Development of an RNase H2 Activity Assay for Clinical Screening
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Approval and Control Group Selection
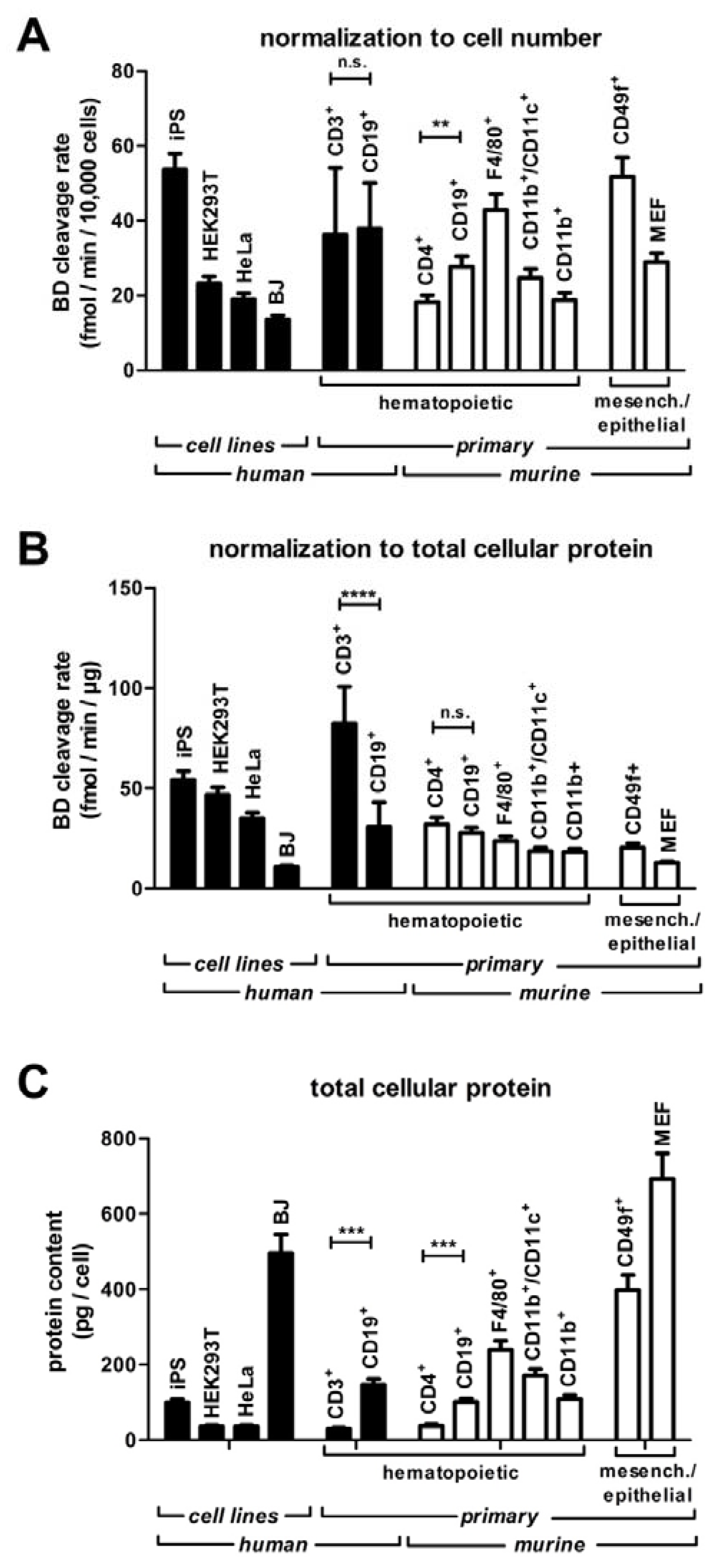
2.2. Cell Culture
2.3. Isolation of Primary Cells from Human Blood and Mice
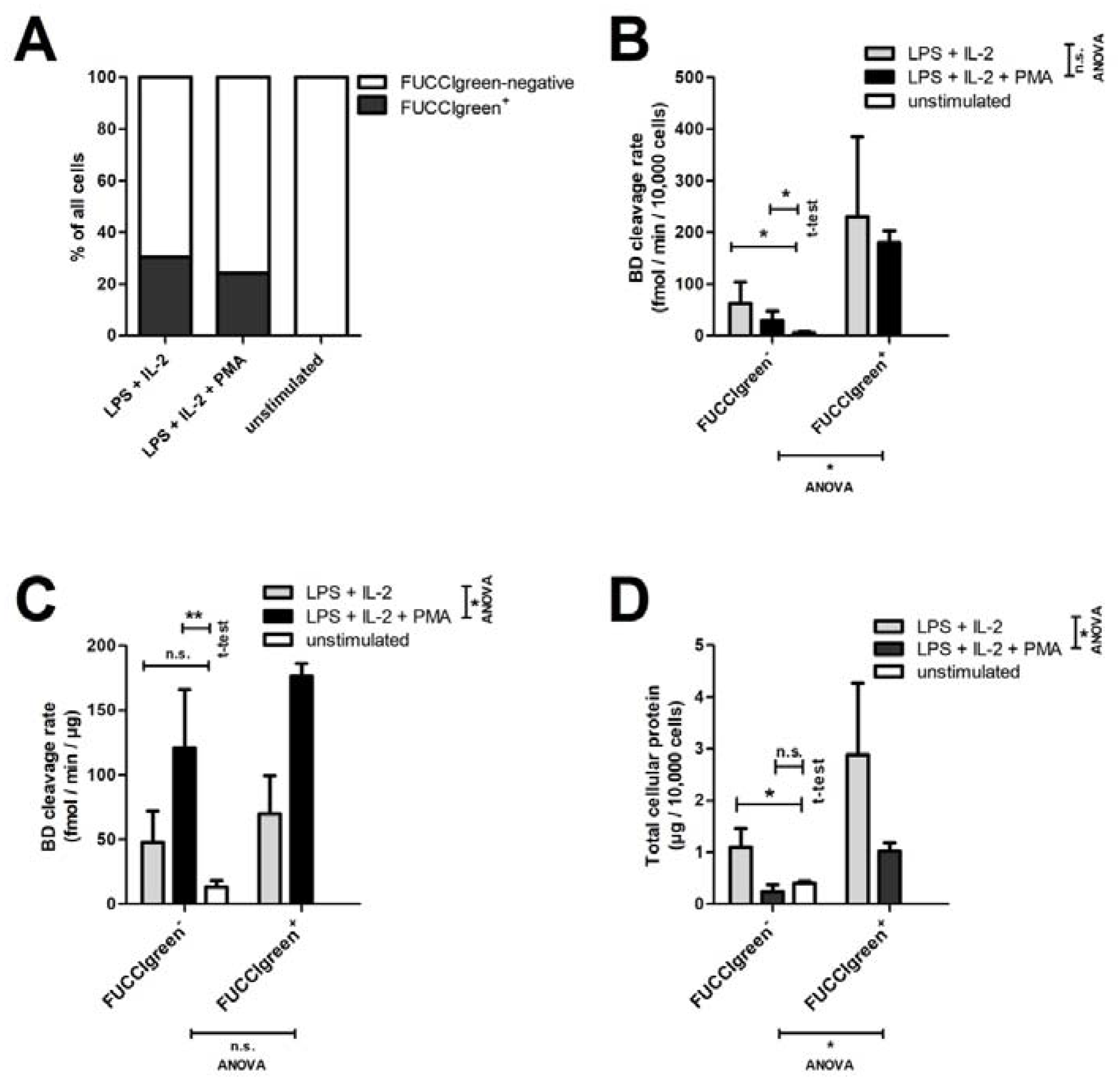
2.4. Short-Time Culture of Spleen B Cells from FUCCI Mice
2.5. Flow Cytometric Cell Sorting
2.6. Cell Lysis and Protein Quantification
2.7. RNase H2 Activity Assay and Standard Conditions
2.8. Statistics

3. Results
3.1. Implementation of Controls and Standard Curves
3.2. Sensitivity and Ruggedness
3.3. Steady-State Kinetics and Assay Endpoints
3.4. Precision
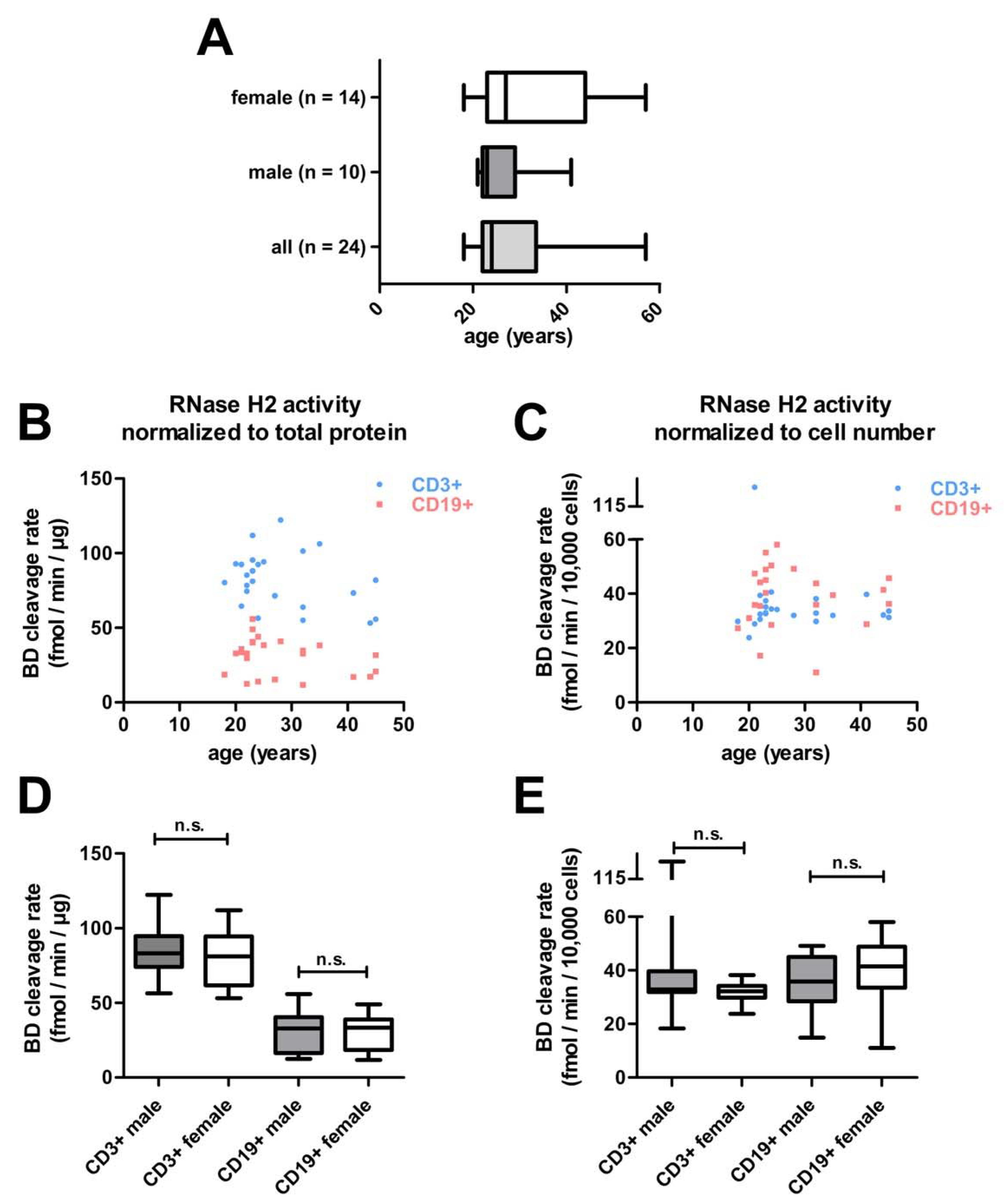
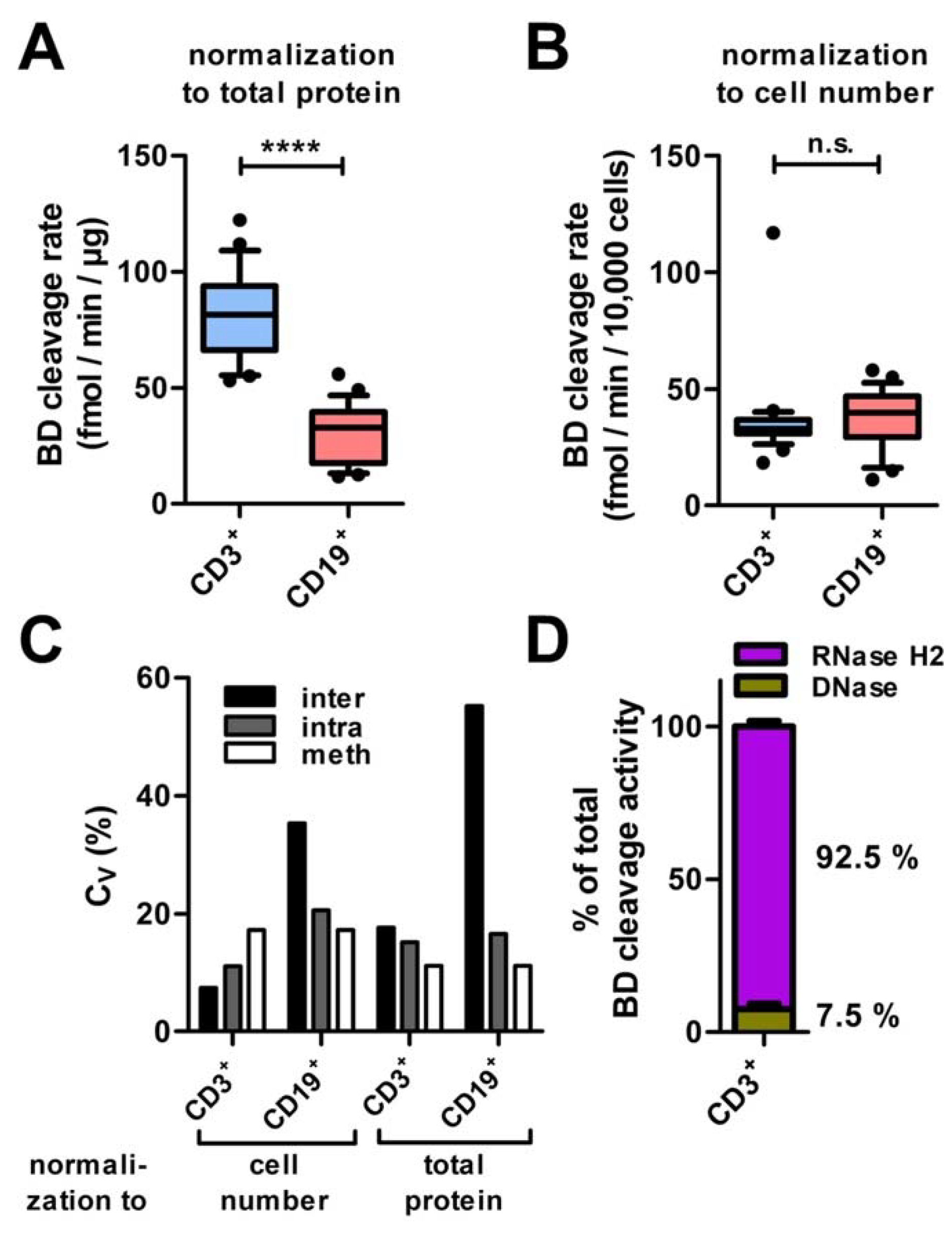
3.5. Screening RNase H2 Activity in Human Lymphocytes
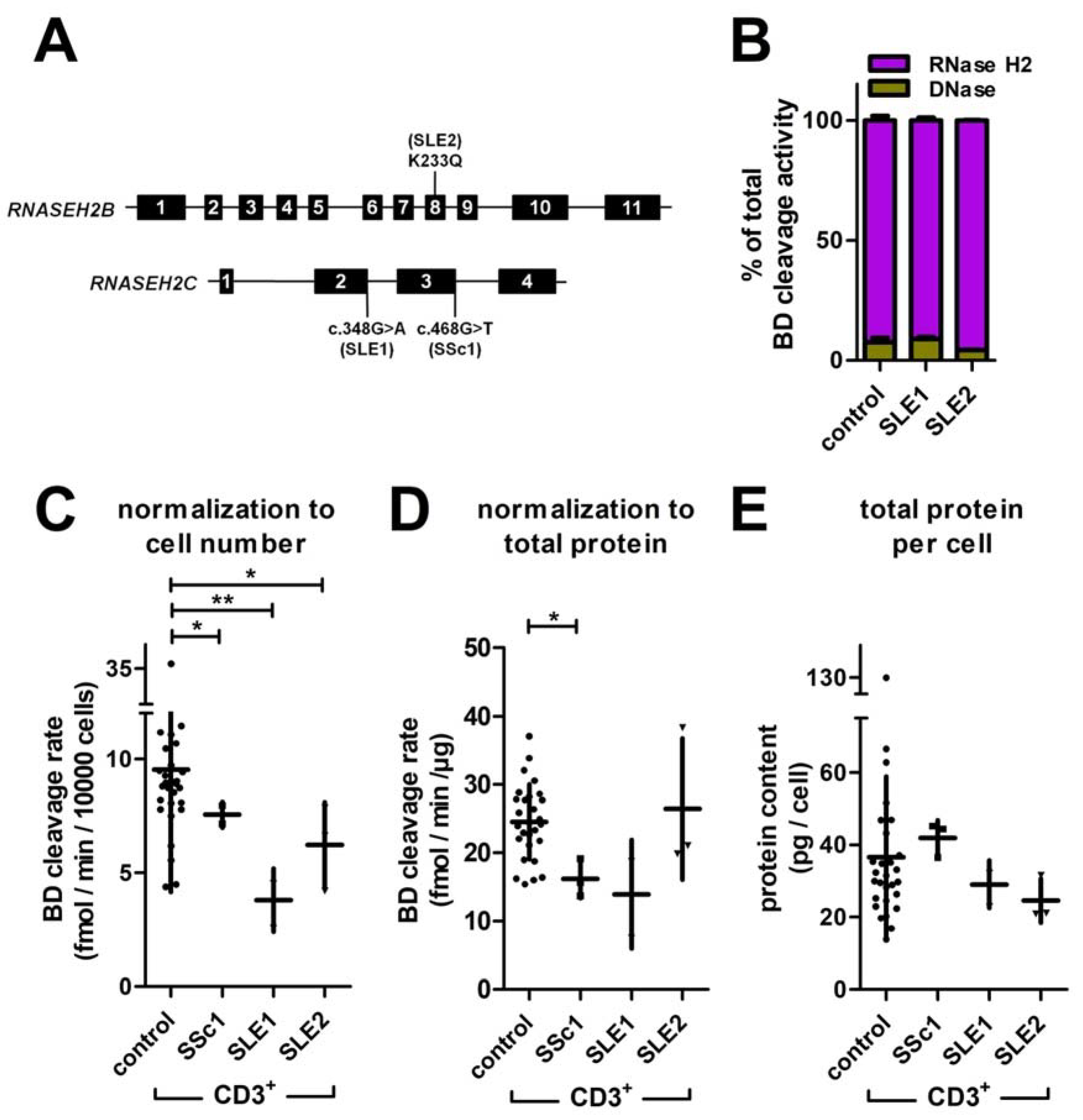
3.6. Reduced Rnase H2 Activity in T Cells of Patients with Systemic Autoimmunity
4. Discussions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Organism | Cell Type | Minimum Amount of Protein/Well (µg) | Maximum Amount of Protein/Well (µg) | Optimal Amount of Protein/Well (µg) | Number of Cells Yielding 1 µg Total Protein |
|---|---|---|---|---|---|
| murine | CD4+ spleen T cells | 1.1 | 26.2 | 2.7–13.3 | 2.6× 104 |
| CD11b+ spleen macrophages | 2.9 | 70.6 | 7.2–35.8 | 8.2× 103 | |
| CD11b+/CD11c+ spleen dendritic cells | 2.8 | 67.2 | 6.8–34.1 | 5.8× 103 | |
| CD19+ spleen B cells | 1.3 | 32.6 | 3.3–16.6 | 9.7× 104 | |
| F4/80+ peritoneal macrophages | 1.7 | 42.1 | 4.3–21.3 | 8.2× 103 | |
| CD49f+ epidermal stem cells | 2.3 | 55.3 | 5.6–28.1 | 2.0× 104 | |
| murine embryonic fibroblasts (MEFs) | 8.6 | 210.2 | 21.3–106.7 | 1.4× 104 | |
| human | BJ cell line | 32.1 | 782.4 | 79.4–397.1 | 2.4× 103 |
| HeLa cell line | 0.9 | 23.1 | 2.3–11.7 | 4.3× 103 | |
| HEK293T cell line | 0.6 | 15.8 | 1.6–8.0 | 4.4× 103 | |
| CD3+ peripheral blood T cells | 0.5 | 13.4 | 1.4–6.8 | 5.4× 104 | |
| CD19+ peripheral blood B cells | 1.5 | 37.1 | 3.8–18.8 | 9.4× 103 | |
| iPS cell line | 0.5 | 13.1 | 1.3–6.7 | 1.0× 105 |
References
- Gustafson, M.A.; Sullivan, E.D.; Copeland, W.C. Consequences of Compromised Mitochondrial Genome Integrity. DNA Repair 2020, 93, 102916. [Google Scholar] [CrossRef] [PubMed]
