Clinical Outcomes and Complication Rate after Single-Stage Hardware Removal and Total Hip Arthroplasty: A Matched-Pair Controlled Study
Abstract
1. Introduction
2. Materials and Methods
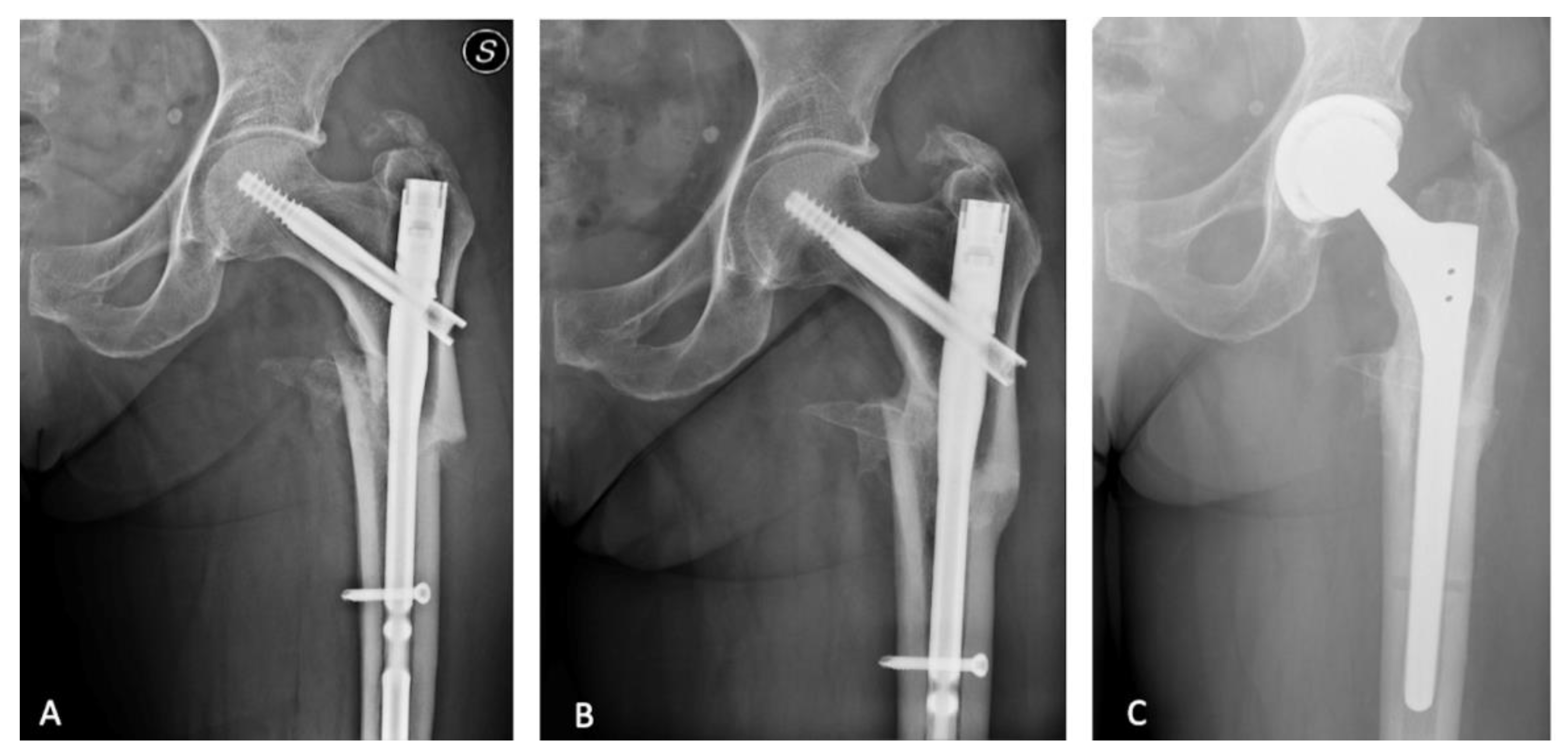
2.1. Surgical Procedure
2.2. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gallazzi, E.; Morelli, I.; Peretti, G.; Zagra, L. What Is the Impact of a Previous Femoral Osteotomy on THA? A Systematic Review. Clin. Orthop. Relat. Res. 2019, 477, 1176–1187. [Google Scholar] [CrossRef]
- Madariaga, S.; Vargas-Reverón, C.; Tornero, E.; Alías, A.; Capurro, B.; Combalia, A.; Fernández-Valencia, J.Á.; Muñoz-Mahamud, E. Outcomes of Hip Arthroplasty with Concomitant Hardware Removal: Influence of the Type of Implant Retrieved and Impact of Positive Intraoperative Cultures. Arch. Orthop. Trauma Surg. 2021, 141, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Schwarzkopf, R.; Baghoolizadeh, M. Conversion Total Hip Arthroplasty: Primary or Revision Total Hip Arthroplasty. World J. Orthop. 2015, 6, 750–753. [Google Scholar] [CrossRef] [PubMed]
- Kovar, F.M.; Strasser, E.; Jaindl, M.; Endler, G.; Oberleitner, G. Complications Following Implant Removal in Patients with Proximal Femur Fractures—An Observational Study over 16years. Orthop. Traumatol. Surg. Res. 2015, 101, 785–789. [Google Scholar] [CrossRef]
- Scholten, R.; Füssenich, W.; Somford, M.P.; van Susante, J.L.C. High Incidence of Early Periprosthetic Joint Infection Following Total Hip Arthroplasty with Concomitant or Previous Hardware Removal. Arch. Orthop. Trauma Surg. 2019, 139, 1051–1056. [Google Scholar] [CrossRef] [PubMed]
- Klatte, T.O.; Meinicke, R.; O’Loughlin, P.; Rueger, J.M.; Gehrke, T.; Kendoff, D. Incidence of Bacterial Contamination in Primary Tha and Combined Hardware Removal: Analysis of Preoperative Aspiration and Intraoperative Biopsies. J. Arthroplast. 2013, 28, 1677–1680. [Google Scholar] [CrossRef] [PubMed]
- Moussa, F.W.; Anglen, J.O.; Gehrke, J.C.; Christensen, G.; Simpson, W.A. The Significance of Positive Cultures from Orthopedic Fixation Devices in the Absence of Clinical Infection. Am. J. Orthop. 1997, 20, 617–620. [Google Scholar]
- Cichos, K.H.; Christie, M.C.; Heatherly, A.R.; McGwin, G.; Quade, J.H.; Ghanem, E.S. The Value of Serological Screening Prior to Conversion Total Hip Arthroplasty. J. Arthroplast. 2020, 35, S319–S324. [Google Scholar] [CrossRef]
