Impulse Oscillometry as a Diagnostic Test for Pulmonary Emphysema in a Clinical Setting
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Data Collection
2.3. Computed Tomography Scans
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Excluded Patients (n = 12) | Included Patients (n = 88) | p * | |
|---|---|---|---|
| Clinical variables | n (%) | n (%) | |
| Female sex | 8 (67) | 45 (33) | 0.37 |
| Positive smoking history | 8 (67) | 42 (47) | 0.36 |
| Dyspnea | 9 (75) | 51 (58) | 0.35 |
| Cough | 1 (8) | 26 (30) | 0.17 |
| Median (IQR) | Median (IQR) | ||
| Age (years) | 65 (51–70) | 63 (49–72) | 0.71 |
| BMI | 29.0 (24.6–32.1) | 26.4 (23.3–29.8) | 0.46 |
| Body Plethysmography | Median (IQR) | Median (IQR) | |
| FEV1 % of predicted | 91 (72–112) | 95 (76–103) | 0.49 |
| FVC % of predicted | 94 (91–123) | 102 (93–112) | 0.59 |
| FEV1/FVC | 73 (66–80) | 73 (65–79) | 0.77 |
| TLC % of predicted | 109 (88–114) | 99 (91–110) | 0.94 |
| RV % of predicted | 129 (91–144) | 120 (96–145) | 0.94 |
| RV/TLC | 0.44 (0.36–0.49) | 0.44 (0.34–0.51) | 0.95 |
| DLCOc % of predicted | 87 (71–100) | 70 (58–79) | <0.01 |
| Impulse Oscillometry | Median (IQR) | Median (IQR) | |
| R5 % of predicted | 101 (74–117) | 99 (77–126) | 0.57 |
| R5–R20 | 0.08 (0.04–0.11) | 0.07 (0.03–0.12) | 0.93 |
| X5 | −0.11 (−0.14–−0.09) | −0.1 (−0.16–-0.07) | 0.63 |
| Fres | 15.20 (11.25–18.01) | 15.13 (10.87–20.00) | 0.79 |
| Ax | 0.45 (0.24–0.66) | 0.42 (0.17–1.07) | 0.99 |
| Model 1 (BP) | Model 2 (IOS) | |
|---|---|---|
| AUC | 0.905 (0.811–0.998) | 0.847 (0.751–0.943) |
| Sensitivity, % | 78.9 (60.0–92.3 *) | 70.0 (44.4–88.2 *) |
| PPV, % | 50.0 (37.5–61.9 *) | 50.0 (36.0–70.0 *) |
| Specificity, % | 81.3 (64.7–85.1 *) | 82.5 (66.2–94.1 *) |
| NPV, % | 94.2 (89.4–97.2 *) | 91.7 (86.0–95.8 *) |
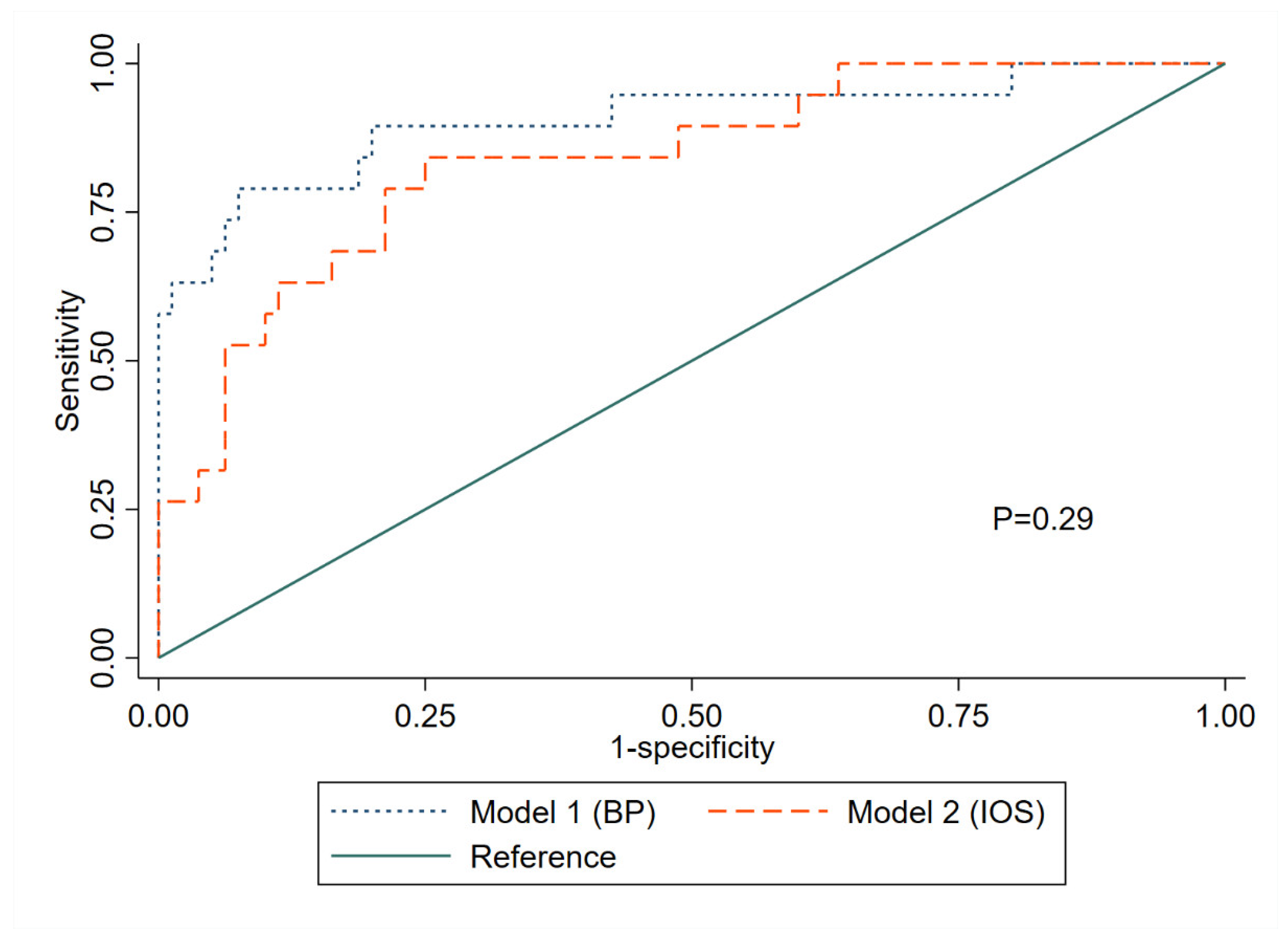
References
- Global Initiative for Chronic Obstructive Lung Disease. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease; 2020 report; Global Initiative for Chronic Obstructive Lung Disease: Brussels, Belgium, 2020. [Google Scholar]
- Marques, A.; Souto-Miranda, S.; Machado, A.; Oliveira, A.; Jácome, C.; Cruz, J.; Enes, V.; Afreixo, V.; Martins, V.; Andrade, L.; et al. COPD profiles and treatable traits using minimal resources: Identification, decision tree and stability over time. Respir. Res. 2022, 23, 30. [Google Scholar] [CrossRef]
