Differentiation of Adipose-Derived Stem Cells into Smooth Muscle Cells in an Internal Anal Sphincter-Targeting Anal Incontinence Rat Model
Abstract
1. Introduction
2. Materials and Methods
2.1. In Vitro Differentiation of hADScs into SMCs
2.2. RNA Isolation and Quantitative Real-Time Polymerase Chain Reaction (RT–PCR)
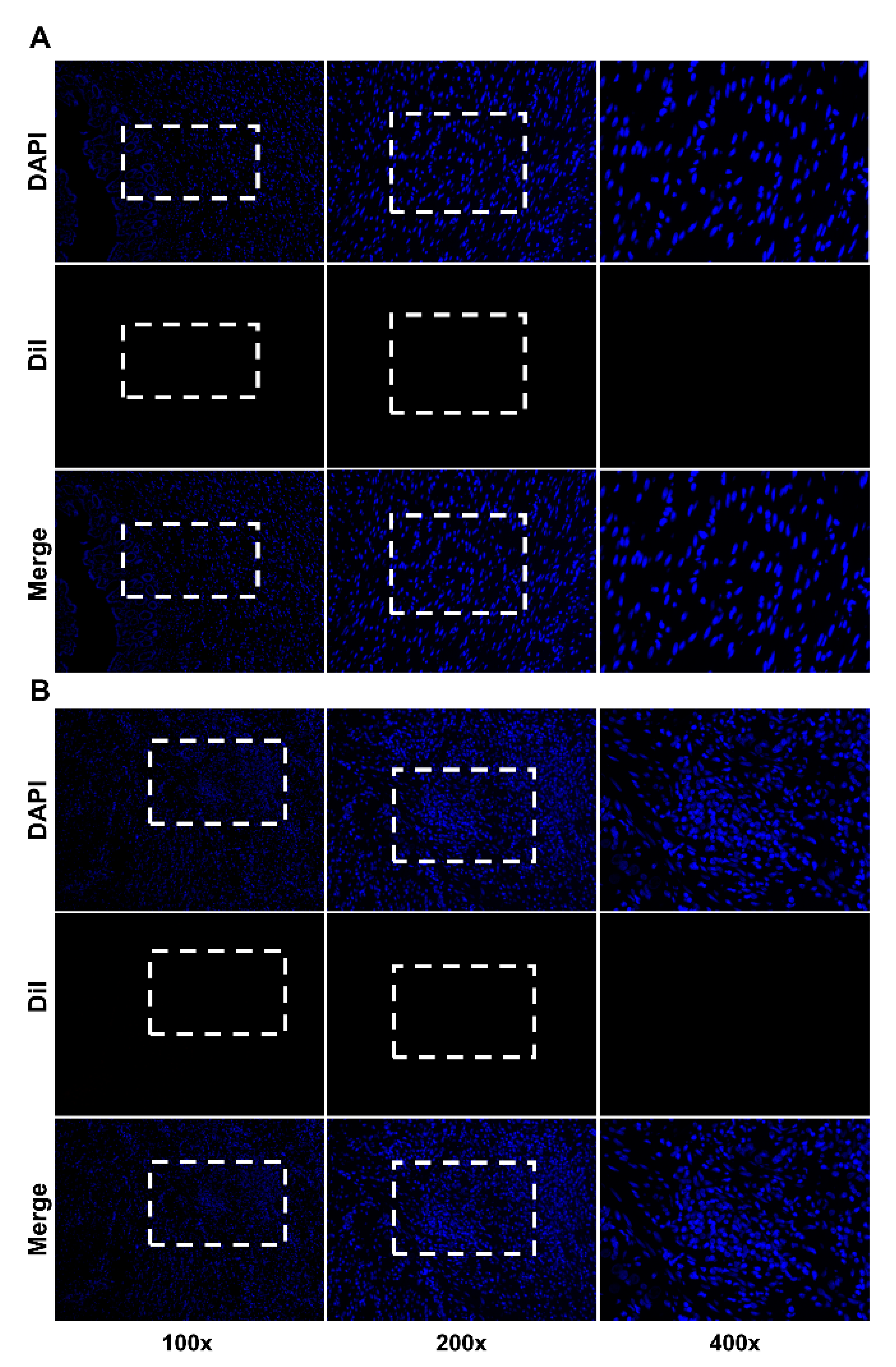
2.3. Immunofluorescence Staining
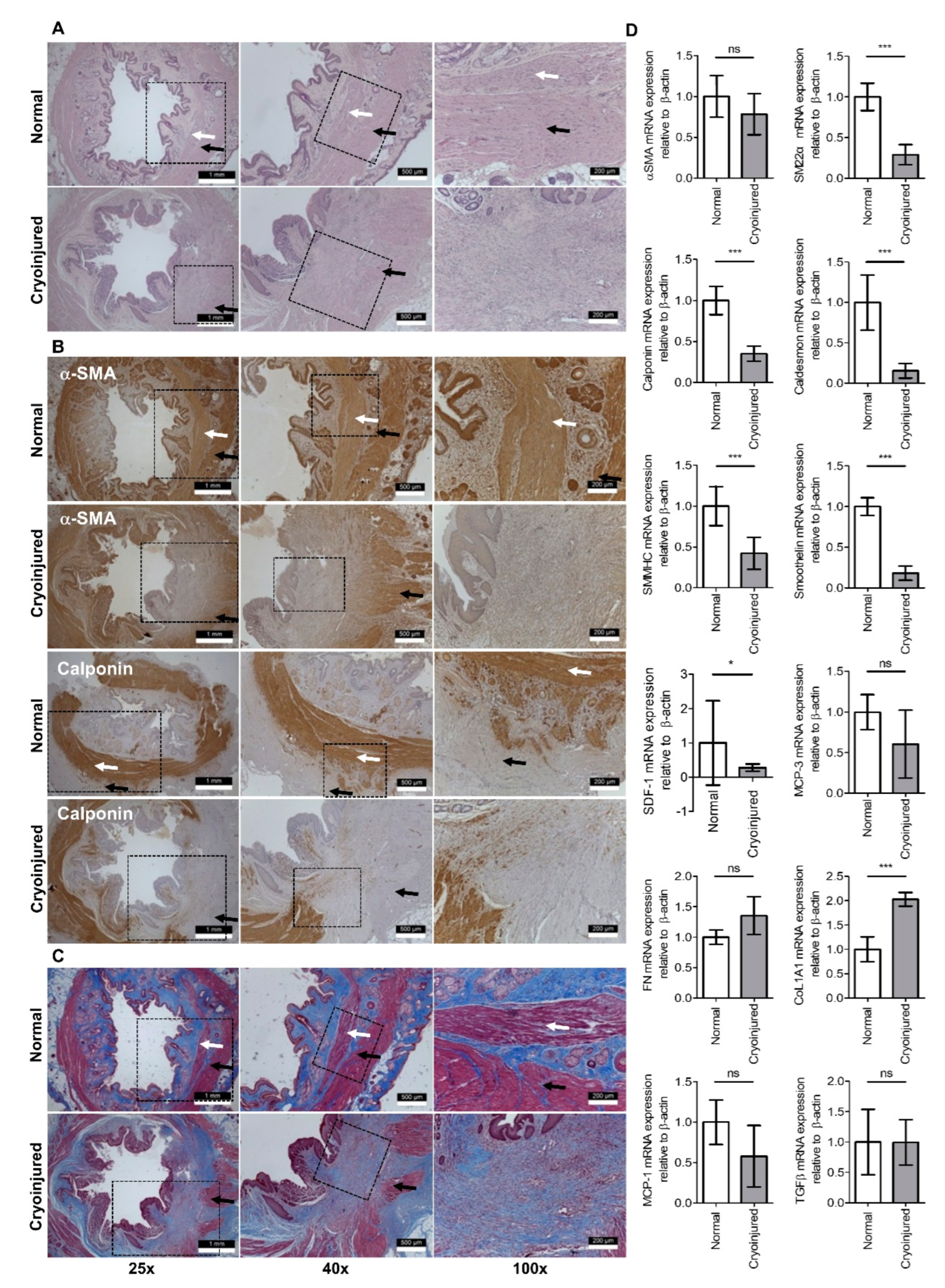
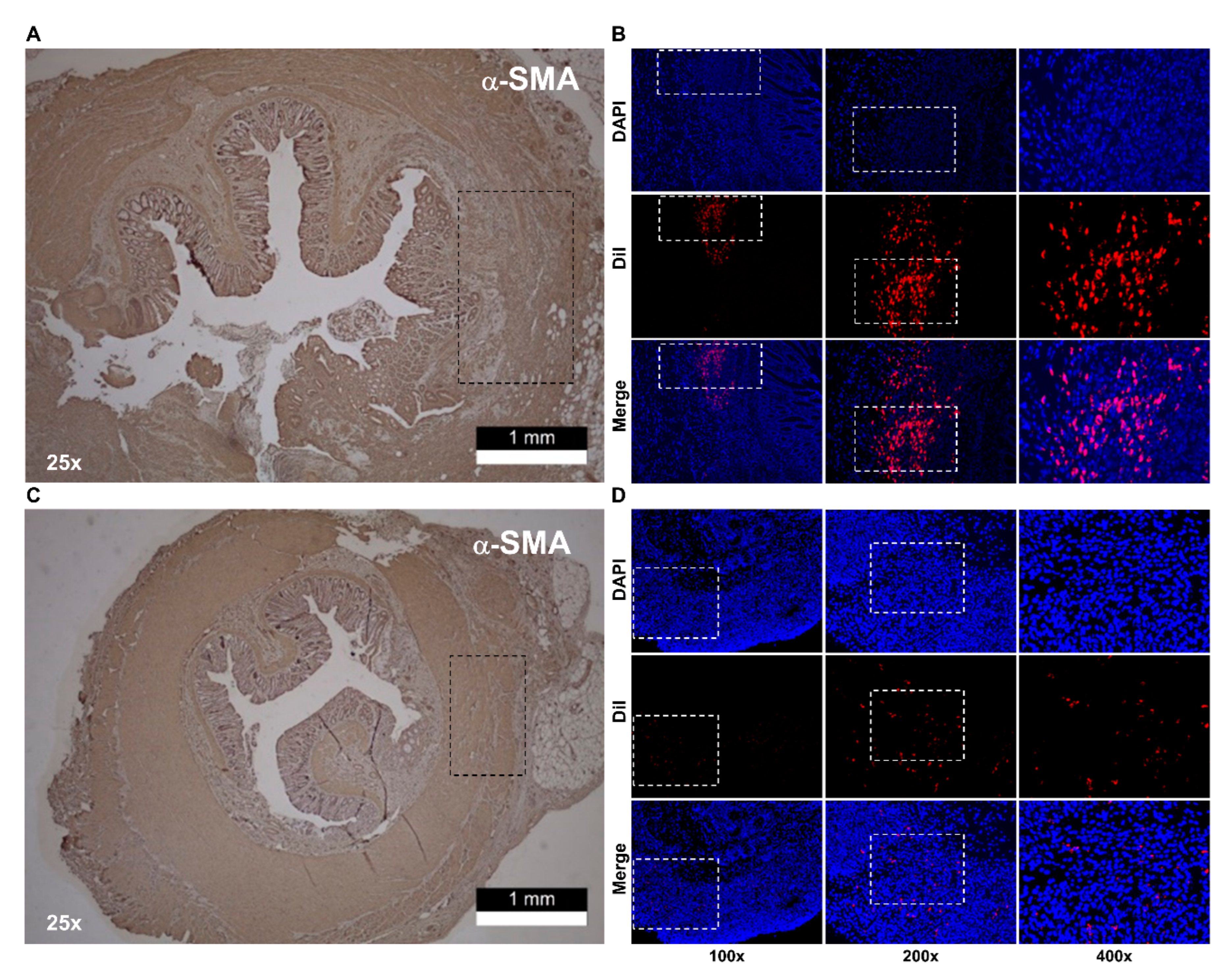
2.4. Histologic Staiing
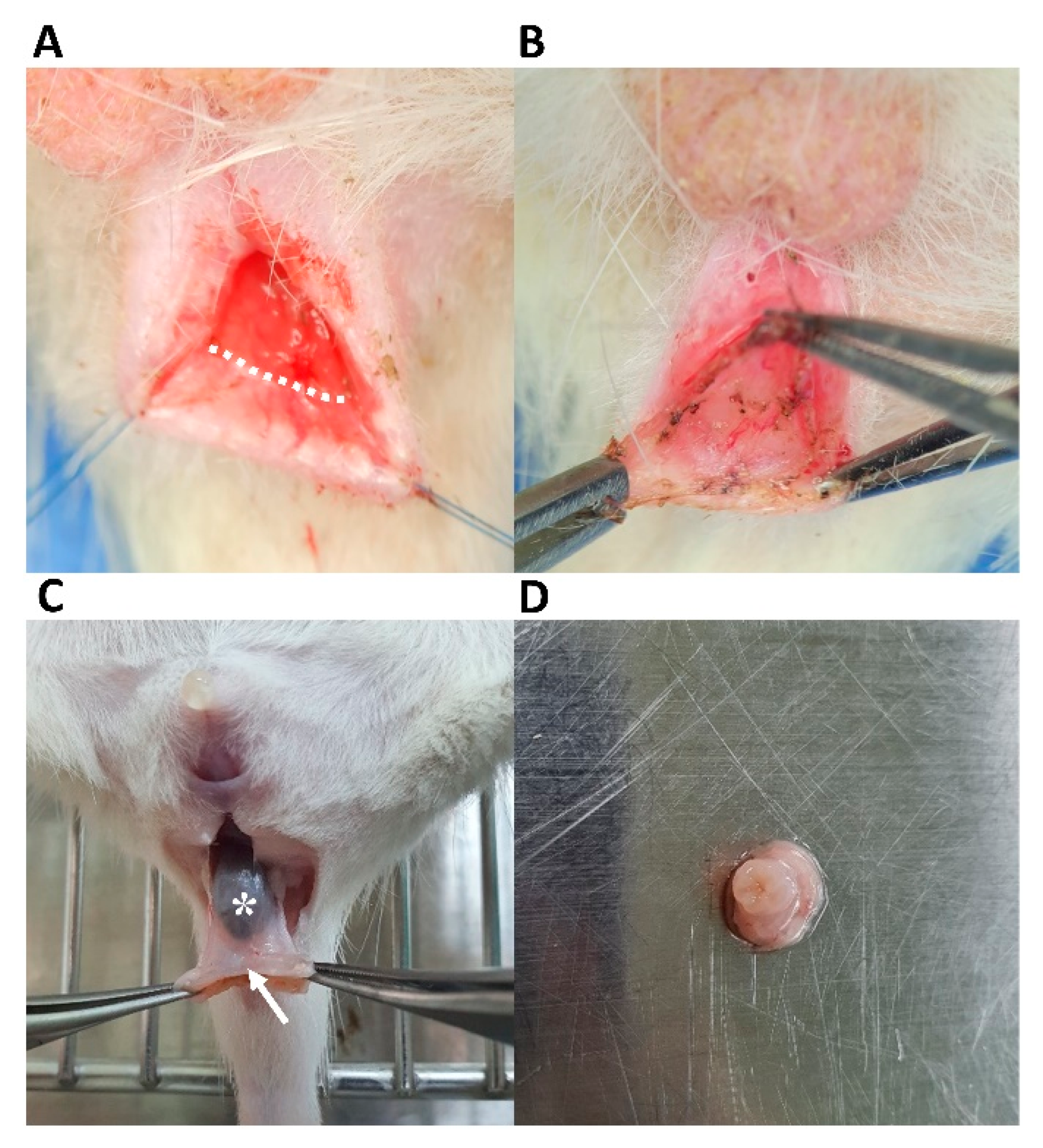
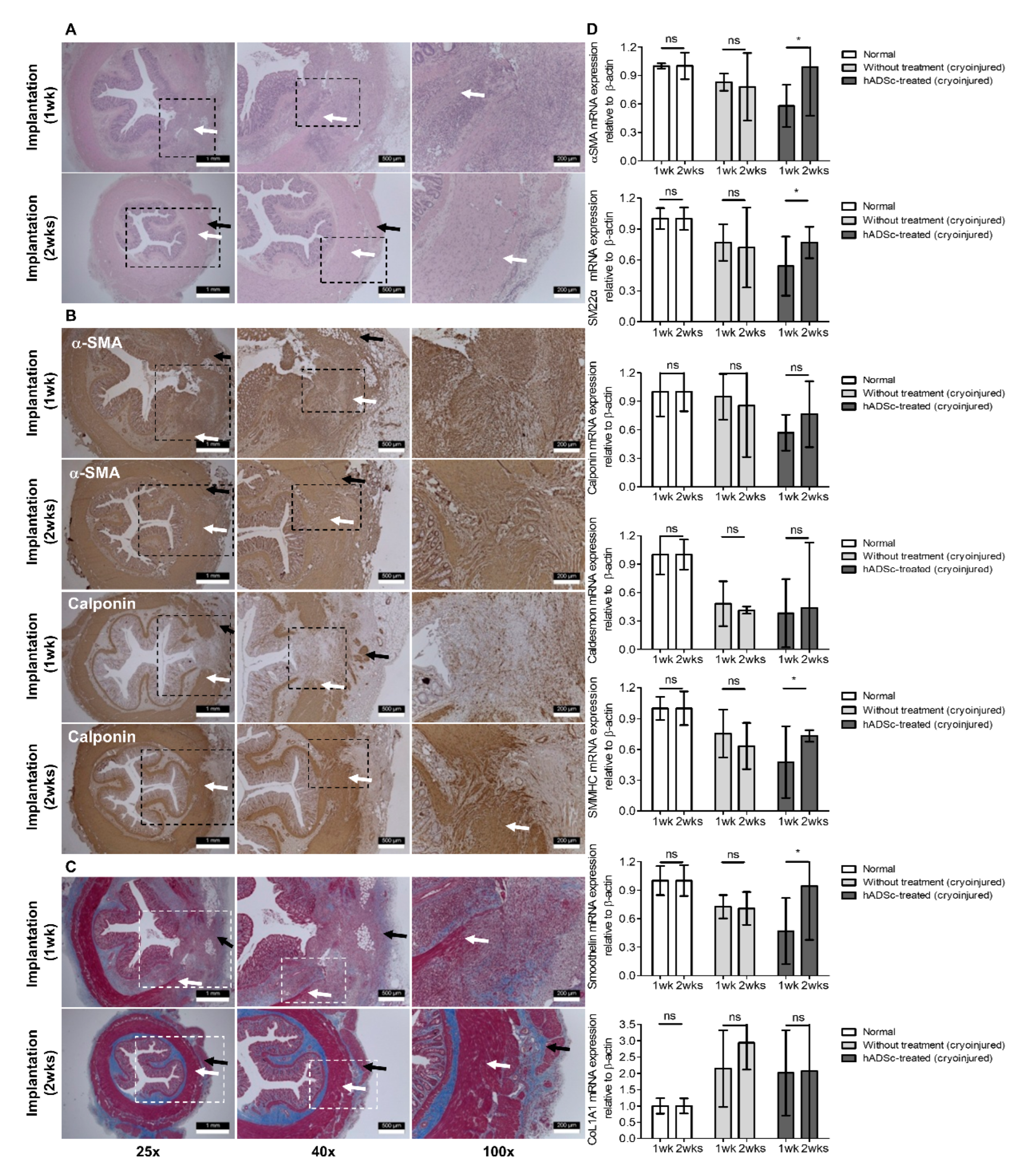
2.5. IAS-Specific Incontinence Model Development and hADSc Implantation
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Trébol, J.; Carabias-Orgaz, A.; García-Arranz, M.; García-Olmo, D. Stem cell therapy for faecal incontinence: Current state and future perspectives. World J. Stem Cells 2018, 10, 82–105. [Google Scholar] [CrossRef]
- Evers, J.; Jones, J.F.X.; O’Connell, P.R. Systematic review of animal models used in research of origins and treatments of fecal incontinence. Dis. Colon Rectum 2017, 60, 614–626. [Google Scholar] [CrossRef] [PubMed]
- De Ligny, W.R.; Kerkhof, M.H.; Ruiz-Zapata, A.M. Regenerative medicine as a therapeutic option for fecal incontinence: A systematic review of preclinical and clinical studies. Am. J. Obstet. Gynecol. 2019, 220, 142–154.e2. [Google Scholar] [CrossRef] [PubMed]
- Frudinger, A.; Kölle, D.; Schwaiger, W.; Pfeifer, J.; Paede, J.; Halligan, S. Muscle-derived cell injection to treat anal incontinence due to obstetric trauma: Pilot study with 1 year follow-up. Gut 2010, 59, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Frudinger, A.; Pfeifer, J.; Paede, J.; Kolovetsiou-Kreiner, V.; Marksteiner, R.; Halligan, S. Autologous skeletal-muscle-derived cell injection for anal incontinence due to obstetric trauma: A 5-year follow-up of an initial study of 10 patients. Colorectal Dis. 2015, 17, 794–801. [Google Scholar] [CrossRef]
