Chitotriosidase and Neopterin as Potential Biomarkers for the Evaluation of Complicated Cholecystitis—A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Setting and Study Design
2.2. Participants
2.3. Data Source and Collection
2.4. Statistical Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sorrentino, S.A. Gallstones. Radiology Reference Article. Available online: https://radiopaedia.org/articles/gallstones-1 (accessed on 27 November 2022).
- Stinton, L.M.; Shaffer, E.A. Epidemiology of Gallbladder Disease: Cholelithiasis and Cancer. Gut Liver 2012, 6, 172–187. [Google Scholar] [CrossRef]
- Sakorafas, G.H.; Milingos, D.; Peros, G. Asymptomatic Cholelithiasis: Is Cholecystectomy Really Needed? A Critical Reappraisal 15 Years after the Introduction of Laparoscopic Cholecystectomy. Dig. Dis. Sci. 2007, 52, 1313–1325. [Google Scholar] [CrossRef]
- Bortoff, G.A.; Chen, M.Y.; Ott, D.J.; Wolfman, N.T.; Routh, W.D. Gallbladder Stones: Imaging and Intervention. Radiographics 2000, 20, 751–766. [Google Scholar] [CrossRef]
- Hassler, K.R.; Collins, J.T.; Philip, K.; Jones, M.W. Laparoscopic Cholecystectomy; StatPearls Publishing: St. Petersburg, FL, USA, 2022. [Google Scholar]
- Friedman GD: Natural history of asymptomatic and symptomatic gallstones. Am. J. Surg. 1993, 165, 399–404. [CrossRef]
- Önder, A.; Kapan, M.; Ülger, B.V.; Oğuz, A.; Türkoğlu, A.; Uslukaya, Ö. Gangrenous Cholecystitis: Mortality and Risk Factors. Int. Surg. 2015, 100, 254–260. [Google Scholar] [CrossRef]
- Hunt, D.R.; Chu, F.C. Gangrenous Cholecystitis in the Laparoscopic Era. Aust. N. Z. J. Surg. 2000, 70, 428–430. [Google Scholar] [CrossRef]
- Cutaș, A.; Drugan, C.; Roman, G.; Rusu, A.; Cătană, C.S.; Achimaș-Cadariu, A.; Drugan, T. Evaluation of Chitotriosidase and Neopterin as Biomarkers of Microvascular Complications in Patients with Type 1 Diabetes Mellitus. Diagnostics 2021, 11, 263. [Google Scholar] [CrossRef]
