Association between Mean Arterial Pressure during the First 24 Hours and Clinical Outcome in Critically Ill Stroke Patients: An Analysis of the MIMIC-III Database
Abstract
1. Introduction
2. Methods
2.1. Data Sources
2.2. Study Population
2.3. Data Extraction and Variables
2.4. Endpoints and Definitions
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics
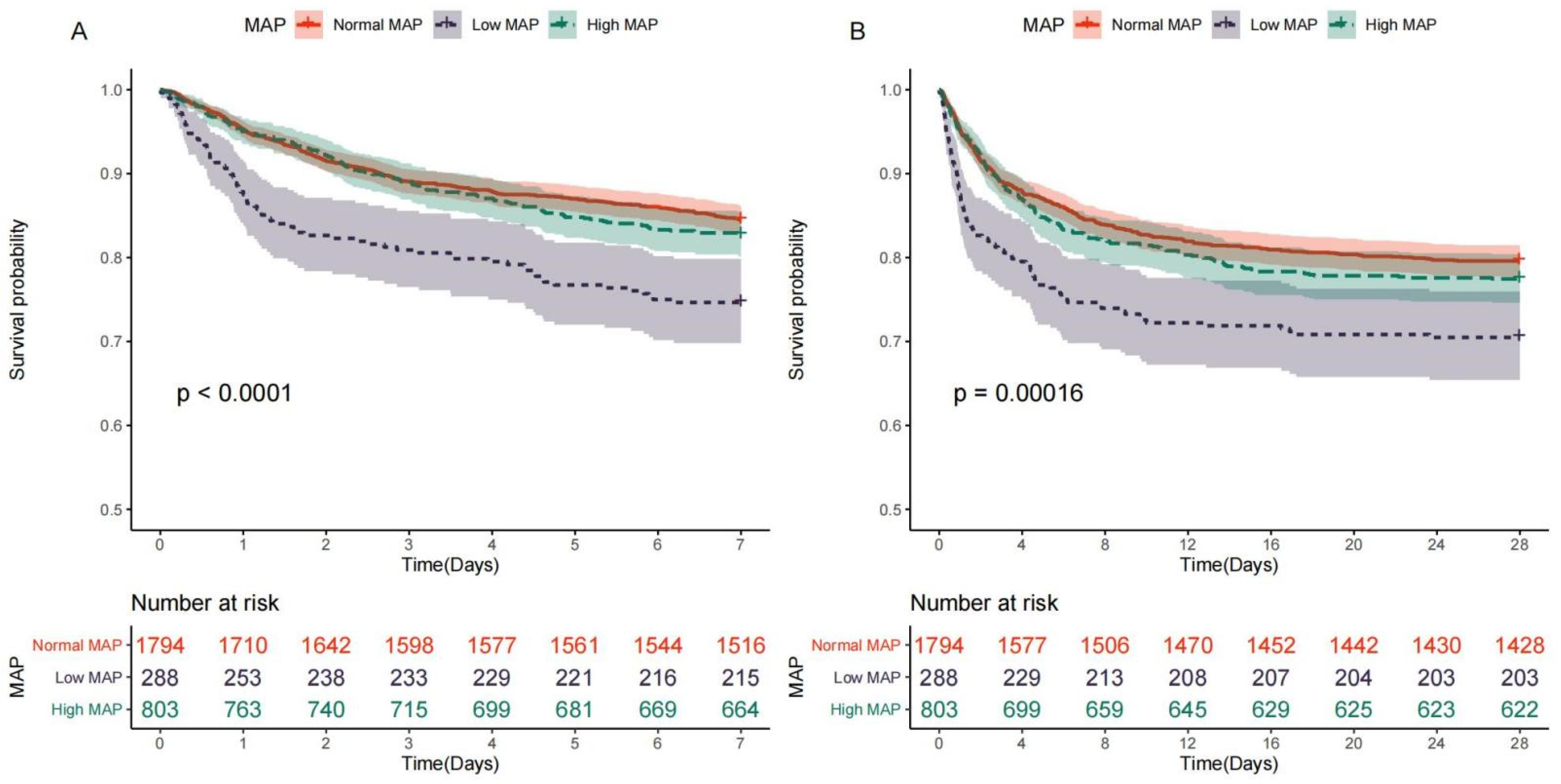
3.2. Relationship between MAP and Mortality
3.3. Non-Linear Association between MAP and Outcomes
3.4. Sensitivity Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Campbell, B.; De Silva, D.A.; Macleod, M.R.; Coutts, S.B.; Schwamm, L.H.; Davis, S.M.; Donnan, G.A. Ischaemic stroke. Nature reviews. Dis. Prim. 2019, 5, 70. [Google Scholar] [CrossRef]
- Campbell, B.; Khatri, P. Stroke. Lancet 2020, 396, 129–142. [Google Scholar] [CrossRef] [PubMed]
- Ikram, M.A.; Wieberdink, R.G.; Koudstaal, P.J. International epidemiology of intracerebral hemorrhage. Curr. Atheroscler. Rep. 2012, 14, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Labovitz, D.L.; Sacco, R.L. Intracerebral hemorrhage: Update. Curr. Opin. Neurol. 2001, 14, 103–108. [Google Scholar] [CrossRef]
- Toyoda, K.; Koga, M.; as the SAMURAI Investigators. Controlling blood pressure soon after intracerebral hemorrhage: The SAMURAI-ICH Study and its successors. Hypertens. Res. Off. J. Jpn. Soc. Hypertens. 2022, 45, 583–590. [Google Scholar] [CrossRef]
- Lim, N.K.; Park, H.Y.; Kim, W.H.; Mancia, G.; Cho, M.C. The U-shaped association between achieved blood pressure and risk of cardiovascular events and mortality in elderly and younger patients. J. Hypertens. 2020, 38, 1559–1566. [Google Scholar] [CrossRef]
- Bangalore, S.; Schwamm, L.; Smith, E.E.; Hellkamp, A.S.; Suter, R.E.; Xian, Y.; Schulte, P.J.; Fonarow, G.C.; Bhatt, D.L. With the Guidelines-Stroke Steering Committee and Investigators. Blood pressure and in-hospital outcomes in patients presenting with ischaemic stroke. Eur. Heart J. 2017, 38, 2827–2835. [Google Scholar] [CrossRef]
- Leonardi-Bee, J.; Bath, P.M.; Phillips, S.J.; Sandercock, P.A.; IST Collaborative Group. Blood pressure and clinical outcomes in the International Stroke Trial. Stroke 2002, 33, 1315–1320. [Google Scholar] [CrossRef]
