Utility of Doppler-Ultrasound and Liver Elastography in the Evaluation of Patients with Suspected Pregnancy-Related Liver Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Data Collection and Definitions
2.3. Ultrasound Evaluation
2.4. Liver Elastography
2.5. Statistical Analysis
3. Results
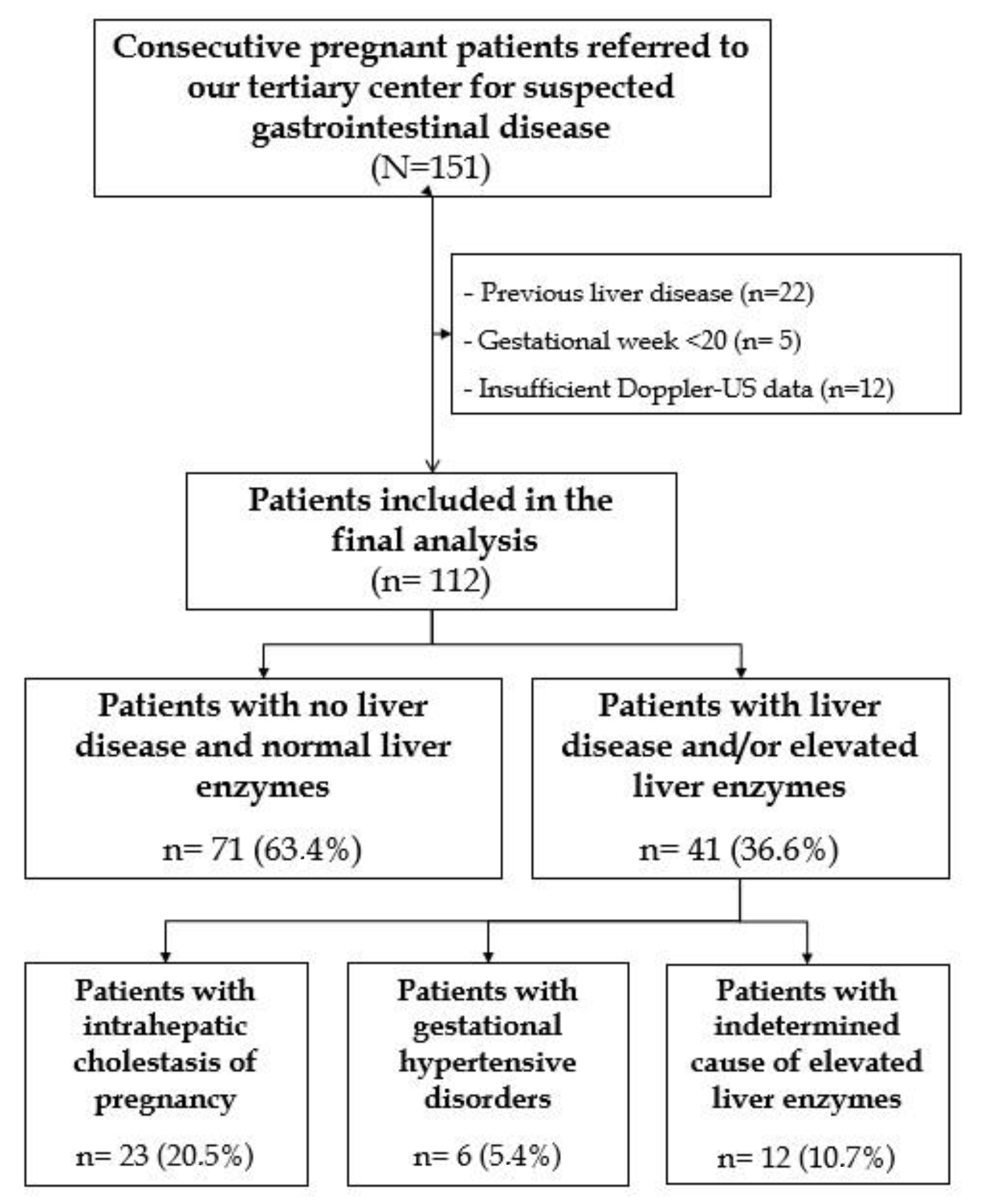
3.1. Patients’ Selection and Characteristics
3.2. Role of Doppler-US and Liver Elastography in the Differential Diagnosis of Pregnancy-Related Liver Dysfunction
3.3. Performance of Liver Elastography in the Diagnosis of Pregnancy-Related Liver Dysfunction
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Joshi, D.; James, A.; Quaglia, A.; Westbrook, R.H.; Heneghan, M.A. Liver disease in pregnancy. Lancet 2010, 375, 594–605. [Google Scholar] [CrossRef]
- Westbrook, R.H.; Dusheiko, G.; Williamson, C. Pregnancy and liver disease. J. Hepatol. 2016, 64, 933–945. [Google Scholar] [CrossRef] [Green Version]
- Hay, J.E. Liver disease in pregnancy. Hepatology 2008, 47, 1067–1076. [Google Scholar] [CrossRef]
- Lim, E.; Mouyis, M.; MacKillop, L. Liver diseases in pregnancy. Clin. Med. 2021, 21, E441–E445. [Google Scholar] [CrossRef]
- Azzaroli, F.; Mazzella, G.; Marchesini, G.; Brodosi, L.; Petroni, M.L. Fatty liver in pregnancy: A narrative review of two distinct conditions. Expert Rev. Gastroenterol. Hepatol. 2020, 14, 127–135. [Google Scholar] [CrossRef]
- Mohr-Sasson, A.; Schiff, E.; Suday, R.R.; Hayman, Z.; Kleinbaum, Y.; Kalter-Farber, A.; Mashiach, R.; Yinon, Y.; Dulitzki, M.; Sivan, E.; et al. The Yield of Abdominal Ultrasound in the Evaluation of Elevated Liver Enzymes during the Second and the Third Trimester of Pregnancy. Gynecol. Obstet. Investig. 2017, 82, 517–520. [Google Scholar] [CrossRef]
- Donet, A.; Girault, A.; Pinton, A.; Lepercq, J. Intrahepatic cholestasis of pregnancy: Is a screening for differential diagnoses necessary? J. Gynecol. Obstet. Hum. Reprod. 2020, 49, 101907. [Google Scholar] [CrossRef]
- Gyselaers, W.; Mullens, W.; Tomsin, K.; Mesens, T.; Peeters, L. Role of dysfunctional maternal venous hemodynamics in the pathophysiology of pre-eclampsia: A review. Ultrasound Obstet. Gynecol. 2011, 38, 123–129. [Google Scholar] [CrossRef]