- Kumar, A.; Zhang, K.Y.J. Human Chitinases: Structure, Function, and Inhibitor Discovery. Adv. Exp. Med. Biol. 2019, 1142, 221–251. [Google Scholar] [CrossRef]
- Van Eijk, M.; van Roomen, C.P.A.A.; Renkema, G.H.; Bussink, A.P.; Andrews, L.; Blommaart, E.F.C.; Sugar, A.; Verhoeven, A.J.; Boot, R.G.; Aerts, J.M.F.G. Characterization of Human Phagocyte-Derived Chitotriosidase, a Component of Innate Immunity. Int. Immunol. 2005, 17, 1505–1512. [Google Scholar] [CrossRef]
- Malaguarnera, L.; Musumeci, M.; Di Rosa, M.; Scuto, A.; Musumeci, S. Interferon-Gamma, Tumor Necrosis Factor-Alpha, and Lipopolysaccharide Promote Chitotriosidase Gene Expression in Human Macrophages. J. Clin. Lab. Anal. 2005, 19, 128–132. [Google Scholar] [CrossRef]
- Elmonem, M.A.; van den Heuvel, L.P.; Levtchenko, E.N. Immunomodulatory Effects of Chitotriosidase Enzyme. Enzym. Res. 2016, 2016, e2682680. [Google Scholar] [CrossRef]
- Renkema, H.; Boot, R.; Muijsers, A.O.; Donker-Koopman, W.E.; Aerts, J. Purification and Characterization of Human Chitotriosidase, a Novel Member of the Chitinase Family of Proteins. J. Biol. Chem. 1995, 270, 2198–2202. [Google Scholar] [CrossRef]
- Mazur, M.; Zielińska, A.; Grzybowski, M.M.; Olczak, J.; Fichna, J. Chitinases and Chitinase-Like Proteins as Therapeutic Targets in Inflammatory Diseases, with a Special Focus on Inflammatory Bowel Diseases. Int. J. Mol. Sci. 2021, 22, 6966. [Google Scholar] [CrossRef]
- Pingle, S.K.; Tumane, R.G.; Jawade, A.A. Neopterin: Biomarker of Cell-Mediated Immunity and Potent Usage as Biomarker in Silicosis and Other Occupational Diseases. Indian J. Occup. Environ. Med. 2008, 12, 107–111. [Google Scholar] [CrossRef]
- Voet, D.; Voet, J.G. Biochemistry, 3rd ed.; John Wiley and Sons: Hoboken, NJ, USA, 2004; pp. 39223–39225. [Google Scholar]