- Lange, P.; Halpin, D.M.; O’Donnell, D.E.; MacNee, W. Diagnosis, assessment, and phenotyping of COPD: Beyond FEV1. Int. J. Chronic Obstr. Pulm. Dis. 2016, 11, 3–12. [Google Scholar] [CrossRef] [Green Version]
- Yousuf, A.; McAuley, H.; Elneima, O.; Brightling, C.E. The different phenotypes of COPD. Br. Med. Bull. 2021, 137, 82–97. [Google Scholar] [CrossRef]
- Pahal, P.; Avula, A.; Sharma, S. Emphysema. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2022. [Google Scholar]
- Barr, R.G.; Berkowitz, E.A.; Bigazzi, F.; Bode, F.; Bon, J.; Bowler, R.P.; Chiles, C.; Crapo, J.D.; Criner, G.J.; Curtis, J.L.; et al. A combined pulmonary-radiology workshop for visual evaluation of COPD: Study design, chest CT findings and concordance with quantitative evaluation. COPD J. Chronic Obstr. Pulm. Dis. 2012, 9, 151–159. [Google Scholar] [CrossRef]
- Newell, J.D., Jr.; Hogg, J.C.; Snider, G.L. Report of a workshop: Quantitative computed tomography scanning in longitudinal studies of emphysema. Eur. Respir. J. 2004, 23, 769–775. [Google Scholar] [CrossRef] [Green Version]
- Smith, B.M.; Pinto, L.; Ezer, N.; Sverzellati, N.; Muro, S.; Schwartzman, K. Emphysema detected on computed tomography and risk of lung cancer: A systematic review and meta-analysis. Lung Cancer 2012, 77, 58–63. [Google Scholar] [CrossRef]
- Johannessen, A.; Skorge, T.D.; Bottai, M.; Grydeland, T.B.; Nilsen, R.M.; Coxson, H.; Dirksen, A.; Omenaas, E.; Gulsvik, A.; Bakke, P. Mortality by level of emphysema and airway wall thickness. Am. J. Respir. Crit. Care. Med. 2013, 187, 602–608. [Google Scholar] [CrossRef] [Green Version]
- Kitaguchi, Y.; Fujimoto, K.; Kubo, K.; Honda, T. Characteristics of COPD phenotypes classified according to the findings of HRCT. Respir. Med. 2006, 100, 1742–1752. [Google Scholar] [CrossRef] [Green Version]
- Kahnert, K.; Jobst, B.; Biertz, F.; Biederer, J.; Watz, H.; Huber, R.M.; Behr, J.; Grenier, P.A.; Alter, P.; Vogelmeier, C.F.; et al. Relationship of spirometric, body plethysmographic, and diffusing capacity parameters to emphysema scores derived from CT scans. Chronic Respir. Dis. 2019, 16, 1479972318775423. [Google Scholar] [CrossRef]