- Okumura, K.; Ohya, Y.; Maehara, A.; Wakugami, K.; Iseki, K.; Takishita, S. Effects of blood pressure levels on case fatality after acute stroke. J. Hypertens. 2005, 23, 1217–1223. [Google Scholar] [CrossRef]
- Willmot, M.; Leonardi-Bee, J.; Bath, P.M. High blood pressure in acute stroke and subsequent outcome: A systematic review. Hypertension (Dallas, Tex. 1979) 2004, 43, 18–24. [Google Scholar] [CrossRef]
- Lu, Z.; Zhang, J.; Hong, J.; Wu, J.; Liu, Y.; Xiao, W.; Hua, T.; Yang, M. Development of a Nomogram to Predict 28-Day Mortality of Patients With Sepsis-Induced Coagulopathy: An Analysis of the MIMIC-III Database. Front. Med. 2021, 8, 661710. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.E.; Tong, X.; George, M.G.; Coleman King, S.M.; Yin, X.; O’Brien, S.; Ibrahim, G.; Liskay, A.; the Paul Coverdell National Acute Stroke Program team; Wiltz, J.L. Trends and Factors Associated With Concordance Between International Classification of Diseases, Ninth and Tenth Revision, Clinical Modification Codes and Stroke Clinical Diagnoses. Stroke 2019, 50, 1959–1967. [Google Scholar] [CrossRef] [PubMed]
- Douvris, A.; Malhi, G.; Hiremath, S.; McIntyre, L.; Silver, S.; Bagshaw, S.M.; Wald, R.; Ronco, C.; Sikora, L.; Weber, C.; et al. Interventions to prevent hemodynamic instability during renal replacement therapy in critically ill patients: A systematic review. Crit. Care 2019, 22, 41. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, P.; Furie, K.L.; Davis, S.M.; Donnan, G.A.; Norrving, B. World Stroke Organization global stroke services guidelines and action plan. Int. J. Stroke Off. J. Int. Stroke Soc. 2014, 9 (Suppl. A100), 4–13. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhang, S.; Deng, Y.; Wu, S.; Ren, J.; Sun, G.; Yang, J.; Jiang, Y.; Xu, X.; Wang, T.-D.; et al. Trial of Intensive Blood-Pressure Control in Older Patients with Hypertension. N. Engl. J. Med. 2021, 385, 1268–1279. [Google Scholar] [CrossRef]
- Hemphill, J.C., 3rd; Greenberg, S.M.; Anderson, C.S.; Becker, K.; Bendok, B.R.; Cushman, M.; Fung, G.L.; Goldstein, J.N.; Macdonald, R.L.; Mitchell, P.H.; et al. Guidelines for the Management of Spontaneous Intracerebral Hemorrhage: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 2015, 46, 2032–2060. [Google Scholar] [CrossRef]