- Bisson, A.; Fréret, M.; Drouot, L.; Jean, L.; Le Corre, S.; Gourcerol, G.; Doucet, C.; Michot, F.; Boyer, O.; Lamacz, M. Restoration of anal sphincter function after myoblast cell therapy in incontinent rats. Cell Transplant. 2015, 24, 277–286. [Google Scholar] [CrossRef]
- Romaniszyn, M.; Rozwadowska, N.; Nowak, M.; Malcher, A.; Kolanowski, T.; Walega, P.; Richter, P.; Kurpisz, M. Successful implantation of autologous muscle-derived stem cells in treatment of faecal incontinence due to external sphincter rupture. Int. J. Colorectal Dis. 2013, 28, 1035–1036. [Google Scholar] [CrossRef]
- Romaniszyn, M.; Rozwadowska, N.; Malcher, A.; Kolanowski, T.; Walega, P.; Kurpisz, M. Implantation of autologous muscle-derived stem cells in treatment of fecal incontinence: Results of an experimental pilot study. Tech. Coloproctol. 2015, 19, 685–696. [Google Scholar] [CrossRef]
- Frudinger, A.; Marksteiner, R.; Pfeifer, J.; Margreiter, E.; Paede, J.; Thurner, M. Skeletal muscle-derived cell implantation for the treatment of sphincter-related faecal incontinence. Stem Cell Res. Ther. 2018, 9, 233. [Google Scholar] [CrossRef]
- Kajbafzadeh, A.M.; Elmi, A.; Talab, S.S.; Esfahani, S.A.; Tourchi, A. Functional external anal sphincter reconstruction for treatment of anal incontinence using muscle progenitor cell auto grafting. Dis. Colon Rectum 2010, 53, 1415–1421. [Google Scholar] [CrossRef]
- Kajbafzadeh, A.M.; Kajbafzadeh, M.; Sabetkish, S.; Sabetkish, N.; Tavangar, S.M. Tissue-engineered external anal sphincter using autologous myogenic satellite cells and extracellular matrix: Functional and histological studies. Ann. Biomed. Eng. 2016, 44, 1773–1784. [Google Scholar] [CrossRef]