- Von Siemens, M.S.; Alter, P.; Lutter, J.I.; Kauczor, H.U.; Jobst, B.; Bals, R.; Trudzinski, F.C.; Söhler, S.; Behr, J.; Watz, H.; et al. CAT score single item analysis in patients with COPD: Results from COSYCONET. Respir. Med. 2019, 159, 105810. [Google Scholar] [CrossRef]
- Herth, F.J.F.; Slebos, D.J.; Criner, G.J.; Shah, P.L. Endoscopic Lung Volume Reduction: An Expert Panel Recommendation—Update 2017. Respiration 2017, 94, 380–388. [Google Scholar] [CrossRef]
- Venkatesan, P. GOLD report: 2022 update. Lancet Respir. Med. 2022, 10, e20. [Google Scholar] [CrossRef]
- Kellerer, C.; Jörres, R.A.; Schneider, A.; Alter, P.; Kauczor, H.U.; Jobst, B.; Biederer, J.; Bals, R.; Watz, H.; Behr, J.; et al. Prediction of lung emphysema in COPD by spirometry and clinical symptoms: Results from COSYCONET. Respir. Res. 2021, 22, 242. [Google Scholar] [CrossRef]
- Criée, C.P.; Sorichter, S.; Smith, H.J.; Kardos, P.; Merget, R.; Heise, D.; Berdel, D.; Köhler, D.; Magnussen, H.; Marek, W.; et al. Body plethysmography—Its principles and clinical use. Respir. Med. 2011, 105, 959–971. [Google Scholar] [CrossRef]
- Shirai, T.; Kurosawa, H. Clinical Application of the Forced Oscillation Technique. Intern. Med. 2016, 55, 559–566. [Google Scholar] [CrossRef] [Green Version]
- Bickel, S.; Popler, J.; Lesnick, B.; Eid, N. Impulse oscillometry: Interpretation and practical applications. Chest 2014, 146, 841–847. [Google Scholar] [CrossRef]
- Enright, P.; Lehmann, S. Spirometry in old age: Feasibility and interpretation. In Respiratory Diseases in the Elderly; European Respiratory Society: Lausanne, Switzerland, 2009; pp. 25–34. [Google Scholar]
- Pezzoli, L.; Giardini, G.; Consonni, S.; Dallera, I.; Bilotta, C.; Ferrario, G.; Sandrini, C.M.; Annoni, G.; Vergani, C. Quality of spirometric performance in older people. Age Ageing 2003, 32, 43–46. [Google Scholar] [CrossRef] [Green Version]
- De Filippi, F.; Tana, F.; Vanzati, S.; Balzarini, B.; Galetti, G. Study of respiratory function in the elderly with different nutritional and cognitive status and functional ability assessed by plethysmographic and spirometric parameters. Arch. Gerontol. Geriatr. 2003, 37, 33–43. [Google Scholar] [CrossRef]
- Bellia, V.; Pistelli, R.; Catalano, F.; Antonelli-Incalzi, R.; Grassi, V.; Melillo, G.; Olivieri, D.; Rengo, F. Quality control of spirometry in the elderly. The SA.R.A. study. SAlute Respiration nell’Anziano = Respiratory Health in the Elderly. Am. J. Respir. Crit. Care Med. 2000, 161, 1094–1100. [Google Scholar] [CrossRef]
