.M.; Sprio, A.E.; Baroso, A.; Gallo, F.; Riccardi, E.; Bertolini, F.; Carriero, V.; Arrigo, E.; Ciprandi, G. Characterization of T2-Low and T2-High Asthma Phenotypes in Real-Life. Biomedicines 2021, 9, 1684. [Google Scholar] [CrossRef] [PubMed]
- Pitre, T.; Jassal, T.; Angjeli, A.; Jarabana, V.; Nannapaneni, S.; Umair, A.; Hussain, M.; Leung, G.; Kirsh, S.; Su, J.; et al. A comparison of the effectiveness of biologic therapies for asthma: A systematic review and network meta-analysis. Ann. Allergy Asthma Immunol. 2022, in press. [Google Scholar] [CrossRef] [PubMed]
- Akenroye, A.T.; Segal, J.B.; Zhou, G.; Foer, D.; Li, L.; Alexander, G.C.; Keet, C.A.; Jackson, J.W. Comparative Effectiveness of Omalizumab, Mepolizumab, and Dupilumab in Asthma: A Target Trial Emulation. J. Allergy Clin. Immunol. 2023, in press. [Google Scholar] [CrossRef] [PubMed]
- Kardas, G.; Panek, M.; Kuna, P.; Damiański, P.; Kupczyk, M. Monoclonal antibodies in the management of asthma: Dead ends, current status and future perspectives. Front. Immunol. 2022, 13, 983852. [Google Scholar] [CrossRef]
- Papaioannou, A.I.; Diamant, Z.; Bakakos, P.; Loukides, S. Towards precision medicine in severe asthma: Treatment algorithms based on treatable traits. Respir. Med. 2018, 142, 15–22. [Google Scholar] [CrossRef]
- Song, P.; Adeloye, D.; Salim, H.; Dos Santos, J.P.; Campbell, H.; Sheikh, A.; Rudan, I. Global, regional, and national prevalence of asthma in 2019, A systematic analysis and modelling study. J. Glob. Health 2022, 12, 04052. [Google Scholar] [CrossRef]


| Omalizumab | Mepolizumab | Benralizumab | Dupilumab | Tezepelumab | |
|---|---|---|---|---|---|
| Mechanism of action | Anti-IgE | Anti–IL-5 mAb | Anti–IL-5Rα mAb | Anti–IL-4Rα mAb | Anti-TSLP mAb |
| Asthma US label | Add-on maintenance treatment of adult and adolescent patients (12 years of age and above) with severe persistent allergic asthma who have a positive skin test or in vitro reactivity to a perennial aeroallergen and who have reduced lung function (FEV1 < 80%) | Add-on maintenance treatment of adult and pediatric patients aged ≥6 years with severe asthma and with an eosinophilic phenotype | Add-on maintenance treatment of patients with severe asthma aged ≥12 years, and with an eosinophilic phenotype | Add-on maintenance treatment in adult and pediatric patients aged ≥6 years with moderate-to-severe asthma characterized by an eosinophilic phenotype or with OCS dependent asthma | Add-on maintenance treatment of adult and pediatric patients aged ≥12 years with severe asthma |
| Asthma EU label | Add-on maintenance treatment of adults and pediatric patients 6 years of age and older with moderate to severe persistent asthma who have a positive skin test or in vitro reactivity to a perennial aeroallergen and whose symptoms are inadequately controlled with inhaled corticosteroids | Add-on treatment for severe refractory eosinophilic asthma in adults, adolescents and children aged ≥6 years | Add-on maintenance treatment in adult patients with severe eosinophilic asthma inadequately