- Powers, W.J.; Rabinstein, A.A.; Ackerson, T.; Adeoye, O.M.; Bambakidis, N.C.; Becker, K.; Biller, J.; Brown, M.; Demaerschalk, B.M.; Hoh, B.; et al. Guidelines for the Early Management of Patients With Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 2019, 50, e344–e418. [Google Scholar] [CrossRef]
- WHO publishes definitive atlas on global heart disease and stroke epidemic. Indian J. Med. Sci. 2004, 58, 405–406.
- Vemmos, K.N.; Tsivgoulis, G.; Spengos, K.; Zakopoulos, N.; Synetos, A.; Manios, E.; Konstantopoulou, P.; Mavrikakis, M. U-shaped relationship between mortality and admission blood pressure in patients with acute stroke. J. Intern. Med. 2004, 255, 257–265. [Google Scholar] [CrossRef]
- Hao, Z.; Liu, M.; Wang, D.; Wu, B.; Tao, W.; Chang, X. High blood pressure on admission in relation to poor outcome in acute ischemic stroke with intracranial atherosclerotic stenosis or occlusion. J. Stroke Cerebrovasc. Dis. Off. J. Natl. Stroke Assoc. 2014, 23, 1403–1408. [Google Scholar] [CrossRef]
- Zhao, R.; Liu, F.-D.; Wang, S.; Peng, J.-L.; Tao, X.-X.; Zheng, B.; Zhang, Q.-T.; Yao, Q.; Shen, X.-L.; Li, W.-T.; et al. Blood Pressure Reduction in the Acute Phase of an Ischemic Stroke Does Not Improve Short- or Long-Term Dependency or Mortality: A Meta-Analysis of Current Literature. Medicine 2015, 94, e896. [Google Scholar] [CrossRef]
- Anderson, C.S.; Heeley, E.; Huang, Y.; Wang, J.; Stapf, C.; Delcourt, C.; Lindley, R.; Robinson, T.; Lavados, P.; Neal, B.; et al. Rapid blood-pressure lowering in patients with acute intracerebral hemorrhage. N. Engl. J. Med. 2013, 368, 2355–2365. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, A.I.; Huang, W.; Lobanova, I.; Barsan, W.G.; Hanley, D.F.; Hsu, C.Y.; Lin, C.-L.; Silbergleit, R.; Steiner, T.; Suarez, J.I.; et al. Outcomes of Intensive Systolic Blood Pressure Reduction in Patients With Intracerebral Hemorrhage and Excessively High Initial Systolic Blood Pressure: Post Hoc Analysis of a Randomized Clinical Trial. JAMA Neurol. 2020, 77, 1355–1365. [Google Scholar] [CrossRef] [PubMed]