- Czajkowska-Malinowska, M.; Tomalak, W.; Radliński, J. Quality of spirometry in the elderly. Adv. Respir. Med. 2013, 81, 511–517. [Google Scholar] [CrossRef]
- Frei, J.; Jutla, J.; Kramer, G.; Hatzakis, G.E.; Ducharme, F.M.; Davis, G.M. Impulse oscillometry: Reference values in children 100 to 150 cm in height and 3 to 10 years of age. Chest 2005, 128, 1266–1273. [Google Scholar] [CrossRef]
- Wei, X.; Shi, Z.; Cui, Y.; Mi, J.; Ma, Z.; Ren, J.; Li, J.; Xu, S.; Guo, Y. Impulse oscillometry system as an alternative diagnostic method for chronic obstructive pulmonary disease. Medicine 2017, 96, e8543. [Google Scholar] [CrossRef]
- Piorunek, T.; Kostrzewska, M.; Cofta, S.; Batura-Gabryel, H.; Andrzejczak, P.; Bogdański, P.; Wysocka, E. Impulse oscillometry in the diagnosis of airway resistance in chronic obstructive pulmonary disease. Adv. Exp. Med. Biol. 2015, 838, 47–52. [Google Scholar] [CrossRef]
- Lundblad, L.K.A.; Miletic, R.; Piitulainen, E.; Wollmer, P. Oscillometry in Chronic Obstructive Lung Disease: In vitro and in vivo evaluation of the impulse oscillometry and tremoflo devices. Sci. Rep. 2019, 9, 11618. [Google Scholar] [CrossRef] [Green Version]
- Crim, C.; Celli, B.; Edwards, L.D.; Wouters, E.; Coxson, H.O.; Tal-Singer, R.; Calverley, P.M. Respiratory system impedance with impulse oscillometry in healthy and COPD subjects: ECLIPSE baseline results. Respir. Med. 2011, 105, 1069–1078. [Google Scholar] [CrossRef] [Green Version]
- Frantz, S.; Nihlén, U.; Dencker, M.; Engström, G.; Löfdahl, C.G.; Wollmer, P. Impulse oscillometry may be of value in detecting early manifestations of COPD. Respir. Med. 2012, 106, 1116–1123. [Google Scholar] [CrossRef] [Green Version]
- Mandrekar, J.N. Receiver operating characteristic curve in diagnostic test assessment. J. Thorac. Oncol. 2010, 5, 1315–1316. [Google Scholar] [CrossRef] [Green Version]
- Porojan-Suppini, N.; Fira-Mladinescu, O.; Marc, M.; Tudorache, E.; Oancea, C. Lung Function Assessment by Impulse Oscillometry in Adults. Ther. Clin. Risk Manag. 2020, 16, 1139–1150. [Google Scholar] [CrossRef]
- Kalhoff, H.; Breidenbach, R.; Smith, H.J.; Marek, W. Impulse oscillometry in preschool children and association with body mass index. Respirology 2011, 16, 174–179. [Google Scholar] [CrossRef]