- McKinnon, P.J. Genome Integrity and Disease Prevention in the Nervous System. Genes Dev. 2017, 31, 1180–1194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jackson, S.P.; Bartek, J. The DNA-Damage Response in Human Biology and Disease. Nature 2009, 461, 1071–1078. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chow, H.M.; Herrup, K. Genomic Integrity and the Ageing Brain. Nat. Rev. Neurosci. 2015, 16, 672–684. [Google Scholar] [CrossRef] [PubMed]
- Madabhushi, R.; Pan, L.; Tsai, L.H. DNA Damage and Its Links to Neurodegeneration. Neuron 2014, 83, 266–282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rohman, M.S.; Koga, Y.; Takano, K.; Chon, H.; Crouch, R.J.; Kanaya, S. Effect of the Disease-Causing Mutations Identified in Human Ribonuclease (RNase) H2 on the Activities and Stabilities of Yeast RNase H2 and Archaeal RNase HII. FEBS J. 2008, 275, 4836–4849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heider, M.R.; Burkhart, B.W.; Santangelo, T.J.; Gardner, A.F. Defining the RNaseH2 Enzyme-Initiated Ribonucleotide Excision Repair Pathway in Archaea. J. Biol. Chem. 2017, 292, 8835–8845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kellner, V.; Luke, B. Molecular and Physiological Consequences of Faulty Eukaryotic Ribonucleotide Excision Repair. EMBO J. 2020, 39, e102309. [Google Scholar] [CrossRef]
- Feng, S.; Cao, Z. Is the Role of Human RNase H2 Restricted to Its Enzyme Activity? Prog. Biophys. Mol. Biol. 2016, 121, 66–73. [Google Scholar] [CrossRef]
- Eder, P.S.; Walder, J.A. Ribonuclease H from K562 Human Erythroleukemia Cells: Purification, Characterization, and Substrate Specificity. J. Biol. Chem. 1991, 266, 6472–6479. [Google Scholar] [CrossRef]
- Nowotny, M.; Gaidamakov, S.A.; Crouch, R.J.; Yang, W. Crystal Structures of RNase H Bound to an RNA/DNA Hybrid: Substrate Specificity and Metal-Dependent Catalysis. Cell 2005, 121, 1005–1016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lujan, S.A.; Williams, J.S.; Clausen, A.R.; Clark, A.B.; Kunkel, T.A. Ribonucleotides Are Signals for Mismatch Repair of Leading-Strand Replication Errors. Mol. Cell 2013, 50, 437–443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sassa, A.; Yasui, M.; Honma, M. Current Perspectives on Mechanisms of Ribonucleotide Incorporation and Processing in Mammalian DNA. Genes Environ. 2019, 41, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sparks, J.L.; Chon, H.; Cerritelli, S.M.; Kunkel, T.A.; Johansson, E.; Crouch, R.J.; Burgers, P.M. RNase H2-Initiated Ribonucleotide Excision Repair. Mol. Cell 2012, 47, 980–986. [Google Scholar] [CrossRef] [Green Version]