- Murr, C.; Widner, B.; Wirleitner, B.; Fuchs, D. Neopterin as a Marker for Immune System Activation. Curr. Drug Metab. 2002, 3, 175–187. [Google Scholar] [CrossRef]
- Hoffmann, G.; Wirleitner, B.; Fuchs, D. Potential Role of Immune System Activation-Associated Production of Neopterin Derivatives in Humans. Inflamm. Res. 2003, 52, 313–321. [Google Scholar] [CrossRef]
- Gieseg, S.P.; Baxter-Parker, G.; Lindsay, A. Neopterin, Inflammation, and Oxidative Stress: What Could We Be Missing? Antioxidants 2018, 7, 80. [Google Scholar] [CrossRef]
- Werner, E.R.; Bichler, A.; Daxenbichler, G.; Fuchs, D.; Fuith, L.C.; Hausen, A.; Hetzel, H.; Reibnegger, G.; Wachter, H. Determination of Neopterin in Serum and Urine. Clin. Chem. 1987, 33, 62–66. [Google Scholar] [CrossRef]
- Acar, A.; Keskek, M.; Işman, F.K.; Kucur, M.; Tez, M. Serum Chitotriosidase Activity in Acute Appendicitis: Preliminary Results. Am. J. Emerg. Med. 2012, 30, 775–777. [Google Scholar] [CrossRef]
- Coşkun, K.; Mentes, O.; Atak, A.; Aral, A.; Eryilmaz, M.; Onguru, O.; Balkan, M.; Kozak, O.; Cetiner, S. Is Neopterin a Diagnostic Marker of Acute Appendicitis? Ulus. Travma Ve Acil Cerrahi Derg. 2012, 18, 1–4. [Google Scholar] [CrossRef]
- Kamal, Z.B.; Naji, R.E.; Ali, H.A. Comparative Study between Neopterin and Alvarado Score in the Diagnosis of Acute Appendicitis and Its Severity. Open Access Maced. J. Med. Sci. 2021, 9, 42–47. [Google Scholar] [CrossRef]
- Dal, F. Role of Alvarado Score and Biological Indicators of C-Reaktif Protein, Procalicitonin and Neopterin in Diagnosis of Acute Appendicitis. Ulus. Travma Ve Acil Cerrahi Derg. 2018, 25, 229–237. [Google Scholar] [CrossRef]
- Hirota, M.; Takada, T.; Kawarada, Y.; Nimura, Y.; Miura, F.; Hirata, K.; Mayumi, T.; Yoshida, M.; Strasberg, S.; Pitt, H.; et al. Diagnostic Criteria and Severity Assessment of Acute Cholecystitis: Tokyo Guidelines. J. Hepatobiliary Pancreat. Surg. 2007, 14, 78–82. [Google Scholar] [CrossRef]