- Bednarek, M.; Grabicki, M.; Piorunek, T.; Batura-Gabryel, H. Current place of impulse oscillometry in the assessment of pulmonary diseases. Respir. Med. 2020, 170, 105952. [Google Scholar] [CrossRef]
- Liu, Z.; Lin, L.; Liu, X. Clinical application value of impulse oscillometry in geriatric patients with COPD. Int. J. Chronic Obstr. Pulm. Dis. 2017, 12, 897–905. [Google Scholar] [CrossRef] [Green Version]
- Nakagawa, M.; Hattori, N.; Haruta, Y.; Sugiyama, A.; Iwamoto, H.; Ishikawa, N.; Fujitaka, K.; Murai, H.; Tanaka, J.; Kohno, N.; et al. Effect of increasing respiratory rate on airway resistance and reactance in COPD patients. Respirology 2015, 20, 87–94. [Google Scholar] [CrossRef] [Green Version]
- Jetmalani, K.; Thamrin, C.; Farah, C.S.; Bertolin, A.; Chapman, D.G.; Berend, N.; Salome, C.M.; King, G.G. Peripheral airway dysfunction and relationship with symptoms in smokers with preserved spirometry. Respirology 2018, 23, 512–518. [Google Scholar] [CrossRef]
- Desiraju, K.; Agrawal, A. Impulse oscillometry: The state-of-art for lung function testing. Lung India 2016, 33, 410–416. [Google Scholar] [CrossRef]
- McDonough, J.E.; Yuan, R.; Suzuki, M.; Seyednejad, N.; Elliott, W.M.; Sanchez, P.G.; Wright, A.C.; Gefter, W.B.; Litzky, L.; Coxson, H.O.; et al. Small-airway obstruction and emphysema in chronic obstructive pulmonary disease. N. Engl. J. Med. 2011, 365, 1567–1575. [Google Scholar] [CrossRef] [Green Version]
- Su, Z.Q.; Guan, W.J.; Li, S.Y.; Ding, M.; Chen, Y.; Jiang, M.; Chen, X.B.; Zhong, C.H.; Tang, C.L.; Zhong, N.S. Significances of spirometry and impulse oscillometry for detecting small airway disorders assessed with endobronchial optical coherence tomography in COPD. Int. J. Chronic Obstr. Pulm. Dis. 2018, 13, 3031–3044. [Google Scholar] [CrossRef] [Green Version]
- Hira, H.; Munjal, J.; Zachariah, S.; Chauhan, M.; Singh, A. The site of airway obstruction among patients of emphysema: Role of impulse oscillometry. Lung India 2008, 25, 8–13. [Google Scholar] [CrossRef]
- Peng, J.; Wu, F.; Tian, H.; Yang, H.; Zheng, Y.; Deng, Z.; Wang, Z.; Xiao, S.; Wen, X.; Huang, P.; et al. Clinical characteristics of and risk factors for small airway dysfunction detected by impulse oscillometry. Respir. Med. 2021, 190, 106681. [Google Scholar] [CrossRef]