- Aalirezaie, A.; Anoushiravani, A.; Cashman, J.; Choon, D.; Danoff, J.; Dietz, M.; Gold, P.; Schwarzkopf, R.; Sheehan, E.; Vigante, D. General Assembly, Prevention, Host Risk Mitigation—Local Factors: Proceedings of International Consensus on Orthopedic Infections. J. Arthroplast. 2019, 34, S37–S41. [Google Scholar] [CrossRef]
- Cizmic, Z.; Feng, J.E.; Huang, R.; Iorio, R.; Komnos, G.; Kunutsor, S.K.; Metwaly, R.G.; Saleh, U.H.; Sheth, N.; Sloan, M. Hip and Knee Section, Prevention, Host Related: Proceedings of International Consensus on Orthopedic Infections. J. Arthroplast. 2019, 34, S255–S270. [Google Scholar] [CrossRef]
- Bondarenko, S.; Chang, C.B.; Cordero-Ampuero, J.; Kates, S.; Kheir, M.; Klement, M.R.; McPherson, E.; Morata, L.; Silibovsky, R.; Skaliczki, G.; et al. General Assembly, Prevention, Antimicrobials (Systemic): Proceedings of International Consensus on Orthopedic Infections. J. Arthroplast. 2019, 34, S61–S73. [Google Scholar] [CrossRef] [PubMed]
- Harris, W.H. Traumatic Arthritis of the Hip after Dislocation and Acetabular Fractures: Treatment by Mold Arthroplasty. An End-Result Study Using a New Method of Result Evaluation. JBJS 1969, 51, 737–755. [Google Scholar] [CrossRef]
- Naal, F.D.; Impellizzeri, F.M.; Leunig, M. Which Is the Best Activity Rating Scale for Patients Undergoing Total Joint Arthroplasty? Clin. Orthop. Relat. Res. 2009, 467, 958–965. [Google Scholar] [CrossRef] [PubMed]
- Loppini, M.; Longo, U.G.; Caldarella, E.; Rocca, A.D.; Denaro, V.; Grappiolo, G. Femur First Surgical Technique: A Smart Non-Computer-Based Procedure to Achieve the Combined Anteversion in Primary Total Hip Arthroplasty. BMC Musculoskelet. Disord. 2017, 18, 331. [Google Scholar] [CrossRef] [PubMed]
- La Camera, F.; Loppini, M.; della Rocca, A.; de Matteo, V.; Grappiolo, G. Total Hip Arthroplasty With a Monoblock Conical Stem in Dysplastic Hips: A 20-Year Follow-Up Study. J. Arthroplast. 2020, 35, 3242–3248. [Google Scholar] [CrossRef] [PubMed]
- Gruen, T.A.; McNeice, G.M.; Amstutz, H.C. “Modes of Failure” of Cemented Stem-Type Femoral Components. A Radiographic Analysis of Loosening. Clin. Orthop. Relat. Res. 1979, 141, 17–27. [Google Scholar] [CrossRef]
- Sierra, R.J.; Timperley, J.A.; Gie, G.A. Contemporary Cementing Technique and Mortality During and After Exeter Total Hip Arthroplasty. J. Arthroplast. 2009, 24, 325–332. [Google Scholar] [CrossRef]
- Ryan, S.P.; DiLallo, M.; Attarian, D.E.; Jiranek, W.A.; Seyler, T.M. Conversion vs Primary Total Hip Arthroplasty: Increased Cost of Care and Perioperative Complications. J. Arthroplast. 2018, 33, 2405–2411. [Google Scholar] [CrossRef]