- Hollak, C.E.; van Weely, S.; van Oers, M.H.; Aerts, J.M. Marked elevation of plasma chitotriosidase activity. A novel hallmark of Gaucher disease. J. Clin. Investig. 1994, 93, 1288–1292. [Google Scholar] [CrossRef]
- Prakash, G.; Hasan, M. The Accuracy of Neutrophil-to-Lymphocyte Ratio and Abdominal Computed Tomography to Predict the Severity of Acute Cholecystitis. Cureus 2022, 14, e32243. [Google Scholar] [CrossRef]
- Sugrue, M.; Sahebally, S.M.; Ansaloni, L.; Zielinski, M.D. Grading Operative Findings at Laparoscopic Cholecystectomy—A New Scoring System. World J. Emerg. Surg. 2015, 10, 14. [Google Scholar] [CrossRef]
| All Subjects (n = 51) | Simple Cholecystitis (n = 36) | Complicated Cholecystitis (n = 15) | p-Value | |
|---|---|---|---|---|
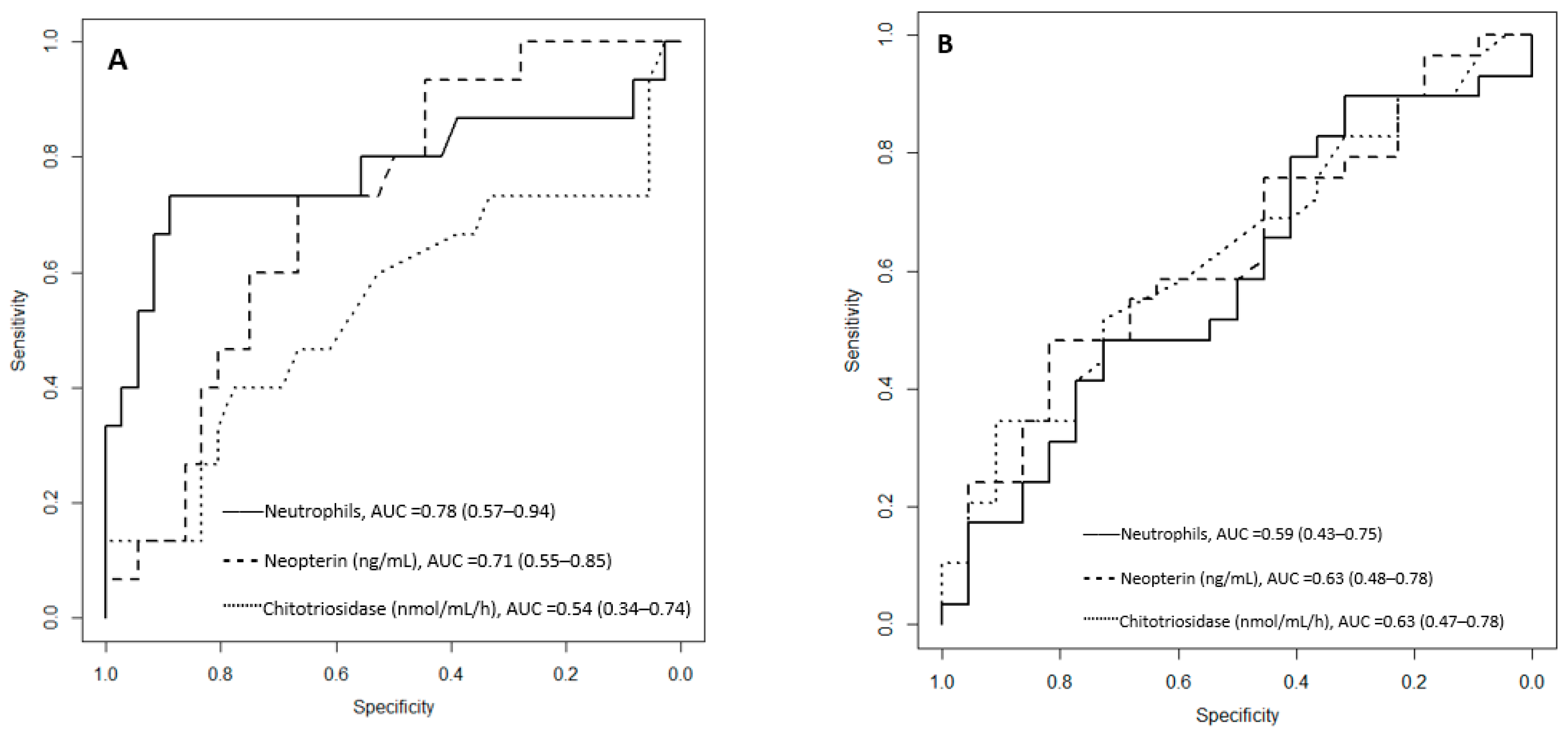
| White blood cells | 7.34 (5.82–10.13) | 7.01 (5.71–8.24) | 10.91 (7.26–13.14) | 0.0131 |
| Neutrophils | 4.90 (3.50–6.48) | 4.29 (3.31–5.53) | 7.63 (5.40–10.19) | 0.00147 |
| Chitotriosidase baseline (nmol/mL/h) | 170.00 (110.00–230.00) | 160.00 (110.00–212.50) | 170.00 (90.00–295.00) | 0.66 |
| Neopterin baseline (nmol/L) | 13.03 (7.78–18.34) | 11.92 (6.79–16.39) | 16.82 (13.53–21.36) | 0.0177 |
| Chitotriosidase fd * (nmol/mL/h) | 140.00 (110.00–195.00) | 145.00 (110.00–172.50) | 140.00 (75.00–210.00) | 0.82 |
| Neopterin fd * (nmol/L) | 12.26 (8.84–20.63) | 13.06 (8.73–19.98) | 10.94 (9.04–20.02) | 0.87 |
| AST/GOT (U/L) | 22.00 (17.00–26.50) | 21.50 (17.00–26.25) | 24.00 (17.50–26.50) | 0.766 |
| ALT/GPT (U/L) | 21.00 (16.00–28.50) | 21.00 (15.75–29.25) | 22.00 (16.50–27.00) | 0.862 |
| Amylase (U/L) | 51.0 (42.00–63.50) | 56.00 (42.00–64.25) | 46.00 (39.50–57.00) | 0.361 |
| Total bilirubin (mg/dL) | 0.60 (0.50–0.80) | 0.60 (0.40–0.80) | 0.70 (0.60–0.95) | 0.119 |
| Direct bilirubin (mg/dL) | 0.27 (0.22–0.35) | 0.25 (0.18–0.32) | 0.31 (0.28–0.46) | 0.0089 |