- Dietrich, C.F.; Bamber, J.; Berzigotti, A.; Bota, S.; Cantisani, V.; Castera, L.; Cosgrove, D.; Ferraioli, G.; Friedrich-Rust, M.; Gilja, O.H.; et al. EFSUMB Guidelines and Recommendations on the Clinical Use of Liver Ultrasound Elastography, Update 2017 (Long Version). Ultraschall Medizin 2017, 38, e16–e47. [Google Scholar]
- Ammon, F.J.; Kohlhaas, A.; Elshaarawy, O.; Mueller, J.; Bruckner, T.; Sohn, C.; Fluhr, G.; Fluhr, H.; Mueller, S. Liver stiffness reversibly increases during pregnancy and independently predicts preeclampsia. World J. Gastroenterol. 2018, 24, 4393–4402. [Google Scholar] [CrossRef]
- Brown, M.A.; Magee, L.A.; Kenny, L.C.; Karumanchi, S.A.; McCarthy, F.P.; Saito, S.; Hall, D.R.; Warren, C.E.; Adoyi, G.; Ishaku, S. The hypertensive disorders of pregnancy: ISSHP classification, diagnosis & management recommendations for international practice. Pregnancy Hypertens. 2018, 13, 291–310. [Google Scholar] [CrossRef]
- Lee, R.H.; Greenberg, M.; Metz, T.Z.; Pettker, C.M.; Lee, R.H. Society for Maternal-Fetal Medicine Consult Series #53: Intrahepatic cholestasis of pregnancy: Replaces Consult #13, April 2011. Am. J. Obstet. Gynecol. 2021, 224, B2–B9. [Google Scholar] [CrossRef]
- Colecchia, A.; Ravaioli, F.; Sessa, M.; Alemanni, V.L.; Dajti, E.; Marasco, G.; Vestito, A.; Zagari, R.M.; Barbato, F.; Arpinati, M.; et al. Liver Stiffness Measurement Allows Early Diagnosis of Veno-Occlusive Disease/Sinusoidal Obstruction Syndrome in Adult Patients Who Undergo Hematopoietic Stem Cell Transplantation: Results from a Monocentric Prospective Study. Biol. Blood Marrow Transplant. 2019, 25, 995–1003. [Google Scholar] [CrossRef] [Green Version]
- Dajti, E.; Ravaioli, F.; Colecchia, A.; Marasco, G.; Vestito, A.; Festi, D. Liver and Spleen Stiffness Measurements for Assessment of Portal Hypertension Severity in Patients with Budd Chiari Syndrome. Can. J. Gastroenterol. Hepatol. 2019, 2019, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Arena, U.; Vizzutti, F.; Corti, G.; Ambu, S.; Stasi, C.; Bresci, S.; Moscarella, S.; Boddi, V.; Petrarca, A.; Laffi, G.; et al. Acute viral hepatitis increases liver stiffness values measured by transient elastography. Hepatology 2007, 47, 380–384. [Google Scholar] [CrossRef] [Green Version]