- Moullaali, T.J.; Wang, X.; Martin, R.H.; Shipes, V.B.; Robinson, T.G.; Chalmers, J.; Suarez, J.I.; Qureshi, A.I.; Palesch, Y.Y.; Anderson, C.S. Blood pressure control and clinical outcomes in acute intracerebral haemorrhage: A preplanned pooled analysis of individual participant data. Lancet Neurol. 2019, 18, 857–864. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, M.; Muñoz-Venturelli, P.; Billot, L.; Wang, X.; Song, L.; Arima, H.; Lavados, P.M.; Hackett, M.L.; Olavarría, V.V.; Brunser, A.; et al. Low blood pressure and adverse outcomes in acute stroke: HeadPoST study explanations. J. Hypertens. 2021, 39, 273–279. [Google Scholar] [CrossRef]
- Verschoof, M.A.; Groot, A.E.; Vermeij, J.-D.; Westendorp, W.F.; Berg, S.A.V.D.; Nederkoorn, P.J.; van de Beek, D.; Coutinho, J.M. Association Between Low Blood Pressure and Clinical Outcomes in Patients With Acute Ischemic Stroke. Stroke 2020, 51, 338–341. [Google Scholar] [CrossRef]
- Greenberg, S.M.; Ziai, W.C.; Cordonnier, C.; Dowlatshahi, D.; Francis, B.; Goldstein, J.N.; Iii, J.C.H.; Johnson, R.; Keigher, K.M.; Mack, W.J.; et al. 2022 Guideline for the Management of Patients With Spontaneous Intracerebral Hemorrhage: A Guideline From the American Heart Association/American Stroke Association. Stroke 2022, 53, e282–e361. [Google Scholar] [CrossRef]
- Qureshi, A.I.; Palesch, Y.Y.; Barsan, W.G.; Hanley, D.F.; Hsu, C.Y.; Martin, R.L.; Moy, C.S.; Silbergleit, R.; Steiner, T.; Suarez, J.I.; et al. Intensive Blood-Pressure Lowering in Patients with Acute Cerebral Hemorrhage. N. Engl. J. Med. 2016, 375, 1033–1043. [Google Scholar] [CrossRef]
- Flack, J.M.; Adekola, B. Blood pressure and the new ACC/AHA hypertension guidelines. Trends Cardiovasc. Med. 2020, 30, 160–164. [Google Scholar] [CrossRef]





| Characteristics | Overall | Normal MAP Group (70 mmHg > MAP ≤ 90 mmHg) | Low MAP Group (MAP ≤ 70 mmHg) | High MAP Group (MAP > 90 mmHg) | p Value for Overall | p Value for Normal MAP VS. Low MAP | p Value for Normal MAP VS. High MAP |
|---|---|---|---|---|---|---|---|
| n = 2885 | n = 1794 | n = 289 | n = 802 | ||||
| Male | 1490 (51.6%) | 914 (50.9%) | 128 (44.3%) | 448 (55.9%) | 0.002 | 0.042 | 0.035 |
| Age, years | 69.9 (57.7;79.6) | 70.9 (58.1;79.7) | 75.4 (66.5;81.3) | 65.6 (54.7;77.6) | <0.001 | <0.001 | <0.001 |
| White | 2105 (73.0%) | 1340 (74.7%) | 230 (79.6%) | 535 (66.7%) | 0.001 | 0.493 | 0.003 |