- Noy, A.; Pérez, A.; Lankas, F.; Javier Luque, F.; Orozco, M. Relative Flexibility of DNA and RNA: A Molecular Dynamics Study. J. Mol. Biol. 2004, 343, 627–638. [Google Scholar] [CrossRef]
- Reijns, M.A.M.; Rabe, B.; Rigby, R.E.; Mill, P.; Astell, K.R.; Lettice, L.A.; Boyle, S.; Leitch, A.; Keighren, M.; Kilanowski, F.; et al. Enzymatic Removal of Ribonucleotides from DNA Is Essential for Mammalian Genome Integrity and Development. Cell 2012, 149, 1008–1022. [Google Scholar] [CrossRef] [Green Version]
- Williams, J.S.; Lujan, S.A.; Kunkel, T.A. Processing Ribonucleotides Incorporated during Eukaryotic DNA Replication. Nat. Rev. Mol. Cell Biol. 2016, 17, 350–363. [Google Scholar] [CrossRef] [Green Version]
- Hiller, B.; Achleitner, M.; Glage, S.; Naumann, R.; Behrendt, R.; Roers, A. Mammalian RNase H2 Removes Ribonucleotides from DNA to Maintain Genome Integrity. J. Exp. Med. 2012, 209, 1419–1426. [Google Scholar] [CrossRef]
- MacKenzie, K.J.; Carroll, P.; Martin, C.A.; Murina, O.; Fluteau, A.; Simpson, D.J.; Olova, N.; Sutcliffe, H.; Rainger, J.K.; Leitch, A.; et al. CGAS Surveillance of Micronuclei Links Genome Instability to Innate Immunity. Nature 2017, 548, 461–465. [Google Scholar] [CrossRef] [Green Version]
- Crow, Y.J.; Manel, N. Aicardi-Goutières Syndrome and the Type I Interferonopathies. Nat. Rev. Immunol. 2015, 15, 429–440. [Google Scholar] [CrossRef]
- Lee-Kirsch, M.A.; Wolf, C.; Kretschmer, S.; Roers, A. Type I Interferonopathies—An Expanding Disease Spectrum of Immunodysregulation. Semin. Immunopathol. 2015, 37, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Volpi, S.; Picco, P.; Caorsi, R.; Candotti, F.; Gattorno, M. Type I Interferonopathies in Pediatric Rheumatology. Pediatr. Rheumatol. 2016, 14, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crow, Y.J.; Rehwinkel, J. Aicardi-Goutie’res Syndrome and Related Phenotypes: Linking Nucleic Acid Metabolism with Autoimmunity. Hum. Mol. Genet. 2009, 18, 130–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee-Kirsch, M.A.; Wolf, C.; Günther, C. Aicardi-Goutières Syndrome: A Model Disease for Systemic Autoimmunity. Clin. Exp. Immunol. 