1. Introduction
In a modern era of minimally invasive procedures, non-intubated video-assisted thoracoscopic surgery (VATS) has been introduced in an effort to reduce the adverse effects of general anesthesia and tracheal intubation in conventional VATS. Non-intubated VATS has been demonstrated in several thoracic surgeries, from minor procedures such as pleural, lung, or mediastinal biopsies, resections of peripheral nodules, and thymectomies to major pulmonary resections [
1]. The outcomes of these operations have encouraged further use of non-intubated anesthetic techniques in selected patients [
2,
3,
4].
Intravenous anesthesia combined with a regional block—such as the intercostal nerve or paravertebral block—is a commonly used anesthetic protocol for non-intubated VATS [
5]. Respiratory management in patients who undergo non-intubated one-lung ventilation (OLV) with sedation is challenging for anesthesiologists because ventilation cannot be mechanically controlled in these patients. Although oxygenation is typically well maintained, maintaining an appropriate partial pressure of arterial carbon dioxide (PaCO
2) during non-intubated VATS is a demanding task. A “permissive hypercapnia” strategy (PaCO
2 up to 70 mmHg), regarded as generally tolerable, is typically implemented [
5]. This means that immediate adjustment of the infusion rates of anesthetic drugs in conjunction with manual ventilation is required when PaCO
2 rises to >60 mmHg to maintain it within the permissive range [
6]. If PaCO
2 exceeds 80 mmHg, despite efforts to reverse hypercapnia, conversion to general anesthesia with endotracheal intubation should be considered [
5]. Detecting an increase in PaCO
2 to >60 mmHg is thus crucial to preventing excessive hypercapnia.
While the gold standard for determining hypercapnia during general anesthesia is the measurement of PaCO
2 by arterial blood gas analysis (ABGA), this method is invasive and intermittent. End-tidal CO
2 partial pressure (PetCO
2) monitoring is preferred for the noninvasive and continuous monitoring of PaCO
2. When patients breathe spontaneously during non-intubated VATS, a nasal PetCO
2 monitoring device connected to the nasal cannula is commonly used. However, it has an inherently limited ability to accurately measure PaCO
2 compared to devices connected to the endotracheal tube due to increased dead space, which could be exacerbated during OLV [
7]. Due to this limitation of nasal PetCO
2 monitoring, transcutaneous CO
2 partial pressure (PtcCO
2), which continuously measures PaCO
2 through arterialized capillary blood in tissues, has been suggested as a reliable tool to detect hypercapnia during invasive procedures requiring moderate-to-deep sedation that can induce hypoventilation [
8,
9,
10]. In addition, the previous studies showed that PtcCO
2 could be superior to PetCO
2 monitoring during OLV, which induces a ventilation-perfusion mismatch that lowers the accuracy of PetCO
2 monitoring [
1,
11].
To date, PtcCO
2 monitoring during non-intubated VATS has not been sufficiently validated. Notably, the intraoperative hypotension, especially by a sudden deepening of anesthesia after intercostal and vagal block, lateral decubitus positioning, and mediastinal shift (caused by iatrogenic pneumothorax and the CO
2 gas inflation to facilitate lung collapse and improve the visual field) are conditions frequently confronted in non-intubated VATS and can influence the accuracy of PtcCO
2 monitoring [
12]. In addition, vasoconstrictors administered to treat intraoperative hypotension that may lead to peripheral hypoperfusion, and consistently higher PaCO
2 levels during non-intubated VATS, could reduce the accuracy of PtcCO
2 monitoring, altogether necessitating its validation in non-intubated VATS before routine application [
11,
13]. Therefore, we aimed to compare the accuracy of two noninvasive (PtcCO
2 and PetCO
2) monitoring methods and evaluate the predictive power and sensitivity when using PaCO
2 > 60 mmHg as the threshold at which respiratory intervention should be initiated to prevent the development of excessive hypercapnia.
2. Materials and Methods
2.1. Study Design and Participants
We retrospectively reviewed electronic medical records of patients who underwent non-intubated VATS to treat lung cancer between December 2019 and May 2021 at Ewha Womans University Seoul Hospital (Seoul, Republic of Korea). While we did not have specific exclusion criteria for this retrospective analysis, our institutional acceptance criteria for non-intubated VATS excluded patients with the following conditions at the planning stage of their surgery: (i) body mass index (BMI) > 30 kg/m2; (ii) requirement for vasopressors to maintain a mean arterial blood pressure greater than 65 mmHg; (iii) anticipated difficult airway management, neuromuscular disease, phrenic nerve palsy, persistent cough, or persistent sputum; (iv) anticipated severe adhesion of the operated lung, (v) a history of thoracic surgery; and (vi) any contraindication for permissive hypercapnia, such as increased intracranial pressure and right ventricular failure.