- Xie, X.; de Jong, P.A.; Oudkerk, M.; Wang, Y.; Ten Hacken, N.H.; Miao, J.; Zhang, G.; de Bock, G.H.; Vliegenthart, R. Morphological measurements in computed tomography correlate with airflow obstruction in chronic obstructive pulmonary disease: Systematic review and meta-analysis. Eur. Radiol. 2012, 22, 2085–2093. [Google Scholar] [CrossRef] [Green Version]
- Garfield, J.L.; Marchetti, N.; Gaughan, J.P.; Steiner, R.M.; Criner, G.J. Total lung capacity by plethysmography and high-resolution computed tomography in COPD. Int. J. Chron. Obstruct. Pulmon. Dis. 2012, 7, 119–126. [Google Scholar] [CrossRef] [Green Version]
- Zaporozhan, J.; Ley, S.; Eberhardt, R.; Weinheimer, O.; Iliyushenko, S.; Herth, F.; Kauczor, H.U. Paired inspiratory/expiratory volumetric thin-slice CT scan for emphysema analysis: Comparison of different quantitative evaluations and pulmonary function test. Chest 2005, 128, 3212–3220. [Google Scholar] [CrossRef] [Green Version]
- Gierada, D.S.; Hakimian, S.; Slone, R.M.; Yusen, R.D. MR analysis of lung volume and thoracic dimensions in patients with emphysema before and after lung volume reduction surgery. Am. J. Roentgenol. 1998, 170, 707–714. [Google Scholar] [CrossRef] [Green Version]
- Coxson, H.O.; Nasute Fauerbach, P.V.; Storness-Bliss, C.; Müller, N.L.; Cogswell, S.; Dillard, D.H.; Finger, C.L.; Springmeyer, S.C. Computed tomography assessment of lung volume changes after bronchial valve treatment. Eur. Respir. J. 2008, 32, 1443–1450. [Google Scholar] [CrossRef] [Green Version]
- Mets, O.M.; Murphy, K.; Zanen, P.; Gietema, H.A.; Lammers, J.W.; van Ginneken, B.; Prokop, M.; de Jong, P.A. The relationship between lung function impairment and quantitative computed tomography in chronic obstructive pulmonary disease. Eur. Radiol. 2012, 22, 120–128. [Google Scholar] [CrossRef] [Green Version]
- Eda, S.; Kubo, K.; Fujimoto, K.; Matsuzawa, Y.; Sekiguchi, M.; Sakai, F. The relations between expiratory chest CT using helical CT and pulmonary function tests in emphysema. Am. J. Respir. Crit. Care Med. 1997, 155, 1290–1294. [Google Scholar] [CrossRef]
- Tylén, U.; Boijsen, M.; Ekberg-Jansson, A.; Bake, B.; Löfdahl, C.G. Emphysematous lesions and lung function in healthy smokers 60 years of age. Respir. Med. 2000, 94, 38–43. [Google Scholar] [CrossRef] [Green Version]
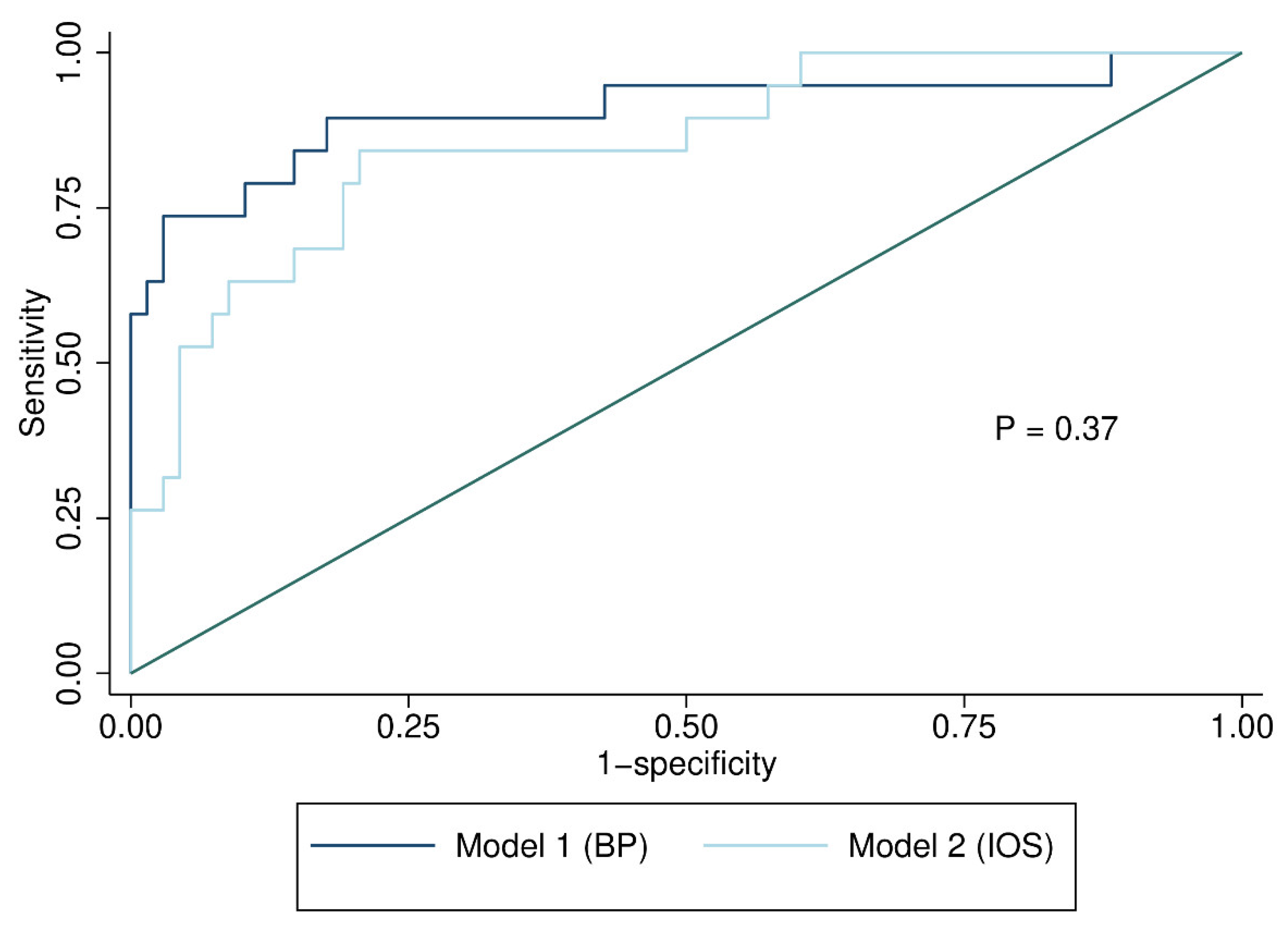