- Boyer, O.; Bridoux, V.; Giverne, C.; Bisson, A.; Koning, E.; Leroi, A.M.; Chambon, P.; Déhayes, J.; Le Corre, S.; Jacquot, S.; et al. Autologous myoblasts for the treatment of fecal incontinence: Results of a Phase 2 randomized placebo-controlled study (MIAS). Ann. Surg. 2018, 267, 443–450. [Google Scholar] [CrossRef]
- Son, I.T.; Lee, H.S.; Ihn, M.H.; Lee, K.H.; Kim, D.W.; Lee, K.W.; Kim, J.S.; Kang, S.B. Isolation of internal and external sphincter progenitor cells from the human anal sphincter with or without radiotherapy. Colorectal Dis. 2019, 21, 38–47. [Google Scholar] [CrossRef]
- Kim, M.; Oh, B.Y.; Lee, J.S.; Yoon, D.; Chun, W.; Son, I.T. A systematic review of translation and experimental studies on internal anal sphincter for fecal incontinence. Ann. Coloproctol. 2022, 38, 183–196. [Google Scholar] [CrossRef]
- Herreros, M.D.; Garcia-Arranz, M.; Guadalajara, H.; De-La-Quintana, P.; Garcia-Olmo, D. Autologous expanded adipose-derived stem cells for the treatment of complex cryptoglandular perianal fistulas: A phase III randomized clinical trial (FATT 1: Fistula Advanced Therapy Trial 1) and long-term evaluation. Dis. Colon Rectum 2012, 55, 762–772. [Google Scholar] [CrossRef]
- Cho, Y.B.; Lee, W.Y.; Park, K.J.; Kim, M.; Yoo, H.W.; Yu, C.S. Autologous adipose tissue-derived stem cells for the treatment of Crohn's fistula: A phase I clinical study. Cell Transplant. 2013, 22, 279–285. [Google Scholar] [CrossRef]
- Park, E.J.; Kang, J.; Baik, S.H. Treatment of faecal incontinence using allogeneic-adipose-derived mesenchymal stem cells: A study protocol for a pilot randomised controlled trial. BMJ Open 2016, 6, e010450. [Google Scholar] [CrossRef]