- Schwarzkopf, R.; Chin, G.; Kim, K.; Murphy, D.; Chen, A.F. Do Conversion Total Hip Arthroplasty Yield Comparable Results to Primary Total Hip Arthroplasty? J. Arthroplast. 2017, 32, 862–871. [Google Scholar] [CrossRef]
- Khurana, S.; Nobel, T.B.; Merkow, J.S.; Walsh, M.; Egol, K.A. Total Hip Arthroplasty for Posttraumatic Osteoarthritis of the Hip Fares Worse Than THA for Primary Osteoarthritis. Am. J. Orthop. 2015, 44, 321–325. [Google Scholar]
- Mortazavi, S.M.J.; Greenky, M.R.; Bican, O.; Kane, P.; Parvizi, J.; Hozack, W.J. Total Hip Arthroplasty After Prior Surgical Treatment of Hip Fracture. Is It Always Challenging? J. Arthroplast. 2012, 27, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Loppini, M.; Pisano, A.; Di Maio, M.; La Camera, F.; Casana, M.; Grappiolo, G. Outcomes of patients with unexpected diagnosis of infection at total hip or total knee arthroplasty revisions. Int. Orthop. 2021, 45, 2791–2796. [Google Scholar] [CrossRef] [PubMed]
- Parvizi, J.; Tan, T.L.; Goswami, K.; Higuera, C.; Della Valle, C.; Chen, A.F.; Shohat, N. The 2018 Definition of Periprosthetic Hip and Knee Infection: An Evidence-Based and Validated Criteria. J. Arthroplasty. 2018, 33, 1309–1314.e2. [Google Scholar] [CrossRef] [PubMed]


| Variables | Hardware Removal Group | Control Group | p-Value |
|---|---|---|---|
| Gender, n (%) | |||
| Male | 47 (38.2%) | 47 (38.2%) | 0.923 |
| Female | 76 (61.8%) | 76 (61.8%) | |
| Age at surgery | 61.9 ± 2.9 (16.6–94.4) | 63.9 ± 2.9 (34.4–87.4) | 0.24 |
| BMI | 27.9 ± 1.1 (15.3–43.3) | 27.1 ± 1.1 (17.1–42.3) | 0.64 |
| FU | 4.5 ± 0.3 years (2–11.4) | 4.5 ± 0.5 years (2–11.4) | 0.89 |
| Surgery duration | 108.9 ± 20.2 min (53–226) | 58.3 ± 16.6 min (34–90) | <0.001 |
| Preoperative Hb level | 13.5 ± 0.31 mmol/L (1.7–5.9) | 13.9 ± 0.25 mmol/L (1.9–6.1) | 0.064 |
| Preoperative CRP | 0.381 ± 0.11 mg/dL (0.01–0.97) | 0.471 ± 0.17 mg/dL (0.02–0.98) | 0.38 |
| LoS | 5.26 ± 1.59 (3–12) | 5.13 ± 1.6 (3–14) | 0.36 |
| Final HHS | 90.2 ± 15.6 (38–100) | 91.4 ± 13.05 (40–100) | 0.51 |
| Final UCLA Activity Score | 5.72 ± 1.83 (1–9) | 5.9 ± 1.79 (2–9) | 0.43 |
| Post-Traumatic Group | Post-Preventive Group | |
|---|---|---|
| Number of hips | 87 | 40 |
| Index surgery | ||
| Subtrochanteric fracture | 34 (39%) | - |
| Pertrochanteric fracture | 45 (51%) | - |
| Shaft fracture | 7 (8%) | - |
| Acetabular fracture | 1 (1%) | - |
| Hip dysplasia | - | 23 (57%) |
| Epiphysiolysis | - | 14 (35%) |
| Osteonecrosis | - | 1 (2%) |
| Arthrodesis | - | 2 (5%) |
| Hardware removed | ||
| Intramedullary nail | 31 (24%) | - |
| Küntscher nail | 1 (1%) | - |