controlled despite high-dose inhaled corticosteroids plus long-acting β-agonists | Add-on therapy in adults and adolescents aged ≥12 years who have severe asthma with type 2 inflammation characterized by raised blood EOS and/or raised FeNO, who are inadequately controlled with high-dose ICS plus another medicinal product for maintenance treatment | Add-on maintenance treatment of adults and adolescents (12 years of age and older) with severe asthma that is not adequately controlled by a combination of high-dose corticosteroids taken by inhalation plus another asthma medicine |
| Dosing in asthma | The recommended dosage for treatment of asthma is 75 mg to 150 mg by subcutaneous injection every 2 or 4 weeks based on serum total IgE level (IU/mL) measured before the start of treatment and by body weight (kg) (0.016 mg/kg per IU/mL of IgE) | 100 mg SC q4w | 30 mg SC q4w (first 3 doses) then q8w | 400 or 600 mg SC loading dose, then 200 or 300 mg SC q2w | 210 mg SC q4w |
| Administration | HCP or patient/caregiver (Prefilled syringe) | HCP or patient/caregiver (AI/prefilled syringe) | HCP or patient/caregiver (AI/prefilled syringe) | HCP or patient/caregiver (AI/prefilled syringe) | HCP (Prefilled syringe) |
| Devices available |
|
|
|
|
|
| Other US- and/or EU-approved indications |
|
| N/A |
| N/A |
| Target | Pivotal Phase 3 | Open Label Extension | OCS Sparing | Lung Function | Clinical Development for New Indications | ||
|---|---|---|---|---|---|---|---|
| Omalizumab | Anti IgE | INNOVATE | NA | EXALT | NA | NA | EXPERIENCE: Real-world evidence PERSIST: Real-world evidence |
| Mepolizumab | Anti-IL-5 | MENSA | COSMOS COSMEX COLUMBA | SIRIUS | CHOOSEBETWEENAMAB: Ph4 mepo vs oma REMOMEPO: Airway remodeling | MUSCA: QoL COMET: Discontinuation REALITI-A: Real-world evidence | |
| Benralizumab | Anti-IL-5Rα | SIROCCO CALIMA | BORA MELTEMI | ZONDA | SOLANA | ANANKE: Real-world evidence TATE: Ages 6–11 HAYATE: OCS reduction PROs (BEEPS, POWER, BE-REAL, imPROve) PONENTE: OCS use (open label) | MIRACLE: Med-high ICS/LABA SHAMAL: ICS reduction CHINOOK: Airway remodeling AERFLO: MRI pilot study |
| Dupilumab | Anti-IL4Rα | QUEST | TRAVERSE TRAVERSE Extension | VENTURE | ATLAS | VESTIGE: Airway remodeling MORPHEO: Sleep disturbances RAPID: Real-world patient registry | EVEREST: Coexisting CRSwNP REVEAL: Real-world patient registry |
| Tezepelumab | Anti-TSLP | NAVIGATOR | DESTINATION | SOURCE | PATH-HOME: Home use study CASCADE: Ph2 MOA/biopsy study PATHWAY: Ph2 | SUNRISE: OCS sparing (double blind, placebo controlled) WAYFINDER: OCS use (open label) | |
| Parameter | Omalizumab 75 mg or 150 mg SC q2W or q4W | Mepolizumab 75 mg IV or 100 mg SC q4w | Benralizumab 30 mg SC q4w or q8w | Dupilumab 200 or 300 mg SC q2w | Tezepelumab 210 mg SC q4w |
|---|---|---|---|---|---|
| INNOVATE n = 419 | MENSA n = 576 | SIROCCO n = 1204 | QUEST n = 1902 | NAVIGATOR n = 1061 | |
| Treatment duration | 28 weeks | 32 weeks | 48 weeks | 52 weeks | 52 weeks |