2014, 175, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Günther, C.; Kind, B.; Reijns, M.A.M.; Berndt, N.; Martinez-Bueno, M.; Wolf, C.; Tüngler, V.; Chara, O.; Lee, Y.A.; Hübner, N.; et al. Defective Removal of Ribonucleotides from DNA Promotes Systemic Autoimmunity. J. Clin. Investig. 2015, 125, 413–424. [Google Scholar] [CrossRef]
- Mackenzie, K.J.; Carroll, P.; Lettice, L.; Tarnauskaitė, Ž.; Reddy, K.; Dix, F.; Revuelta, A.; Abbondati, E.; Rigby, R.E.; Rabe, B.; et al. Ribonuclease H2 Mutations Induce a CGAS/STING -dependent Innate Immune Response. EMBO J. 2016, 35, 831–844. [Google Scholar] [CrossRef]
- Pokatayev, V.; Hasin, N.; Chon, H.; Cerritelli, S.M.; Sakhuja, K.; Ward, J.M.; Douglas Morris, H.; Yan, N.; Crouch, R.J. RNase H2 Catalytic Core Aicardi-Goutières Syndrome-Related Mutant Invokes CGAS-STING Innate Immunesensing Pathway in Mice. J. Exp. Med. 2016, 213, 329–336. [Google Scholar] [CrossRef] [Green Version]
- Bartsch, K.; Knittler, K.; Borowski, C.; Rudnik, S.; Damme, M.; Aden, K.; Spehlmann, M.E.; Frey, N.; Saftig, P.; Chalaris, A.; et al. Absence of RNase H2 Triggers Generation of Immunogenic Micronuclei Removed by Autophagy. Hum. Mol. Genet. 2017, 26, 3960–3972. [Google Scholar] [CrossRef] [Green Version]
- Hiller, B.; Hoppe, A.; Haase, C.; Hiller, C.; Schubert, N.; Müller, W.; Reijns, M.A.M.; Jackson, A.P.; Kunkel, T.A.; Wenzel, J.; et al. Ribonucleotide Excision Repair Is Essential to Prevent Squamous Cell Carcinoma of the Skin. Cancer Res. 2018, 78, 5917–5926. [Google Scholar] [CrossRef] [Green Version]
- Aden, K.; Bartsch, K.; Dahl, J.; Reijns, M.A.M.; Esser, D.; Sheibani-Tezerji, R.; Sinha, A.; Wottawa, F.; Ito, G.; Mishra, N.; et al. Epithelial RNase H2 Maintains Genome Integrity and Prevents Intestinal Tumorigenesis in Mice. Gastroenterology 2019, 156, 145–159.e19. [Google Scholar] [CrossRef] [Green Version]
- Zimmermann, M.; Murina, O.; Reijns, M.A.M.; Agathanggelou, A.; Challis, R.; Tarnauskaite, Ž.; Muir, M.; Fluteau, A.; Aregger, M.; McEwan, A.; et al. CRISPR Screens Identify Genomic Ribonucleotides as a Source of PARP-Trapping Lesions. Nature 2018, 559, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Mottaghi-Dastjerdi, N.; Soltany-Rezaee-Rad, M.; Sepehrizadeh, Z.; Roshandel, G.; Ebrahimifard, F.; Setayesh, N. Identification of Novel Genes Involved in Gastric Carcinogenesis by Suppression Subtractive Hybridization. Hum. Exp. Toxicol. 