| Dynamic hip screw | 14 (11%) | - |
| Screws | 35 (27%) | 13 (10%) |
| plate and screws | 6 (5%) | 9 (7%) |
| Angled blade plate | - | 12 (10%) |
| Tantalum rod | - | 1 (1%) |
| K wires | - | 1 (1%) |
| Camber | - | 4 (3%) |
| Type of femoral stem used for conversion THA | ||
| Cementless | ||
| Conventional tapered | 31 (24%) | 7 (6%) |
| Short tapered | 16 (13%) | 8 (6%) |
| Conical | 4 (3%) | 21 (17%) |
| Long revision | 31 (24%) | 4 (3%) |
| Resurfacing | 1 (1%) | - |
| Cemented | ||
| Conventional tapered | 4 (3%) | - |
| Surface-bearing | ||
| Cer on poly | 70 (55%) | 28 (22%) |
| Met on poly | 2 (1%) | - |
| Met on met | 8 (6%) | - |
| Cer on cer | 7 (5%) | 12 (9%) |
| Event | Hardware Removal Group (N: 123) | Control Group (N: 123) | p-Value |
|---|---|---|---|
| Intraoperative calcar fracture | 3 | 1 | 0.622 |
| Postoperative periprosthetic fracture | 1 | 0 | 1.000 |
| Early postoperative dislocation | 5 | 1 | 0.213 |
| Intraoperative trochanteric fracture | 1 | 0 | 1.000 |
| Leg length discrepancy | 1 | 1 | 1.000 |
| Early postoperative stem subsidence | 1 | 0 | 1.000 |
| Reoperation/revision | 5 | 0 | 0.060 |
| Overall complications | 17 | 3 | 0.002 |
| Blood transfusion | 22 | 3 | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
La Camera, F.; de Matteo, V.; Di Maio, M.; Verrazzo, R.; Grappiolo, G.; Loppini, M. Clinical Outcomes and Complication Rate after Single-Stage Hardware Removal and Total Hip Arthroplasty: A Matched-Pair Controlled Study. J. Clin. Med. 2023, 12, 1666. https://doi.org/10.3390/jcm12041666
La Camera F, de Matteo V, Di Maio M, Verrazzo R, Grappiolo G, Loppini M. Clinical Outcomes and Complication Rate after Single-Stage Hardware Removal and Total Hip Arthroplasty: A Matched-Pair Controlled Study. Journal of Clinical Medicine. 2023; 12(4):1666. https://doi.org/10.3390/jcm12041666
Chicago/Turabian StyleLa Camera, Francesco, Vincenzo de Matteo, Marco Di Maio, Raffaele Verrazzo, Guido Grappiolo, and Mattia Loppini. 2023. "Clinical Outcomes and Complication Rate after Single-Stage Hardware Removal and Total Hip Arthroplasty: A Matched-Pair Controlled Study" Journal of Clinical Medicine 12, no. 4: 1666. https://doi.org/10.3390/jcm12041666
APA StyleLa Camera, F., de Matteo, V., Di Maio, M., Verrazzo, R., Grappiolo, G., & Loppini, M. (2023). Clinical Outcomes and Complication Rate after Single-Stage Hardware Removal and Total Hip Arthroplasty: A Matched-Pair Controlled Study. Journal of Clinical Medicine, 12(4), 1666. https://doi.org/10.3390/jcm12041666