| Emphysema (n = 20) | No Emphysema (n = 68) | p * | |
|---|---|---|---|
| Clinical variables | n (%) | n (%) | |
| Female sex | 9 (45) | 36 (53) | 0.62 |
| Positive smoking history | 16 (80) | 26 (38) | <0.01 |
| Dyspnea | 16 (80) | 35 (51) | 0.04 |
| Cough | 8 (40) | 18 (26) | 0.27 |
| Comorbidities | |||
| 7 (35) | 27 (40) | 0.80 |
| 2 (4) | 3 (10) | 0.32 |
| 3 (15) | 11 (16) | 1.00 |
| 4 (20) | 7 (10) | 0.26 |
| 3 (15) | 8 (12) | 0.71 |
| 5 (7) | 2 (10) | 0.66 |
| 2 (3) | 1 (5) | 0.37 |
| CT scan results | |||
| 10 (50) | 19 (28) | 0.10 |
| 11 (55) | 20 (29) | 0.06 |
| 0 (0) | 11 (16) | 0.06 |
| 1 (5) | 6 (9) | 1.00 |
| 5 (25) | 14 (21) | 0.76 |
| Median (IQR) | Median (IQR) | ||
| Age (years) | 63 (52–72) | 62 (47–72) | 0.60 |
| BMI | 26.6 (23.3–31.2) | 26.3 (23.3–29.7) | 0.50 |
| Body Plethysmography | Median (IQR) | Median (IQR) | |
| FEV1 | 1.7 (0.9–2.6) | 2.8 (2.2–3.2) | <0.01 |
| FEV1 % of predicted | 67 (43–83) | 97 (83–107) | <0.01 |
| FVC | 3.1 (2.2–4.1) | 3.6 (3.2–4.2) | 0.07 |
| FVC % of predicted | 99 (77–112) | 104 (95–112) | 0.02 |
| FEV1/FVC | 52.7 (46.2–63.3) | 75.3 (70.2–80.4) | <0.01 |
| TLC | 7.0 (5.3–7.8) | 5.8 (5.2–6.8) | 0.04 |
| TLC % of predicted | 109 (100–132) | 97 (88–107) | <0.01 |
| RV | 3.5 (2.6–4.7) | 2.4 (2.0–3.0) | <0.01 |
| RV % of predicted | 153 (129–217) | 114 (93–131) | <0.01 |
| RV/TLC | 0.52 (0.44–0.64) | 0.40 (0.34–0.48) | <0.01 |
| DLCOc % of predicted | 56 (43–63) | 73 (65–82) | <0.01 |
| Impulse Oscillometry | Median (IQR) | Median (IQR) | |
| R5 % of predicted | 108 (80–141) | 98 (77–120) | 0.06 |
| R5-20 | 0.14 (0.06–0.25) | 0.06 (0.03–0.10) | <0.01 |
| X5 | −0.15 (−0.28–−0.09) | −0.10 (−0.13–−0.07) | <0.01 |
| Fres | 21.17 (15.46–24.16) | 14.08 (10.50–18.16) | <0.01 |
| Ax | 1.18 (0.29–2.90) | 0.32 (0.16–0.81) | <0.01 |
| Sensitivity, % (95% CI) | PPV, % (95% CI) | Specificity, % (95% CI) | NPV, % (95% CI) | AUC (95% CI) | |
|---|---|---|---|---|---|
| Clinical variables | |||||
| Age | - | - | - | - | 0.539 (0.408–0.670) |
| BMI | - | - | - | - | 0.547 (0.399–0.696) |
| Female sex | 45 (23–69) | 20 (10–35) | 47 (35–60) | 74 (59–87) | - |
| Dyspnea | 80 (56–94) | 31 (19–46) | 49 (36–61) | 89 (75–97) | - |
| Cough | 40 (19–64) | 31 (14–52) | 74 (61–84) | 81 (69–90) | - |
| Positive smoking history | 80 (56–94) | 38 (24–54) | 61 (48–72) | 91 (78–98) | - |