2015, 34, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Flanagan, J.M.; Funes, J.M.; Henderson, S.; Wild, L.; Carey, N.; Boshoff, C. Genomics Screen in Transformed Stem Cells Reveals RNASEH2A, PPAP2C, and ADARB1 as Putative Anticancer Drug Targets. Mol. Cancer Ther. 2009, 8, 249–260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, K.A.; Lee, M.; Hu, Y.; Andreas, J.; Patel, S.J.; Zhang, S.; Chines, P.; Elkahloun, A.; Chandrasekharappa, S.; Gutkind, J.S.; et al. A Systems Genetics Approach Identifies CXCL14, ITGAX, and LPCAT2 as Novel Aggressive Prostate Cancer Susceptibility Genes. PLoS Genet. 2014, 10, e1004809. [Google Scholar] [CrossRef] [Green Version]
- White, R.R.; Vijg, J. Do DNA Double-Strand Breaks Drive Aging? Mol. Cell 2016, 63, 729–738. [Google Scholar] [CrossRef] [Green Version]
- Zhu, L.-S.; Wang, D.-Q.; Cui, K.; Liu, D.; Zhu, L.-Q. Emerging Perspectives on DNA Double-Strand Breaks in Neurodegenerative Diseases. Curr. Neuropharmacol. 2019, 17, 1146–1157. [Google Scholar] [CrossRef]
- Storci, G.; De Carolis, S.; Papi, A.; Bacalini, M.G.; Gensous, N.; Marasco, E.; Tesei, A.; Fabbri, F.; Arienti, C.; Zanoni, M.; et al. Genomic Stability, Anti-Inflammatory Phenotype, and up-Regulation of the RNAseH2 in Cells from Centenarians. Cell Death Differ. 2019, 26, 1845–1858. [Google Scholar] [CrossRef] [Green Version]
- Rychlik, M.P.; Chon, H.; Cerritelli, S.M.; Klimek, P.; Crouch, R.J.; Nowotny, M. Crystal Structures of Rnase H2 in Complex with Nucleic Acid Reveal the Mechanism of RNA-DNA Junction Recognition and Cleavage. Mol. Cell 2010, 40, 658–670. [Google Scholar] [CrossRef]
- Shaban, N.M.; Harvey, S.; Perrino, F.W.; Hollis, T. The Structure of the Mammalian RNase H2 Complex Provides Insight into RNA??DNA Hybrid Processing to Prevent Immune Dysfunction. J. Biol. Chem. 2010, 285, 3617–3624. [Google Scholar] [CrossRef] [Green Version]
- Reijns, M.A.M.; Bubeck, D.; Gibson, L.C.D.; Graham, S.C.; Baillie, G.S.; Jones, E.Y.; Jackson, A.P. The Structure of the Human RNase H2 Complex Defines Key Interaction Interfaces Relevant to Enzyme Function and Human Disease. J. Biol. Chem. 2011, 286, 10530–10539. [Google Scholar] [CrossRef] [Green Version]