- Taniguchi, T.; Ohtani, T.; Kioka, H.; Tsukamoto, Y.; Onishi, T.; Nakamoto, K.; Katsimichas, T.; Sengoku, K.; Chimura, M.; Hashimoto, H.; et al. Liver Stiffness Reflecting Right-Sided Filling Pressure Can Predict Adverse Outcomes in Patients With Heart Failure. JACC Cardiovasc. Imaging 2019, 12, 955–964. [Google Scholar] [CrossRef]
- Millonig, G.; Reimann, F.M.; Friedrich, S.; Fonouni, H.; Mehrabi, A.; Büchler, M.W.; Seitz, H.K.; Mueller, S. Extrahepatic cholestasis increases liver stiffness (FibroScan) irrespective of fibrosis. Hepatology 2008, 48, 1718–1723. [Google Scholar] [CrossRef]

| Variables | All Patients (n = 112) | Patients with No Liver Disease (n = 71) | Patients with Liver Disease (n = 41) | p-Value |
|---|---|---|---|---|
| Age (years) | 35 (31–38) | 34 (30–38) | 36 (32–38) | 0.370 |
| BMI (kg/m2) | 23.9 (21.4–27) | 23 (21.3–25.4) | 25 (21.5–28) | 0.113 |
| Overweight | 52 (46.4%) | 28 (39.4%) | 24 (58.5%) | 0.051 |
| Relative weight gain (%) | 18.6 (14.4–25.4) | 20.6 (16.4–28.1) | 15 (11.3–22) | 0.039 |
| Week of gestation | 34 (32–36) | 35 (33–36) | 33 (29–36) | 0.040 |
| Parity | 1 (1–1) | 1 (1–2) | 1 (1–1) | 0.038 |
| Biochemical tests | ||||
| AST (UI/L) | 21 (17–38) | 19 (15–23) | 46 (39–75) | <0.0001 |
| ALT (UI/L) | 18 (11–45) | 14 (10–21) | 79 (41–126) | <0.0001 |
| Bilirubin (mg/dL) | 0.42 (0.36–0.55) | 0.41 (0.36–0.52) | 0.43 (0.35–0.58) | 0.651 |
| γ-GT (UI/L) | 12 (8–19) | 12 (8–18) | 14 (9–20) | 0.125 |
| Bile acid levels (µmol/L) (n = 78) | 6.7 (3–15.5) | 3.2 (2.1–6) | 16 (9.3–24.1) | <0.0001 |
| Liver-related dysfunction | ||||
| Elevated liver enzymes | 41 (36.6%) | |||
| Intrahepatic cholestasis of pregnancy | 23 (20.5%) | |||
| Hypertension/pre-eclampsia/ HELLP syndrome | 6 (5.4%) | |||
| Unknown | 12 (10.7%) | |||
| Metabolic complications | ||||
| Gestational diabetes | 15 (13.4%) | 7 (9.9%) | 8 (19.5%) | 0.148 |
| Perinatal outcomes | ||||
| Birth weight (g) | 3050 (2740–3380) | 3050 (2800–3380) | 3085 (2695–3383) | 0.547 |
| Unfavourable outcomes (n = 100) | 11 (11%) | 5 (7.8%) | 6 (16.7%) | 0.174 |