- Sarveazad, A.; Newstead, G.L.; Mirzaei, R.; Joghataei, M.T.; Bakhtiari, M.; Babahajian, A.; Mahjoubi, B. A new method for treating fecal incontinence by implanting stem cells derived from human adipose tissue: Preliminary findings of a randomized double-blind clinical trial. Stem Cell Res. Ther. 2017, 8, 40. [Google Scholar] [CrossRef]
- El-Said, M.M.; Emile, S.H. Comment on “A new method for treating fecal incontinence by implanting stem cells derived from human adipose tissue: Preliminary findings of a randomized double-blind clinical trial”. Stem Cell Res. Ther. 2018, 9, 115. [Google Scholar] [CrossRef]
- Inoue, Y.; Fujita, F.; Yamaguchi, I.; Kinoe, H.; Kawahara, D.; Sakai, Y.; Kuroki, T.; Eguchi, S. Improvement of anal function by adipose-derived stem cell sheets. Dig. Surg. 2018, 35, 64–69. [Google Scholar] [CrossRef]
- Salcedo, L.; Mayorga, M.; Damaser, M.; Balog, B.; Butler, R.; Penn, M.; Zutshi, M. Mesenchymal stem cells can improve anal pressures after anal sphincter injury. Stem Cell Res. 2013, 10, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Salcedo, L.; Penn, M.; Damaser, M.; Balog, B.; Zutshi, M. Functional outcome after anal sphincter injury and treatment with mesenchymal stem cells. Stem Cells Transl. Med. 2014, 3, 760–767. [Google Scholar] [CrossRef]
- Kuismanen, K.; Juntunen, M.; Narra Girish, N.; Tuominen, H.; Huhtala, H.; Nieminen, K.; Hyttinen, J.; Miettinen, S. Functional outcome of human adipose stem cell injections in rat anal sphincter acute injury model. Stem Cells Transl. Med. 2018, 7, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Somara, S.; Gilmont, R.R.; Dennis, R.G.; Bitar, K.N. Bioengineered internal anal sphincter derived from isolated human internal anal sphincter smooth muscle cells. Gastroenterology 2009, 137, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Rattan, S. Bioengineered human IAS reconstructs with functional and molecular properties similar to intact IAS. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 303, G713–G722. [Google Scholar] [CrossRef]