| Alkaline phosphatase (U/L) | 188.00 (153.00–242.00) | 204.50 (161.50–242.00) | 181.00 (128.50–233.50) | 0.442 |
| Gamma-glutamyl transpeptidase (U/L) | 41.00 (23.00–64.50) | 40.00 (22.75–61.75) | 41.00 (23.00–68.00) | 0.91 |
| Surgery duration (min) | 45.00 (40.00–57.50) | 42.50 (35.00–55.00) | 60.00 (50.00–70.00) | 0.0012 |
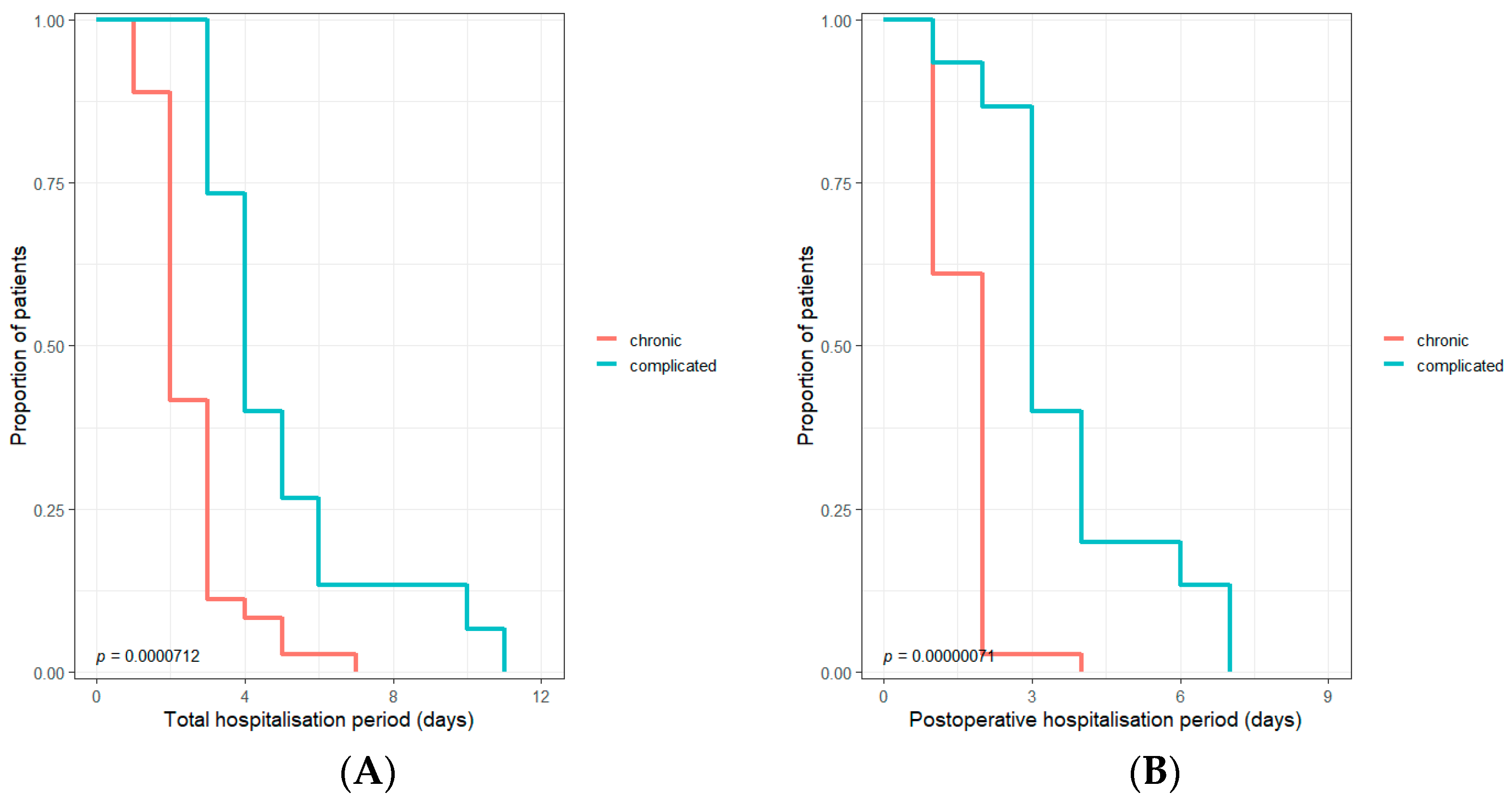
| Hospitalization after surgery (days) | 2.00 (1.00–3.00) | 2.00 (1.00–2.00) | 3.00 (3.00–4.00) | 0.000003 |
| Total hospitalization (days) | 3.00 (2.00–4.00) | 2.00 (2.00–3.00) | 4.00 (3.50–5.50) | 0.000002 |
| Preoperative Values (n = 51) | Neopterin (nmol/L) Yes * | Neopterin (nmol/L) No ** | p-Value | Chitotriosidase (nmol/mL/h) Yes * | Chitotriosidase (nmol/mL/h) No ** | p-Value |
|---|---|---|---|---|---|---|
| Adhesiolisis (n = 23) | 11.65 (6.99–20.82) | 14.46 (8.45–18.09) | 0.79 | 170 (115–400) | 160 (100–205) | 0.23 |
| Drainage (n = 29) | 15.13 (9.56–20.81) | 12.52 (7.11–15.8) | 0.108 | 180 (115–350) | 160 (100–185) | 0.11 |
| Aponeurosis enlargement (n = 11) | 9.56 (7.86–15.8) | 14.69 (7.9–18.09) | 0.504 | 160 (160–395) | 170 (107.5–212.5) | 0.346 |
| Postoperative values (n = 35) # | ||||||
| Adhesiolisis (n = 13) | 12.99 (6.52–22.63) | 11.93 (9.12–17.54) | 0.959 | 160 (110–350) | 140 (110–170) | 0.322 |
| Drainage (n = 20) | 12.64 (8.69–22.95) | 11.57 (9.64–17.02) | 0.8051 | 145 (107.5–280) | 140 (115–170) | 0.517 |
| Aponeurosis enlargement (n = 7) | 9.95 (7.78–18.17) | 12.64 (9.16–19.95) | 0.635 | 150 (130–310) | 140 (107.5–187.5) | 0.5093 |
| Baseline Neopterin (nmol/L) | Postoperative Neopterin (nmol/L) | p-Value | Baseline Chitotriosidase (nmol/mL/h) | Postoperative Chitotriosidase (nmol/mL/h) | p-Value | |
|---|---|---|---|---|---|---|
| All cases (n = 35) | 14.89 (10.48–21) | 12.25 (8.84–20.62) | 0.22 | 160 (105–225) | 140 (110–195) | 0.00369 |