| GCS | 14.0 (12.0;15.0) | 14.0 (12.0;15.0) | 15.0 (12.0;15.0) | 14.0 (11.0;15.0) | 0.023 | 0.376 | 0.039 |
| PO2, mmHg | 158 (107;237) | 157 (108;238) | 166 (103;257) | 158 (108;227) | 0.723 | 0.689 | 0.689 |
| PCO2, mmHg | 38.0 (34.0;43.0) | 38.0 (33.2;43.0) | 38.0 (34.0;45.0) | 38.0 (34.0;42.0) | 0.042 | 0.028 | 0.693 |
| pH | 7.41 (7.36;7.45) | 7.41 (7.36;7.45) | 7.40 (7.35;7.45) | 7.41 (7.38;7.45) | <0.001 | 0.072 | 0.002 |
| Heart rate, bpm | 77.0 (68.0;87.0) | 76.7 (68.0;86.5) | 71.6 (64.1;82.0) | 79.1 (70.6;90.1) | <0.001 | <0.001 | <0.001 |
| MAP, mmHg | 83.7 (76.3;91.1) | 81.3 (76.5;85.5) | 66.5 (63.4;68.3) | 96.3 (92.8;101) | 0 | <0.001 | 0 |
| Respiratory rate, bpm | 17.7 (15.9;19.9) | 17.5 (15.8;19.6) | 17.8 (16.0;20.0) | 18.0 (16.1;20.2) | 0.001 | 0.286 | <0.001 |
| Temperature, °C | 36.9 (36.5;37.3) | 36.9 (36.5;37.3) | 36.8 (36.4;37.2) | 36.9 (36.5;37.3) | 0.024 | 0.027 | 0.387 |
| Glucose, mg/dL | 133 (115;160) | 135 (116;162) | 131 (111;160) | 130 (113;157) | 0.003 | 0.108 | 0.004 |
| Hemoglobin, g/L | 11.7 (10.3;13.0) | 11.6 (10.3;12.9) | 10.6 (8.90;12.0) | 12.4 (11.0;13.6) | <0.001 | <0.001 | <0.001 |
| Platelet, ×109/L | 214 (170;266) | 212 (170;265) | 213 (157;264) | 220 (176;270) | 0.054 | 0.337 | 0.067 |
| WBC, ×109/L | 11.4 (8.70;14.7) | 11.4 (8.70;14.8) | 11.2 (8.40;15.0) | 11.5 (8.90;14.3) | 0.918 | 0.898 | 0.898 |
| Creatinine, mg/dL | 0.90 (0.80;1.20) | 0.90 (0.80;1.20) | 1.00 (0.80;1.40) | 1.00 (0.80;1.20) | 0.002 | 0.001 | 0.27 |
| BUN, mg/dL | 18.0 (13.0;25.0) | 18.0 (13.0;24.0) | 20.0 (15.0;32.0) | 17.0 (13.0;23.0) | <0.001 | <0.001 | 0.526 |
| Potassium, mmol/L | 3.70 (3.40;4.00) | 3.70 (3.40;4.00) | 3.80 (3.50;4.10) | 3.60 (3.30;4.00) | <0.001 | 0.003 | 0.005 |
| Sodium, mmol/L | 138 (136;140) | 138 (136;140) | 138 (135;140) | 138 (136;140) | 0.005 | 0.565 | 0.007 |
| Chloride, mmol/L | 103 (100;106) | 103 (100;106) | 103 (100;106) | 103 (100;105) | 0.778 | 0.72 | 0.72 |
| Bicarbonate, mmol/L | 23.0 (21.0;26.0) | 23.0 (21.0;26.0) | 23.0 (21.0;26.0) | 24.0 (21.0;26.0) | 0.734 | 0.72 | 0.72 |
| PTT, s | 27.8 (24.9;33.6) | 27.8 (24.9;33.6) | 29.1 (25.8;36.2) | 27.4 (24.7;32.2) | 0.003 | 0.02 | 0.092 |
| PT, s | 13.4 (12.7;14.7) | 13.4 (12.7;14.6) | 13.7 (12.9;16.0) | 13.3 (12.6;14.5) | 0.002 | 0.001 | 0.543 |