- Lo, M.S. Monogenic Lupus. Curr. Rheumatol. Rep. 2016, 18, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Figiel, M.; Chon, H.; Cerritelli, S.M.; Cybulska, M.; Crouch, R.J.; Nowotny, M. The Structural and Biochemical Characterization of Human RNase H2 Complex Reveals the Molecular Basis for Substrate Recognition and Aicardi-Goutières Syndrome Defects. J. Biol. Chem. 2011, 286, 10540–10550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stein, H.; Hausen, P. Enzyme from Calf Thymus Degrading the RNA Moiety of DNA-RNA Hybrids: Effect on DNA-Dependent RNA Polymerase. Science 1969, 166, 393–395. [Google Scholar] [CrossRef]
- Crow, Y.J.; Leitch, A.; Hayward, B.E.; Garner, A.; Parmar, R.; Griffith, E.; Ali, M.; Semple, C.; Aicardi, J.; Babul-Hirji, R.; et al. Mutations in Genes Encoding Ribonuclease H2 Subunits Cause Aicardi-Goutières Syndrome and Mimic Congenital Viral Brain Infection. Nat. Genet. 2006, 38, 910–916. [Google Scholar] [CrossRef] [PubMed]
- Jensen, K.B.; Driskell, R.R.; Watt, F.M. Assaying Proliferation and Differentiation Capacity of Stem Cells Using Disaggregated Adult Mouse Epidermis. Nat. Protoc. 2010, 5, 898–911. [Google Scholar] [CrossRef] [PubMed]
- Ray, A.; Dittel, B.N. Isolation of Mouse Peritoneal Cavity Cells. J. Vis. Exp. 2010, 35, e1488. [Google Scholar] [CrossRef]
- Lim, J.F.; Berger, H.; Su, I.H. Isolation and Activation of Murine Lymphocytes. J. Vis. Exp. 2016, 116, e54596. [Google Scholar] [CrossRef]
- Durkin, M.; Qian, X.; Popescu, N.; Lowy, D. Isolation of Mouse Embryo Fibroblasts. Bio. Protoc. 2013, 3, e908. [Google Scholar] [CrossRef] [Green Version]
- Rigby, R.E. Ribonuclease H2, RNA: DNA Hybrids and Innate Immunity; The University of Edinburgh: Edinburgh, UK, 2010. [Google Scholar]
- Hulley, S.; Cummings, S.; Browner, W.; Grady, D.; Newman, T. Estimating Sample Size and Power: Applications and Examples. In Designing Clinical Research; Hulley, S., Cummings, S., Browner, W., Grady, D., Newman, T., Eds.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2013; pp. 55–83. ISBN 9781608318049. [Google Scholar]