2.2. Data Acquisition
All data were extracted from electronic medical records, including anesthesia records. Data on the following demographic characteristics were extracted: age, sex, height, body weight, American Society of Anesthesiologists (ASA) physical status, and results of pulmonary function test. For anesthetic and procedure associated data, the following were extracted: the duration of anesthesia, administration of anesthetics and vasoconstrictor agents, and type of thoracic procedure. For CO2 data, we collected datasets of intraoperative PetCO2, PtcCO2, and PaCO2 that were simultaneously measured. Our institutional protocol was set to measure PaCO2 at time points as follows: during two-lung ventilation (TLV) preoperatively, 15 min after OLV by creating iatrogenic pneumothorax, after lobectomy, and upon PetCO2 > 55 mmHg or PtcCO2 > 60 mmHg. For cases of conversion to general anesthesia with tracheal intubation performed during surgery, the CO2 datasets acquired prior to the conversion were included in the analysis.
2.3. The Protocol of Anesthesia for Non-Intubated VATS
The below protocol was followed for anesthesia for non-intubated VATS performed at our institution.
2.3.1. Induction and Maintenance of Anesthesia for Non-Intubated VATS
Standard ASA monitoring was applied during the surgery. On arrival at the operating theater, patients were administered 5 mg of dexamethasone and 0.2 mg of glycopyrrolate. Spontaneous breathing was maintained throughout the non-intubated VATS procedure. Continuous dexmedetomidine infusion was administered to all patients at a rate of 0.5–0.7 μg/kg/h following 10 min of a loading dose of 1 μg/kg. Propofol administration was initiated with effective site concentrations of 3.0 μg/mL and titrated to 2.0–4.0 μg/mL. In initially awake patients, the dose was titrated to achieve a modified Ramsay sedation (MRS) score between 4 (appears asleep; purposeful responses to verbal commands louder than a usual conversation or to light glabellar tap) and 5 (asleep; sluggish purposeful responses only to loud verbal commands or strong glabellar tap). After an appropriate sedation level was achieved based on MRS score, the bispectral index was monitored using electroencephalographic analysis (target at levels between 40 and 60) to ensure an adequate sedation level during the surgery. Remifentanil was simultaneously initiated at 0.5 ng/mL and titrated to within a range of 0.5–3.0 ng/mL to maintain a respiratory rate of ≥10 breaths/min.
2.3.2. CO2 Measurements during Non-Intubated VATS