| INR | 1.20 (1.10;1.30) | 1.20 (1.10;1.30) | 1.20 (1.10;1.50) | 1.20 (1.10;1.30) | 0.007 | 0.006 | 0.45 |
| Weight, kg | 75.0 (63.5;88.7) | 75.0 (63.5;88.1) | 70.0 (60.9;81.6) | 76.7 (64.0;91.3) | <0.001 | 0.001 | 0.048 |
| SOFA score | 3.00 (1.00;4.00) | 3.00 (1.00;4.00) | 3.00 (2.00;5.00) | 2.00 (1.00;4.00) | <0.001 | <0.001 | <0.001 |
| SAPS score | 33.0 (25.0;41.0) | 33.0 (25.0;41.0) | 37.0 (30.0;49.0) | 30.0 (23.0;38.0) | <0.001 | <0.001 | <0.001 |
| RRT | 30 (1.04%) | 17 (0.95%) | 7 (2.42%) | 6 (0.75%) | 0.068 | 0.075 | 0.784 |
| Ventilation | 1239 (42.9%) | 772 (43.0%) | 119 (41.2%) | 348 (43.4%) | 0.803 | 0.897 | 0.898 |
| Elixhauser score | 5.00 (0.00;10.0) | 5.00 (0.00;10.0) | 5.00 (0.00;11.0) | 5.00 (0.00;9.00) | 0.77 | 0.763 | 0.87 |
| CHD | 414 (14.4%) | 246 (13.7%) | 56 (19.4%) | 112 (14.0%) | 0.036 | 0.043 | 0.912 |
| Cardiac arrhythmias | 960 (33.3%) | 572 (31.9%) | 103 (35.6%) | 285 (35.5%) | 0.126 | 0.346 | 0.224 |
| Hypertension | 1983 (68.7%) | 1195 (66.6%) | 193 (66.8%) | 595 (74.2%) | <0.001 | 1 | <0.001 |
| Diabetes | 672 (23.3%) | 420 (23.4%) | 92 (31.8%) | 160 (20.0%) | <0.001 | 0.004 | 0.057 |
| Liver disease | 93 (3.22%) | 56 (3.12%) | 14 (4.84%) | 23 (2.87%) | 0.244 | 0.274 | 0.823 |
| Peptic ulcer | 14 (0.49%) | 6 (0.33%) | 7 (2.42%) | 1 (0.12%) | <0.001 | 0.001 | 0.448 |
| Solid tumor | 47 (1.63%) | 28 (1.56%) | 8 (2.77%) | 11 (1.37%) | 0.261 | 0.294 | 0.848 |
| Coagulopathy | 139 (4.82%) | 85 (4.74%) | 15 (5.19%) | 39 (4.86%) | 0.944 | 0.97 | 0.97 |
| Obesity | 73 (2.53%) | 38 (2.12%) | 4 (1.38%) | 31 (3.87%) | 0.014 | 0.55 | 0.046 |
| Length of hospital, days | 6.78 (3.58;12.9) | 6.91 (3.69;13.0) | 4.91 (2.02;9.38) | 7.18 (3.86;13.7) | <0.001 | <0.001 | 0.157 |
| Length of ICU stay, days | 2.34 (1.22;5.84) | 2.34 (1.21;6.08) | 1.89 (1.05;3.72) | 2.69 (1.44;6.00) | <0.001 | <0.001 | 0.146 |
| all-cause mortality | 640 (22.2%) | 370 (20.6%) | 86 (29.8%) | 184 (22.9%) | 0.002 | 0.002 | 0.2 |
| 7-day mortality | 490 (17.0%) | 278 (15.5%) | 74 (25.6%) | 138 (17.2%) | <0.001 | <0.001 | 0.298 |
| 28-day mortality | 632 (21.9%) | 366 (20.4%) | 86 (29.8%) | 180 (22.4%) | 0.002 | 0.001 | 0.259 |
| Model | 7-Day Mortality | 28-Day Mortality | |||
|---|---|---|---|---|---|
| Hazard Ratio (95% CI) | p | Hazard Ratio (95% CI) | p | ||