| Study dosing | Oma 75 mg to 150 mg q2W/q4WSC to provide a dose of at least 0.016 mg/kg per IU/mL of IgE | Mepo 75 mg IV or 100 mg SC q4w PBO IV/SC q4w | Benra 30 mg SC q4w Benra 30 mg SC q8w PBO SC q4w | Dupi 200 or 300 mg SC q2w PBO 200 or 300 mg SC q2w | Teze 210 mg SC q4w PBO SC q4W |
| Patient population | Severe allergic asthma (positive skin prick test to ‡1 perennial aeroallergen and total serum IgE level of ‡30 to 700 IU/mL) | Severe eosinophilic asthma (≥150/uL at BL or ≥300/uL prev 12 mo) | Uncontrolled eosinophilic asthma (no min EOS/FeNO) | Uncontrolled asthma ≥12 mo (no min EOS/FeNO) | Patients aged 12–80 years with severe, uncontrolled asthma |
| Background medication | High-dose ICS/LABA | High-dose ICS | Medium- to high-dose ICS/LABA for >12 mo | Medium- to high-dose ICS + ≤2 additional controller medications | High-dose ICS ≥1 additional controller medication w/(o) OCS |
| Key entry criteria | |||||
| No. of previous exacerbations | ≥2 | ≥2 | ≥2 | ≥1 | ≥2 |
| Pre-BD FEV1, % predicted |
|
|
|
|
|
| Bronchodilator reversibility |
|
|
|
|
|
| Primary end points |
|
|
|
|
|
| Omalizumab (INNOVATE) | Mepolizumab (MENSA) | Benralizumab (SIROCCO) | Dupilumab (QUEST) | Tezepelumab (NAVIGATOR) | |
|---|---|---|---|---|---|
| Age at diagnosis | 44 (12–79) | 51 ± 14.5 | 47 ± 15 | 48±16 | 49.9 ± 16.0 |
| Blood eosinophil count (Median) | 300 cells/µL | 290 cells/µL | 360 cells/µL | 255 cells/µL | 250 cells/µL |
| Atopy % | 100 | 50 | 60 | 83 | 68.6 |
| Exacerbations | 2.1 | 2.8 | 3.8 | 2.0 | 2.0 (58.7); >2 (41.3) |
| OCS mg/day Median | N/A | 12.6 | 15.2 | N/A | N/A |
| OCS use % | 23 | 27 | 17 | NA | 9.3 |
| FEV1 | 61% of predicted | 1.73 L | 1.65 L | 1.70 L | 1.8 L |
| ACQ | 3.0 | 2.3 | 2.8 | 2.7 | 2.8 |
| CRSwNP% | N/A | 14 | 19 | 23 | 17 |
| Omalizumab (INNOVATE) | Mepolizumab (MENSA) | Benralizumab (SIROCCO) | Dupilumab (QUEST) | Tezepelumab (NAVIGATOR) | |
|---|---|---|---|---|---|
| −48% | −52% | −51% | −47% | −50% |
| N/A | −53% | −55% | −67% | −70% |
| N/A | N/A | N/A | −67% | −77% |
| N/A | N/A | N/A | −69% | N/A |
| FEV1 improvement (mL) | +190 | +98 | +159 | +340 | +230 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Menzella, F. Baseline Characteristics of Patients Enrolled in Clinical Trials of Biologics for Severe Asthma as Potential Predictors of Outcomes. J. Clin. Med. 2023, 12, 1546. https://doi.org/10.3390/jcm12041546
Menzella F. Baseline Characteristics of Patients Enrolled in Clinical Trials of Biologics for Severe Asthma as Potential Predictors of Outcomes. Journal of Clinical Medicine. 2023; 12(4):1546. https://doi.org/10.3390/jcm12041546
Chicago/Turabian StyleMenzella, Francesco. 2023. "Baseline Characteristics of Patients Enrolled in Clinical Trials of Biologics for Severe Asthma as Potential Predictors of Outcomes" Journal of Clinical Medicine 12, no. 4: 1546. https://doi.org/10.3390/jcm12041546
APA StyleMenzella, F. (2023). Baseline Characteristics of Patients Enrolled in Clinical Trials of Biologics for Severe Asthma as Potential Predictors of Outcomes. Journal of Clinical Medicine, 12(4), 1546. https://doi.org/10.3390/jcm12041546