| Variables | Patients with Normal Liver Enzymes (n = 71) | Patients with ICP (n = 23) | p-Value | Patients with GH/PE/HELLP (n = 6) | p-Value | Patients with Undetermined Causes of Elevated Liver Enzymes (n = 12) | p-Value |
|---|---|---|---|---|---|---|---|
| Age (years) | 34 (30–38) | 36 (32–38) | 0.303 | 35 (34–35) | 0.900 | 35 (31–39) | 0.747 |
| BMI (kg/m2) | 23 (21.3–25.4) | 25.7 (22.1–29.2) | 0.136 | 21.4 (21.1–24.5) | 0.404 | 26.8 (24.8–26.9) | 0.053 |
| Overweight | 28 (39.4%) | 14 (60.9%) | 0.072 | 1 (16.7%) | 0.269 | 9 (75%) | 0.024 |
| Relative weight gain (%) | 20.6 (16.4–28.1) | 11.9 (9.8–18.7) | 0.005 | 19.7 (15.9–29.7) | 0.917 | 20.3 (16.4–29.4) | 0.917 |
| Week of gestation | 35 (33–36) | 33 (29–36) | 0.156 | 33 (33–33) | 0.188 | 33 (27–35) | 0.150 |
| Parity | 1 (1–2) | 1 (1–1) | 0.035 | 1 (1–2) | 0.443 | 1 (0–1) | 0.115 |
| Gestational diabetes | 7 (9.9%) | 4 (17.4%) | 0.328 | 1 (16.7%) | 0.600 | 3 (25%) | 0.136 |
| Perinatal outcomes | |||||||
| Unfavorable outcomes | 5 (7.8%) | 3 (14.3%) | 0.378 | 2 (33.3%) | 0.107 | 1 (11.1%) | 0.736 |
| Doppler-US findings | |||||||
| Middle-HV flow | 0.831 | 0.998 | 0.225 | ||||
| Monophasic | 24 (33.8%) | 9 (39.1%) | 2 (33%) | 6 (50%) | |||
| Biphasic | 23 (32.4%) | 6 (26.1%) | 2 (33%) | 1 (8.3%) | |||
| Triphasic | 24 (33.8%) | 8 (34.8%) | 2 (33%) | 5 (41.7%) | |||
| Middle-HV flow in the lateral position | 0.702 | 0.449 | 0.126 | ||||
| Monophasic | 8 (11.3%) | 4 (17.4%) | 1 (16.7%) | 4 (33.3%) | |||
| Biphasic | 11 (15.5%) | 4 (17.4%) | 2 (33.3%) | 1 (8.3%) | |||
| Triphasic | 52 (73.2%) | 15 (65.2%) | 3 (50%) | 7 (58.4%) | |||
| Improvement in HV flow after lateral position | 35 (49.3%) | 10 (43.5%) | 0.627 | 2 (33%) | 0.676 | 3 (25%) | 0.209 |
| Right HA-RI | 0.60 (0.57–0.67) | 0.61 (0.59–0.69) | 0.342 | 0.73 (0.64–0.87) | 0.070 | 0.70 (0.66–0. 75) | 0.034 |
| Right HA-RI > 0.7 (yes) | 10 (14.1%) | 6 (26.1%) | 0.208 | 4 (66.7%) | 0.009 | 8 (66.7%) | 0.003 |
| Left HA-RI | 0.63 (0.58–0.69) | 0.60 (0.58–0.71) | 0.880 | 0.63 (0.57–0.69) | 0.990 | 0.71 (0.70–0.74) | 0.011 |
| Left HA-RI > 0.7 (yes) | 16 (22.5%) | 9 (39.1%) | 0.173 | 2 (33.3%) | 0.620 | 10 (83.3%) | 0.001 |