- Smit, J.H.; Van Der Velde, J.H.M.; Huang, J.; Trauschke, V.; Henrikus, S.S.; Chen, S.; Eleftheriadis, N.; Warszawik, E.M.; Herrmann, A.; Cordes, T. On the Impact of Competing Intra- and Intermolecular Triplet-State Quenching on Photobleaching and Photoswitching Kinetics of Organic Fluorophores. Phys. Chem. Chem. Phys. 2019, 21, 3721–3733. [Google Scholar] [CrossRef] [Green Version]
- Torimura, M.; Kurata, S.; Yamada, K.; Yokomaku, T.; Kamataga, Y.; Kanagawa, T.; Kurane, R. Fluorescence-Quenching Phenomenon by Photoinduced Electron Transfer between a Fluorescent Dye and a Nucleotide Base. Anal. Sci. 2001, 17, 155–160. [Google Scholar] [CrossRef] [Green Version]
- Robinson, P.K. Enzymes: Principles and Biotechnological Applications. Essays Biochem. 2015, 59, 1–41. [Google Scholar] [CrossRef] [PubMed]
- Copeland, R.A. Enzymes. A Practical Introduction to Structure, Mechanism and Data Analysis, 2nd ed.; Wiley-VCH, Inc.: New York, NY, USA, 2000; ISBN 1-56081-903-0. [Google Scholar]
- Magnusson, B.; Örnemark, U. Eurachem Guide: The Fitness for Purpose of Analytical Methods—A Laboratory Guide to Method Validation and Related Topics, 2nd ed.; Eurachem: Zug, Switzerland, 2014; ISBN 0-94948926-12-0. [Google Scholar]
- Dowd, J.E.; Riggs, D.S. A Comparison of Estimates of Michaelis-Menten Kinetic Constants From Various Linear Transformations. J. Biol. Chem. 1965, 240, 863–869. [Google Scholar] [CrossRef] [PubMed]
- Goods, B.A.; Sansing, L.; Vahey, J.M.; Christopher Love, J.; Steinschneider, A.F.; Askenase, M.H. Correction to: Blood Handling and Leukocyte Isolation Methods Impact the Global Transcriptome of Immune Cells. BMC Immunol. 2018, 19, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grievink, H.W.; Luisman, T.; Kluft, C.; Moerland, M.; Malone, K.E. Comparison of Three Isolation Techniques for Human Peripheral Blood Mononuclear Cells: Cell Recovery and Viability, Population Composition, and Cell Functionality. Biopreserv. Biobank 2016, 14, 410–415. [Google Scholar] [CrossRef] [PubMed]
- Chon, H.; Vassilev, A.; Depamphilis, M.L.; Zhao, Y.; Zhang, J.; Burgers, P.M.; Crouch, R.J.; Cerritelli, S.M. Contributions of the Two Accessory Subunits, RNASEH2B and RNASEH2C, to the Activity and Properties of the Human RNase H2 Complex. Nucleic. Acids Res. 2009, 37, 96–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grützkau, A.; Radbruch, A. Small but Mighty: How the MACS1-Technology Based on Nanosized Superparamagnetic Particles Has Helped to Analyze the Immune System within the Last 20 Years. Cytom. Part A 2010, 77, 643–647. [Google Scholar] [CrossRef]