- González-Rosa, J.M.; Mercader, N. Cryoinjury as a myocardial infarction model for the study of cardiac regeneration in the zebrafish. Nat. Protoc. 2012, 7, 782–788. [Google Scholar] [CrossRef]
- Kang, S.B.; Lee, H.N.; Lee, J.Y.; Park, J.S.; Lee, H.S.; Lee, J.Y. Sphincter contractility after muscle-derived stem cells autograft into the cryoinjured anal sphincters of rats. Dis. Colon Rectum 2008, 51, 1367–1373. [Google Scholar] [CrossRef]
- Truebestein, L.; Elsner, D.J.; Fuchs, E.; Leonard, T.A. A molecular ruler regulates cytoskeletal remodelling by the Rho kinases. Nat. Commun. 2015, 6, 10029. [Google Scholar] [CrossRef]
- Gao, D.; Critser, J.K. Mechanisms of cryoinjury in living cells. ILAR J. 2000, 41, 187–196. [Google Scholar] [CrossRef]
- Gagnard, C.; Godlewski, G.; Prat, D.; Lan, O.; Cousineau, J.; Maklouf, Y. The nerve branches to the external anal sphincter: The macroscopic supply and microscopic structure. Surg. Radiol. Anat. 1986, 8, 115–119. [Google Scholar] [CrossRef]
- Zhang, C.H.; Wang, P.; Liu, D.H.; Chen, C.P.; Zhao, W.; Chen, X.; Chen, C.; He, W.Q.; Qiao, Y.N.; Tao, T.; et al. The molecular basis of the genesis of basal tone in internal anal sphincter. Nat. Commun. 2016, 7, 11358. [Google Scholar] [CrossRef]
- Meyer, I.; Blanchard, C.T.; Markland, A.D.; Gibson, E.G.; Richter, H.E. Fecal incontinence symptoms and impact in older versus younger women seeking care. Dis. Colon Rectum 2019, 62, 733–738. [Google Scholar] [CrossRef]
- Schenk, S.; Mal, N.; Finan, A.; Zhang, M.; Kiedrowski, M.; Popovic, Z.; McCarthy, P.M.; Penn, M.S. Monocyte chemotactic protein-3 is a myocardial mesenchymal stem cell homing factor. Stem Cells 2007, 25, 245–251. [Google Scholar] [CrossRef]