| Right HA-PI | 0.97 (0.85–1.17) | 1.10 (0.93–1.33) | 0.104 | 1.12 (0.90–1.52) | 0.355 | 1.19 (1.10–1.44) | 0.165 |
| Right HA-PI > 1.2 (yes) | 54 (76.1%) | 20 (87%) | 0.383 | 6 (100%) | 0.329 | 11 (91.7%) | 0.448 |
| Left HA-PI | 1.02 (0.86–1.30) | 0.96 (0.91–1.49) | 0.513 | 1.04 (0.97–1.38) | 0.596 | 1.44 (1.30–1.45) | 0.033 |
| Left HA-PI > 1.2 (yes) | 24 (33.8%) | 10 (47.6%) | 0.458 | 2 (33.3%) | 0.981 | 8 (66.7%) | 0.051 |
| SA-RI | 0.54 (0.51–0.58) | 0.54 (0.51–0.59) | 0.826 | 0.54 (0.51–0.58) | 1 | 0.60 (0.57–0.72) | 0.021 |
| SA-RI > 0.6 (yes) | 10 (14.1%) | 3 (14.3%) | 0.390 | 1 (16.7%) | 0.862 | 5 (41.7%) | 0.037 |
| SA-PI | 0.80 (0.72–0.87) | 0.79 (0.72–0.92) | 0.962 | 0.76 (0.71–0.88) | 0.794 | 0.90 (0.70–0.95) | 0.594 |
| SA-PI > 0.95 (yes) | 9 (12.7%) | 2 (8.7%) | 0.886 | 1 (16.7%) | 0.579 | 2 (16.7%) | 0.657 |
| Elastography (n = 101) | |||||||
| Liver stiffness (kPa) | 4.5 (4.2–5.3) | 5 (4.3–5.5) | 0.195 | 7.4 (5.1–9.9) | 0.006 | 5.1 (4.2–5.7) | 0.448 |
| Liver stiffness ≥ 7.6 kPa | 2 (2.9%) | 2 (11.8%) | 0.120 | 3 (60%) | 0.003 | 0 (0%) | 0.605 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serra, C.; Dajti, E.; De Molo, C.; Montaguti, E.; Porro, A.; Seidenari, A.; Angilletta, E.; Bernardi, V.; Salsi, G.; Bakken, S.M.; et al. Utility of Doppler-Ultrasound and Liver Elastography in the Evaluation of Patients with Suspected Pregnancy-Related Liver Disease. J. Clin. Med. 2023, 12, 1653. https://doi.org/10.3390/jcm12041653
Serra C, Dajti E, De Molo C, Montaguti E, Porro A, Seidenari A, Angilletta E, Bernardi V, Salsi G, Bakken SM, et al. Utility of Doppler-Ultrasound and Liver Elastography in the Evaluation of Patients with Suspected Pregnancy-Related Liver Disease. Journal of Clinical Medicine. 2023; 12(4):1653. https://doi.org/10.3390/jcm12041653
Chicago/Turabian StyleSerra, Carla, Elton Dajti, Chiara De Molo, Elisa Montaguti, Alberto Porro, Anna Seidenari, Emiliana Angilletta, Vito Bernardi, Ginevra Salsi, Sofia Maria Bakken, and et al. 2023. "Utility of Doppler-Ultrasound and Liver Elastography in the Evaluation of Patients with Suspected Pregnancy-Related Liver Disease" Journal of Clinical Medicine 12, no. 4: 1653. https://doi.org/10.3390/jcm12041653