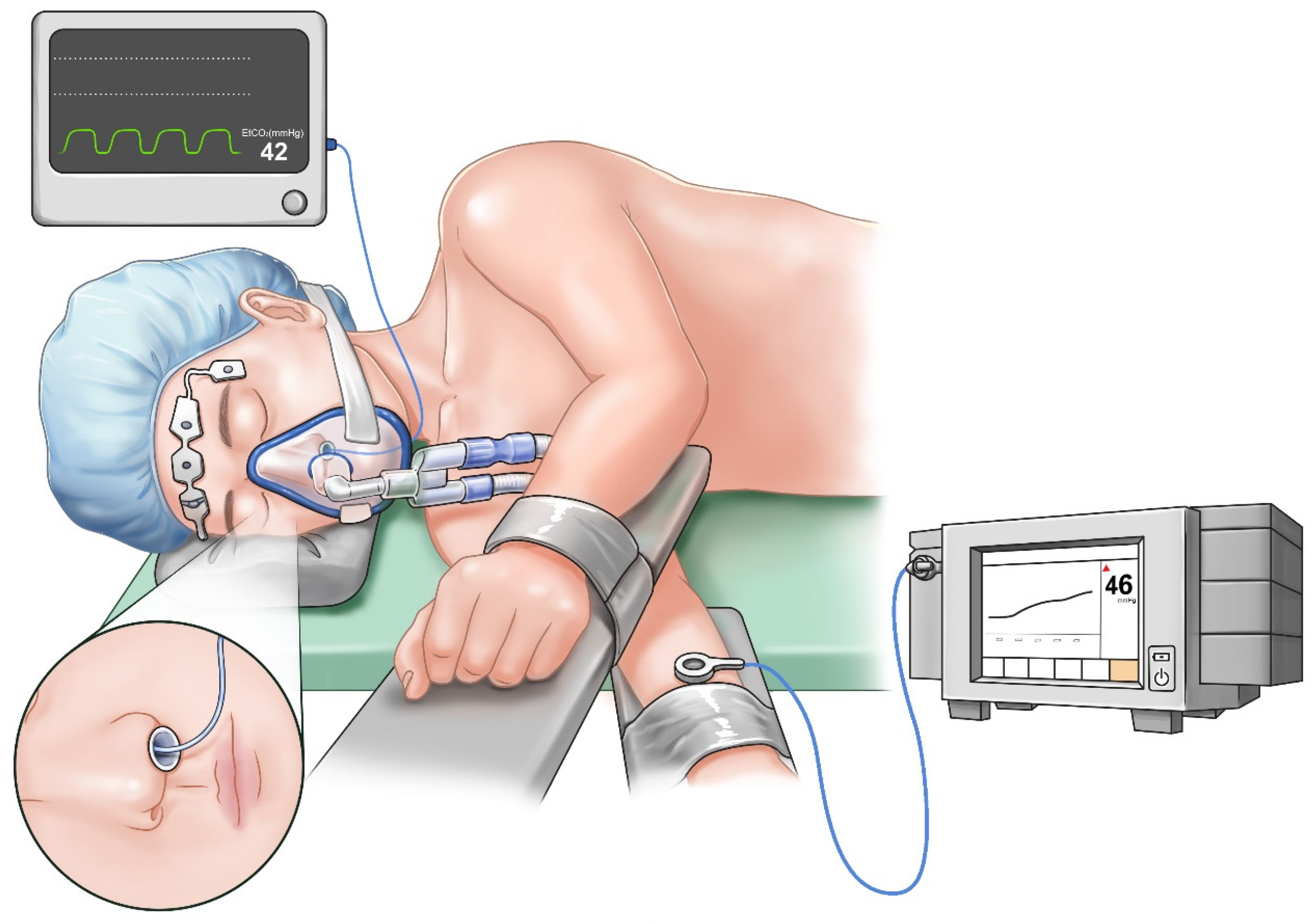
Once a satisfactory sedation level was achieved, a nasopharyngeal airway was inserted. PetCO
2 monitoring was performed using an infrared CO
2 analyzer (Avance CS
2, GE Healthcare, Madison, WI, USA) by inserting a sample line into the nasopharyngeal airway to minimize the potential under-detection of exhaled gas due to airway obstruction (
Figure 1). The radial artery on the non-operated side was cannulated to monitor continuous arterial blood pressure and sample arterial blood for gas analysis. PtcCO
2 was measured using a TCM4
TM device (Radiometer, Copenhagen, Denmark). The transcutaneous monitoring technique was standardized by applying a probe on the forearm ipsilateral to the non-operated lung in the lateral decubitus position (
Figure 1). Before placement, the device was calibrated ex vivo as per the manufacturer’s recommendations. Then, the skin surface where the electrode was placed was swabbed with alcohol to facilitate disc adhesion. Subsequently, the probe was mounted on the electrode with the working temperature set to 42 °C to arterialize the capillary blood flow in the skin. The subsequent in vivo calibration was based on the results of the first ABGA performed after a 10-min equilibration period from the time of the placement of probe on the patient for stabilization of the measurement [
14,
15].
2.3.3. Respiratory Management during Non-Intubated VATS
Six liters per minute of oxygen were supplied via a facial mask to maintain percutaneous oxygen saturation (SpO2) at ≥90%. The facial mask was secured to the patients with an elastic strap, but a full fit was not ensured to allow the collapse of the non-dependent lung. When desaturation (SpO2 < 90%) occurred, the patient was first assessed for airway obstructions, which were relieved by chin lifting and head tilting maneuvers. If desaturation persisted, the sedation level was titrated by adjusting the doses of the sedatives, and manual Ambu-bagging was applied as needed. A decision for conversion to general anesthesia was made in the following situations: an uncontrolled vigorous diaphragmatic movement that hampered the surgical procedure, persistent hypoxemia (SpO2 < 90%) and/or excessive hypercapnia (PaCO2 > 80 mmHg) despite the respiratory management described above, persistent cough, unstable hemodynamics, and conversion to open thoracotomy. Atropine was administered when the heart rate was < 50 beats/min. In addition, an ephedrine bolus or norepinephrine infusion was administered when the systolic blood pressure was <90 mmHg.