- Oh, H.K.; Lee, H.S.; Lee, J.H.; Oh, S.H.; Lim, J.Y.; Ahn, S.; Kang, S.B. Coadministration of basic fibroblast growth factor-loaded polycaprolactone beads and autologous myoblasts in a dog model of fecal incontinence. Int. J. Colorectal Dis. 2015, 30, 549–557. [Google Scholar] [CrossRef]
- Oh, H.K.; Lee, H.S.; Lee, J.H.; Oh, S.H.; Lim, J.Y.; Ahn, S.; Hwang, J.Y.; Kang, S.B. Functional and histological evidence for the targeted therapy using biocompatible polycaprolactone beads and autologous myoblasts in a dog model of fecal incontinence. Dis. Colon Rectum 2015, 58, 517–525. [Google Scholar] [CrossRef]
- Trébol, J.; Georgiev-Hristov, T.; Vega-Clemente, L.; García-Gómez, I.; Carabias-Orgaz, A.; García-Arranz, M.; García-Olmo, D. Rat model of anal sphincter injury and two approaches for stem cell administration. World J. Stem Cells 2018, 10, 1–14. [Google Scholar] [CrossRef]
- Sun, L.; Yeh, J.; Xie, Z.; Kuang, M.; Damaser, M.S.; Zutshi, M. Electrical stimulation followed by mesenchymal stem cells improves anal sphincter anatomy and function in a rat model at a time remote from injury. Dis. Colon Rectum 2016, 59, 434–442. [Google Scholar] [CrossRef]
- Salcedo, L.; Lian, L.; Jiang, H.H.; Sopko, N.; Penn, M.; Damaser, M.; Zutshi, M. Low current electrical stimulation upregulates cytokine expression in the anal sphincter. Int. J. Colorectal Dis. 2012, 27, 221–225. [Google Scholar] [CrossRef]
- Gräs, S.; Tolstrup, C.K.; Lose, G. Regenerative medicine provides alternative strategies for the treatment of anal incontinence. Int. Urogynecol. J. 2017, 28, 341–350. [Google Scholar] [CrossRef]
- Balaphas, A.; Meyer, J.; Meier, R.P.H.; Liot, E.; Buchs, N.C.; Roche, B.; Toso, C.; Bühler, L.H.; Gonelle-Gispert, C.; Ris, F. Cell therapy for anal sphincter incontinence: Where do we stand? Cells 2021, 10, 2086. [Google Scholar] [CrossRef]