| Chronic cholecystitis (n = 23) | 12.94 (8.34–15.91) | 12.98 (8.59–20.62) | 0.78 | 160 (110–200) | 150 (110–175) | 0.07 |
| Complicated cholecystitis (n = 11) | 19.42 (15.07–23.35) | 10.92 (9.05–20.01) | 0.06 | 190 (90–305) | 145 (75–210) | 0.007 |
| Adhesiolisis (n = 13) | 15.25 (11.42–26.35) | 12.99 (6.53–22.66) | 0.27 | 160 (110–480) | 160 (110–350) | 0.11 |
| Subhepatic drainage (n = 20) | 16 (11.6–25.17) | 12.64 (8.7–22.95) | 0.29 | 170 (105–317.5) | 145 (107.5–280) | 0.04 |
| Aponeurosis enlargement (n = 7) | 12.17 (9.01–29.79) | 9.95 (7.78–18.17) | 0.17 | 160 (160–455) | 150 (130–310) | 0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nechita, V.-I.; Hajjar, N.A.; Drugan, C.; Cătană, C.-S.; Moiş, E.; Nechita, M.-A.; Graur, F. Chitotriosidase and Neopterin as Potential Biomarkers for the Evaluation of Complicated Cholecystitis—A Pilot Study. J. Clin. Med. 2023, 12, 1641. https://doi.org/10.3390/jcm12041641
Nechita V-I, Hajjar NA, Drugan C, Cătană C-S, Moiş E, Nechita M-A, Graur F. Chitotriosidase and Neopterin as Potential Biomarkers for the Evaluation of Complicated Cholecystitis—A Pilot Study. Journal of Clinical Medicine. 2023; 12(4):1641. https://doi.org/10.3390/jcm12041641
Chicago/Turabian StyleNechita, Vlad-Ionuţ, Nadim Al Hajjar, Cristina Drugan, Cristina-Sorina Cătană, Emil Moiş, Mihaela-Ancuţa Nechita, and Florin Graur. 2023. "Chitotriosidase and Neopterin as Potential Biomarkers for the Evaluation of Complicated Cholecystitis—A Pilot Study" Journal of Clinical Medicine 12, no. 4: 1641. https://doi.org/10.3390/jcm12041641
APA StyleNechita, V.-I., Hajjar, N. A., Drugan, C., Cătană, C.-S., Moiş, E., Nechita, M.-A., & Graur, F. (2023). Chitotriosidase and Neopterin as Potential Biomarkers for the Evaluation of Complicated Cholecystitis—A Pilot Study. Journal of Clinical Medicine, 12(4), 1641. https://doi.org/10.3390/jcm12041641