2.4. Techniques for Non-Intubated VATS
In all surgeries, uniportal thoracoscopic lobectomy and mediastinal node dissection were performed by a team of surgeons who used the same standardized technique for non-intubated VATS. The patients were placed in the lateral decubitus position. First, a 1:1 mixture of 0.75% ropivacaine and 2% lidocaine was infiltrated into the skin and subcutaneous tissue, followed by a 4 cm incision made along the anterior axillary line of the fourth or fifth intercostal space. Subsequently, iatrogenic pneumothorax was generated by creating an incision through the chest wall and pleura, which caused the ipsilateral lung to collapse gradually (note that surgeons at our institution do not use the CO2 gas inflation technique to facilitate lung collapse). The surgeon then performed an intercostal nerve block from the third to the sixth intercostal space. Additionally, a vagal block on the corresponding side was made with 2 mL of a 1:1 mixture of 0.75% ropivacaine and 2% lidocaine for each nerve under direct visualization through a thoracoscope. On completion of the surgical procedure, the intercostal nerve block was repeated before the closure of the pleura for postoperative analgesia.
2.5. Statistical Analyses
Statistical analyses were performed using SPSS version 26.0 (IBM Corp., Armonk, NY, USA). The primary outcomes were the sensitivity and predictive power of two noninvasive CO
2 monitoring methods (PetCO
2 and PtcCO
2) for hypercapnia (PaCO
2 > 60 mmHg), which was the threshold level at which respiratory intervention was considered to correct hypercapnia. Values were dichotomized into PtcCO
2 > 60 mmHg and PetCO
2 > 55 mmHg. The criterion of PetCO
2 > 55 mmHg was established in consideration of physiological alveolar dead space and the previous study [
15]. They were then compared using the Yates corrected chi-squared method. Predictive power for PaCO
2 > 60 mmHg was compared by constructing a receiver operating characteristic (ROC) curve and calculating the area under the curve (AUC). The secondary outcomes were the agreement between each measure (PetCO
2 and PtcCO
2) and PaCO
2. The bias, i.e., the mean difference, between PaCO
2 and noninvasive monitoring (PtcCO
2 and PetCO
2), precision (standard deviation [SD] of bias), and limit of agreement (LOA; bias ± 1.96SD) were calculated according to the Bland–Altman method. In addition, the relationship between the two noninvasive monitoring and PaCO
2 were evaluated using linear regression analysis with the Pearson correlation coefficient (
r).
In accordance with our retrospective study design, a post-power analysis, i.e., a two-ROC-curve power calculation, was conducted (with the pROC package of R, version 4.2.2) to verify whether our sample size was adequate to compare the predictive power of the two noninvasive monitoring methods. Measurements of PtcCO2 and PetCO2 and outcome values of 1 when hypercapnia (PaCO2 > 60 mmHg) occurred were entered into this calculation. The analysis yielded a power of 0.97, indicating that our sample size was adequate to evaluate the detection power of the two CO2 monitoring systems. Statistical significance was set to p < 0.05.
4. Discussion
This study demonstrates that PtcCO2 monitoring allows anesthesiologists to detect increases in PaCO2 more accurately than PetCO2 monitoring alone, which can prevent excessive hypercapnia and respiratory acidosis. In addition, the study’s results show that PtcCO2 is more in agreement with PaCO2 compared to PetCO2, as indicated by a lower bias and higher precision.
The major concern related to anesthetic techniques for successful non-intubated VATS is to avoid excessive hypercapnia and hypoxemia through effective respiratory management. A previous study reported that an oxygen mask was sufficient to prevent hypoxemia in most non-intubated patients without severe pulmonary comorbidities [
17]. In addition, the SpO
2 levels of patients were generally satisfactory during surgery, and the lowest intraoperative SpO
2 level was comparable to that of intubated patients [
17]. Consistent with this, no patients in the current study experienced persistent hypoxemia during OLV, and the mean PaO
2 was measured to be 198.3 mmHg. Hypercapnia is a central element of non-intubated thoracic surgery related to hypoventilation due to sedation and OLV [
18]. There is a risk of hypercapnic rebreathing effect due to paradoxical respiration that initially occurs and hypoventilation caused by the collapse of the operated lung [
5]. Furthermore, while a PaCO
2 < 70 mmHg is considered “permissive” due to the observed protective effect of improved hemodynamics, ventilation–perfusion match, and reduced inflammatory response, excessive hypercapnia can elevate pulmonary and intracranial pressure and cause cardiac rhythm disturbances [
5,
19]. Considering that VATS is performed more frequently in older patients susceptible to excessive hypercapnia, the accuracy of CO
2 monitoring is crucial to maintaining PaCO
2 within permissive range during non-intubated VATS.