| Model1 | Low MAP | 1.71(1.32–2.12) | <0.001 | 1.51(1.19–1.91) | <0.001 |
| High MAP | 1.18(0.96–1.45) | 0.120 | 1.18(0.99–1.41) | 0.069 | |
| Model2 | Low MAP | 1.79(1.38–2.32) | <0.001 | 1.58(1.25–2.00) | <0.001 |
| High MAP | 1.13(0.92–1.39) | 0.239 | 1.12(0.93–1.34) | 0.221 | |
| Model3 | Low MAP | 1.79(1.38–2.33) | <0.001 | 1.56(1.23–1.99) | <0.001 |
| High MAP | 1.13(0.92–1.39) | 0.241 | 1.12(0.94–1.34) | 0.215 | |
| Model4 | Low MAP | 2.04(1.55–2.69) | <0.001 | 1.76(1.37–2.27) | <0.001 |
| High MAP | 1.04(0.84–1.29) | 0.702 | 1.09(0.90–1.32) | 0.372 | |
| Model5 | Low MAP | 1.64(1.23–2.17) | 0.001 | 1.51(1.17–1.94) | 0.002 |
| High MAP | 1.26(1.01–1.57) | 0.043 | 1.28(1.06–1.55) | 0.012 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.; Cui, Y.-L.; Yu, S.; Shang, W.-F.; Li, J.; Pan, X.-J.; Wen, Z.-L.; Huang, S.-S.; Chen, L.-M.; Shen, X.; et al. Association between Mean Arterial Pressure during the First 24 Hours and Clinical Outcome in Critically Ill Stroke Patients: An Analysis of the MIMIC-III Database. J. Clin. Med. 2023, 12, 1556. https://doi.org/10.3390/jcm12041556
Zhang S, Cui Y-L, Yu S, Shang W-F, Li J, Pan X-J, Wen Z-L, Huang S-S, Chen L-M, Shen X, et al. Association between Mean Arterial Pressure during the First 24 Hours and Clinical Outcome in Critically Ill Stroke Patients: An Analysis of the MIMIC-III Database. Journal of Clinical Medicine. 2023; 12(4):1556. https://doi.org/10.3390/jcm12041556
Chicago/Turabian StyleZhang, Sheng, Yun-Liang Cui, Sheng Yu, Wei-Feng Shang, Jie Li, Xiao-Jun Pan, Zhen-Liang Wen, Si-Si Huang, Li-Min Chen, Xuan Shen, and et al. 2023. "Association between Mean Arterial Pressure during the First 24 Hours and Clinical Outcome in Critically Ill Stroke Patients: An Analysis of the MIMIC-III Database" Journal of Clinical Medicine 12, no. 4: 1556. https://doi.org/10.3390/jcm12041556
APA StyleZhang, S., Cui, Y.-L., Yu, S., Shang, W.-F., Li, J., Pan, X.-J., Wen, Z.-L., Huang, S.-S., Chen, L.-M., Shen, X., Yu, Y.-T., Liu, J., & Chen, D.-C. (2023). Association between Mean Arterial Pressure during the First 24 Hours and Clinical Outcome in Critically Ill Stroke Patients: An Analysis of the MIMIC-III Database. Journal of Clinical Medicine, 12(4), 1556. https://doi.org/10.3390/jcm12041556