| Body Plethysmography | |||||
| FEV1 % of predicted ** | - | - | - | - | 0.842 (0.728–0.955) |
| FEV1/FVC ** | - | - | - | - | 0.874 (0.766–0.981) |
| RV/TLC | - | - | - | - | 0.743 (0.621–0.865) |
| DLCOc % of predicted ** | - | - | - | - | 0.795 (0.682–0.908) |
| Impulse Oscillometry | |||||
| R5 % of predicted | - | - | - | - | 0.592 (0.431–0.754) |
| R5-20 | - | - | - | - | 0.718 (0.568–0.867) |
| X5 ** | - | - | - | - | 0.630 (0.462–0.798) |
| Fres | - | - | - | - | 0.742 (0.603–0.881) |
| Ax | - | - | - | - | 0.704 (0.537–0.870) |
| Model 1 (BP) | Model 2 (IOS) | |||
|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | |
| Positive smoking history | 1.45 (0.28–7.45) | 0.66 | 4.39 (1.11–17.39) | 0.04 |
| FEV1/FVC | 0.86 (0.79–0.94) | <0.01 | - | - |
| RV/TLC | 5.80 (0.002–20,480.37) | 0.42 | - | - |
| DLCOc % of predicted | 0.95 (0.90–0.995) | 0.04 | - | - |
| R5 % of predicted | - | - | 0.97 (0.94–0.995) | 0.03 |
| Fres | - | - | 1.00 (0.81–1.25) | 0.97 |
| Ax | - | - | 9.12 (1.76–47.36) | <0.01 |
| Diagnostic accuracy (95% CI) | ||||
| AUC | 0.905 (0.805–1.00) | 0.861 (0.767–0.955) | ||
| CV-AUC | 0.892 (0.654–0.943 *) | 0.839 (0.688–0.931 *) | ||
| Sensitivity, % | 84.2 (50.0–94.1 *) | 80.0 (61.9–94.7 *) | ||
| PPV, % | 59.3 (35.5–77.8 *) | 55.2 (40.0–73.1 *) | ||
| Specificity, % | 83.8 (64.3–91.5 *) | 80.9 (53.6–93.2 *) | ||
| NPV, % | 95.0 (85.7–98.2 *) | 93.7 (87.5–97.7 *) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klitgaard, A.; Løkke, A.; Hilberg, O. Impulse Oscillometry as a Diagnostic Test for Pulmonary Emphysema in a Clinical Setting. J. Clin. Med. 2023, 12, 1547. https://doi.org/10.3390/jcm12041547
Klitgaard A, Løkke A, Hilberg O. Impulse Oscillometry as a Diagnostic Test for Pulmonary Emphysema in a Clinical Setting. Journal of Clinical Medicine. 2023; 12(4):1547. https://doi.org/10.3390/jcm12041547
Chicago/Turabian StyleKlitgaard, Allan, Anders Løkke, and Ole Hilberg. 2023. "Impulse Oscillometry as a Diagnostic Test for Pulmonary Emphysema in a Clinical Setting" Journal of Clinical Medicine 12, no. 4: 1547. https://doi.org/10.3390/jcm12041547
APA StyleKlitgaard, A., Løkke, A., & Hilberg, O. (2023). Impulse Oscillometry as a Diagnostic Test for Pulmonary Emphysema in a Clinical Setting. Journal of Clinical Medicine, 12(4), 1547. https://doi.org/10.3390/jcm12041547