- Sakaue-Sawano, A.; Kurokawa, H.; Morimura, T.; Hanyu, A.; Hama, H.; Osawa, H.; Kashiwagi, S.; Fukami, K.; Miyata, T.; Miyoshi, H.; et al. Visualizing Spatiotemporal Dynamics of Multicellular Cell-Cycle Progression. Cell 2008, 132, 487–498. [Google Scholar] [CrossRef] [Green Version]



| Buffer | Reagents |
|---|---|
| reaction buffer (1×) | 60 mM KCl, 50 mM Tris.HCl pH 8.0, 20 mM MgCl2, add fresh Triton X-100 and BSA to a final concentration of 0.01% |
| lysis buffer 1 (1×) | 50 mM TRIS.HCL pH 8.0, 280 mM NaCl, 0.5% v/v NP40, 0.2 mM EDTA, 0.2 mM EGTA, 10% v/v glycerol, 0.1 mM sodium orthovanadate, add fresh 1 mM DTT and 1mM PMSF |
| lysis buffer 2 (1×) | 20 mM HEPES, 10 mM KCl, 1 mM EDTA, 0.1 mM sodium or-thovanadate, add fresh 1 mM DTT and 1mM PMSF |
| FACS buffer | (1×) PBS, 3% FCS |
| Substrate | Sequence |
|---|---|
| oligonucleotide A: 2-O′-methylated RNA | 5′-GAUCUGAGCCUGGGAGCU-fluorescein-3′ |
| oligonucleotide B: DNA with a single ribouncleotide | 5′-GATCTGAGCCTGGG[rA]GCT-fluorescein-3′ |
| oligonucleotide D: DNA | 5′-Dabcyl-AGCTCCCAGGCTCAGATC-3′ |
| oligonucleotide E: DNA | 5′-GATCTGAGCCTGGGAGCT-fluorescein-3′ |
| oligonucleotide K: DNA | 5′-AGCTCCCAGGCTCAGATC-3′ |
| Substrate | LOD | LOQ |
|---|---|---|
| BD | 2.7 fmol/min (0.5 eqU RNase HII or 0.02 eqU DNase) | 27 fmol/min (4.5 eqU RNase HII or 1.5 eqU DNase) |
| ED | 4.5 fmol/min (0.02 eqU DNase) | 45 fmol/min (2 eqU DNase) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schulz, M.S.; Sartorius von Bach, C.B.; Marinkovic, E.; Günther, C.; Behrendt, R.; Roers, A. Development of an RNase H2 Activity Assay for Clinical Screening. J. Clin. Med. 2023, 12, 1598. https://doi.org/10.3390/jcm12041598
Schulz MS, Sartorius von Bach CB, Marinkovic E, Günther C, Behrendt R, Roers A. Development of an RNase H2 Activity Assay for Clinical Screening. Journal of Clinical Medicine. 2023; 12(4):1598. https://doi.org/10.3390/jcm12041598
Chicago/Turabian StyleSchulz, Marian Simon, Cay Bennet Sartorius von Bach, Emilija Marinkovic, Claudia Günther, Rayk Behrendt, and Axel Roers. 2023. "Development of an RNase H2 Activity Assay for Clinical Screening" Journal of Clinical Medicine 12, no. 4: 1598. https://doi.org/10.3390/jcm12041598