The results of the present study suggest that PtcCO
2 monitoring is superior to nasal PetCO
2 monitoring during non-intubated VATS for several reasons. First, as hypo-ventilation occurs during sedation, the partial pressure of CO
2 in the air exhaled from the lung may represent less than the actual PaCO
2 concentration, because the sample taken for PetCO
2 analysis can be diluted with dead space air or supplementary oxygen [
20]. Second, spontaneous breathing during the non-intubated VATS might have favorable effects on the functional residual capacity and perfusion of the dependent lung. Nonetheless, the collapsed operated lung and the lateral decubitus position still contribute to increasing the PetCO
2 to PaCO
2 gradient [
5]. Third, the patient’s abnormal preoperative pulmonary function exacerbates the ventilation–perfusion mismatch [
11].
The accuracy of PtcCO
2 monitoring depends on the patient and technical factors. Two attending anesthesiologists who are proficient at performing PtcCO
2 monitoring managed all patients. Patient factors such as hypercapnia, low cardiac output, and impaired peripheral perfusion, or the administration of vasoconstrictor agents, may cause artificially low PtcCO
2 levels [
11]. In the current study, 34 of our 43 patients (79.1%) were administered norepinephrine to treat hypotension events. Although the accuracy of PtcCO
2 monitoring in patients administered vasoconstrictors has been controversial [
15,
21,
22], in our sample, PtcCO
2 monitoring led to the detection of 49 out of 52 hypercapnia events (PaCO
2 > 60 mmHg), whereas PetCO
2 monitoring allowed for the detection of only 10 such events. The expeditious PtcCO
2-based respiratory management (performed in the 49 of 52 hypercapnia cases) may have contributed to the prevention of the exacerbation of hypercapnia. The result was that persistent severe hypoxemia or hypercapnia with a PaCO
2 > 80 mmHg requiring conversion to general anesthesia did not occur. Among the 43 patients, 2 were converted to general anesthesia in the current study due to excessive diaphragmatic movement, which might cause unsafe surgery.
Kelly et al. [
13] reported that the disagreement between PaCO
2 and PtcCO
2 was exacerbated at higher PaCO
2 levels, suggesting that PtcCO
2 monitoring is a suboptimal tool. In contrast, the data analyzed in this study show that the mean difference between PaCO
2 and PtcCO
2 was as small as −1.6 ± 6.5 mmHg, even when PaCO
2 increased to approximately 60 mmHg. This discrepancy might be attributable to differences among patient samples. Kelly et al.’s study [
13] targeted patients in critical condition with progressive respiratory failure, such as acute pulmonary edema or chronic airway disease. The lung functions of our patients, in contrast, were fairly preserved to maintain spontaneous OLV. The agreement analysis and comparison of hypercapnia detection power strongly indicate that PtcCO
2 monitoring can help prevent excessive hypercapnia, as it allows for the more accurate detection of PaCO
2 levels exceeding the threshold of permissive hypercapnia.
While PtcCO
2 monitoring could offer great advantages, as it continuously surrogates PaCO
2 and saves efforts for serial ABGA, it still has a limitation of requiring in vivo calibration at first. In vivo calibration has been suggested as a prerequisite step for interpreting PtcCO
2 with a higher degree of confidence; however, it requires an invasive arterial blood sampling [
23]. When arterial cannulation is unnecessary for the surgery, an additional invasive procedure for in vivo calibration might reduce a benefit of a non-invasive CO
2 measurement technique.
This study had several limitations. First, the inherent limitations of retrospective chart reviews may include unmeasured confounding factors. Second, lung function was preserved in most of our patients, among whom 65.1% had normal pulmonary function test results, and the average predicted forced expiratory volume in 1 s was 94.1%. Hence, the findings of this study cannot not be generalized to patients with severely impaired lung function. Third, although intraoperative administration of dexmedetomidine extended the recovery time from sedation after surgery, missing data during that period prevented us from determining the efficacy of PtcCO2 monitoring because patients in the post-anesthesia care unit (PACU) of our hospital generally rely on SpO2 monitoring, not on PetCO2/PtcCO2. Thus, whether a good correlation between PtcCO2 and PaCO2 is retained during the period of CO2 elimination (corresponding to the PACU stay after surgery) should be clarified in future studies.