- Kuriyan, A.E.; Albini, T.A.; Townsend, J.H.; Rodriguez, M.; Pandya, H.K.; Leonard, R.E., 2nd; Parrott, M.B.; Rosenfeld, P.J.; Flynn, H.W., Jr.; Goldberg, J.L. Vision loss after intravitreal injection of autologous “stem cells” for AMD. N. Engl. J. Med. 2017, 376, 1047–1053. [Google Scholar] [CrossRef] [PubMed]
- Saraf, S.S.; Cunningham, M.A.; Kuriyan, A.E.; Read, S.P.; Rosenfeld, P.J.; Flynn, H.W., Jr.; Albini, T.A. Bilateral retinal detachments after intravitreal injection of adipose-derived ‘stem cells’ in a patient with exudative macular degeneration. Ophthalmic Surg. Lasers Imaging Retin. 2017, 48, 772–775. [Google Scholar] [CrossRef] [PubMed]
- Seong, M.K.; Park, U.C.; Jung, S.I. Determinant of anal resting pressure gradient in association with continence function. J. Neurogastroenterol. Motil. 2011, 17, 300–304. [Google Scholar] [CrossRef] [PubMed]

| Origin | Gene | F/R | Sequence (5′→3′) |
|---|---|---|---|
| Human | α-SMA | F | ACCCAGCACCATGAAGATCA |
| R | TTTGCGGTGGACAATGGAAG | ||
| Human | Calponin | F | GTGAAGCCCCACGACATTTT |
| R | TGATGTTCCGCCCTTCTCTT | ||
| Human | Smoothelin | F | GTACGGGCTCAGGAGATTGA |
| R | GAAACCTCTGCCTGCTGTTC | ||
| Human | Caldesmon | F | AAAACCTACAAAGCCGGCAG |
| R | AAACCCCTACCTTCAAGCCA | ||
| Human | SM22α | F | TCTTCACTCCTTCCTGCGAG |
| R | TATGATCCACTCCACCAGCC | ||
| Rat | α-SMA | F | ACTGGGACGACATGGAAAAG |
| R | GCCACATACATGGCAGGGACATTG | ||
| Rat | SMMHC | F | GATGTGGTGCAGAAAGCTCA |
| R | TGAGAATCCATCGGAAAAGG | ||
| Rat | Smoothelin | F | TCAAGCAGATGTTGCTGGAC |
| R | ACAGAAAGCCATCCCATCAC | ||
| Rat | Caldesmon | F | TGGAAGCAGAAGAACAAGAAC |
| R | TTCAGCCTCCCTCCTCTC | ||
| Rat | SM22α | F | AGGTGTGGCTGAAGAATG |
| R | CCTGTTCCATCTGCTTGA | ||
| Rat | Calponin | F | ACTTCATGGATGGCCTCAAG |
| R | GTGCCAGTTCTGGGTTGACT | ||
| Rat | SDF-1 | F | CTGAATAGTGGCTCCCAAGGTT |
| R | GTGGATCTCGCTCTTCCCTGAC | ||
| Rat | MCP-3 | F | GCATGGAAGTCTGTGCTGAA |
| R | CCGTTCCTACCCCTTAGGAC | ||
| Rat | Fibronectin | F | GAAAGGCAACCAGCAGAGTC |
| R | CTGGAGTCAAGCCAGACACA | ||
| Rat | MCP-1 | F | CTATGCAGGTCTCTGTCACGCTTC |
| R | CAGCCGACTCATTGGGATCA | ||
| Rat | CoL1A1 | F | ACAGGCGAACAAGGTGACAGAG |
| R | GCCAGGAGAACCAGCAGAGC | ||
| Rat | TGF-β | F | AGGGCTACCATGCCAACTTC |
| R | CCACGTAGTAGACGATGGGC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.; Oh, B.-Y.; Lee, J.-S.; Yoon, D.; Kim, Y.-R.; Chun, W.; Kim, J.W.; Son, I.T. Differentiation of Adipose-Derived Stem Cells into Smooth Muscle Cells in an Internal Anal Sphincter-Targeting Anal Incontinence Rat Model. J. Clin. Med. 2023, 12, 1632. https://doi.org/10.3390/jcm12041632
Kim M, Oh B-Y, Lee J-S, Yoon D, Kim Y-R, Chun W, Kim JW, Son IT. Differentiation of Adipose-Derived Stem Cells into Smooth Muscle Cells in an Internal Anal Sphincter-Targeting Anal Incontinence Rat Model. Journal of Clinical Medicine. 2023; 12(4):1632. https://doi.org/10.3390/jcm12041632
Chicago/Turabian StyleKim, Minsung, Bo-Young Oh, Ji-Seon Lee, Dogeon Yoon, You-Rin Kim, Wook Chun, Jong Wan Kim, and Il Tae Son. 2023. "Differentiation of Adipose-Derived Stem Cells into Smooth Muscle Cells in an Internal Anal Sphincter-Targeting Anal Incontinence Rat Model" Journal of Clinical Medicine 12, no. 4: 1632. https://doi.org/10.3390/jcm12041632
APA StyleKim, M., Oh, B.-Y., Lee, J.-S., Yoon, D., Kim, Y.-R., Chun, W., Kim, J. W., & Son, I. T. (2023). Differentiation of Adipose-Derived Stem Cells into Smooth Muscle Cells in an Internal Anal Sphincter-Targeting Anal Incontinence Rat Model. Journal of Clinical Medicine, 12(4), 1632. https://doi.org/10.3390/jcm12041632





