Abstract
Atopic dermatitis (AD) is a common highly pruritic chronic inflammatory skin disorder affecting 5–20% of children worldwide, while the prevalence in adults varies from 7 to 10%. Patients with AD experience intense pruritus that could lead to sleep disturbance and impaired quality of life. Here, we analyze the pathophysiology of itchiness in AD. We extensively review the histamine-dependent and histamine-independent pruritogens. Several receptors, substance P, secreted molecules, chemokines, and cytokines are involved as mediators in chronic itch. We also, summarize the new emerging antipruritic drugs in atopic dermatitis.
1. Introduction
Atopic dermatitis (AD) is a common, chronic, pruritic, inflammatory skin disease that occurs in nearly 20 percent of children worldwide and largely varies between ethnic groups and countries [1,2,3]. There are limited data regarding the prevalence of AD in adulthood. Population-based studies from northern Europe have reported a prevalence rate of AD in adulthood between 10 and 14 percent [4,5]. A cross-sectional study from the United States, which included almost 1300 adults, reported a prevalence of 7.3 percent (95% CI 5.9–8.8) [6]. The prevalence of AD among patients between 60 and 69 years old in Saarland, Germany, was 4% [7]. In Poland, the prevalence of AD was 2% for those over 60 years old, compared to 5% for children (ages 6–7), 4% for teenagers (ages 13–14), and 3% for adults (20–44 years) [8]. Similarly, 4% of adults (30–39 years) and 3% of elderly patients (60 years or older) who attended a hospital in Tokyo had AD [9], compared to 19% of young patients (7–9 years) in Otsu, Japan [10,11]. Risk factors for AD include various genetic and environmental factors [12,13,14,15,16,17,18]. AD is linked to cutaneous hyperreactivity to triggers in the environment that are safe for healthy, non-atopic people [19].
Increased levels of both total serum IgE and IgE that is specific to common allergens are frequently present in these patients. Based on this hyperreactivity, two separate subtypes of AD have recently been identified: an “intrinsic” form of AD that lacks detectable IgE-mediated sensitization and an “extrinsic” form linked to IgE-mediated sensitization to environmental allergens [20]. According to recent studies, the prevalence of extrinsic and intrinsic AD is, respectively, 70–80% and 20–30%. Environmental allergens including animal dander, grasses, and pollens, as well as certain foods like milk, eggs, and wheat, can exacerbate AD [11]. Children are more susceptible to food allergy-related symptoms than older patients, while adult and elderly AD patients with moderate-to-severe cases of the disease tend to be more sensitive to food allergens than patients with milder AD [11]. In a study with AD patients, six inhaled allergens were most frequently identified in patients with high allergen-specific IgE (44 or 73.3%), with the majority of allergens coming from timothy grass pollen (43.2%). The second allergen most frequently identified (25.0%) was birch pollen. Extremely high levels of total IgE, thymus and activation-regulated chemokine levels, and peripheral blood eosinophil counts were present in elderly patients with AD [21]. Cross-reactions may be the cause of the high incidence of these inhaled allergens, as both timothy grass and birch grass pollen have many elements in common with other allergens. Birch pollen frequently reacts adversely with allergens in fruits, nuts, and vegetables [22]. The levels of total IgE, but the tryptase levels, have been associated with the severity of the symptoms in atopic dermatitis patients [23]. More effective candidate biomarkers for determining the severity of AD may be serum LDH and IL-18 [24]. Older AD patients are less allergic to foods, animal dander, and fungi, and more sensitive to dust mites and pollens, as indicated by elevated serum IgE levels that are specific to these allergens [25]. The relationship between serum IgE and treatment response has been controversial. Some studies have shown that dynamic changes in serum IgE levels may reflect the way AD responds to treatment, while others have shown that there is only a weak relationship between serum IgE and continued disease severity after treatment, suggesting that serum IgE is not the most accurate biomarker of AD [25].
Chronic pruritus is a defined symptom of AD that varies from 87 to 100 percent, but in reality, all AD patients who have the active disease also experience pruritus [26]. In addition to pruritus, AD patients experience skin pain [27]. Uncontrolled pruritus in AD results in impaired quality of life, loss of work productivity, and sleep disturbance [28,29]. Although the pathophysiology of AD’s pruritus still needs to be elucidated, it is widely accepted that the signals’ transmission through unmyelinated, histamine-sensitive, and non-histamine-sensitive peripheral C-nerve fibers plays a crucial role [30,31,32]. Here, we summarize existing knowledge on how keratinocytes, the immune system, and the nervous system contribute to the pathophysiology of chronic itch in AD and discuss possible therapeutic strategies to target these circuits.
2. Pruritogenic Mediators
One of the distinguishing characteristics of atopic dermatitis (AD) is chronic pruritus. Between 87 and 100% of AD patients have chronic pruritus at any given time [33,34]. Studies have distinguished histamine-sensitive and histamine-insensitive pruriceptive sensory nerves in the cutaneous neuronal network (Figure 1, Table 1).
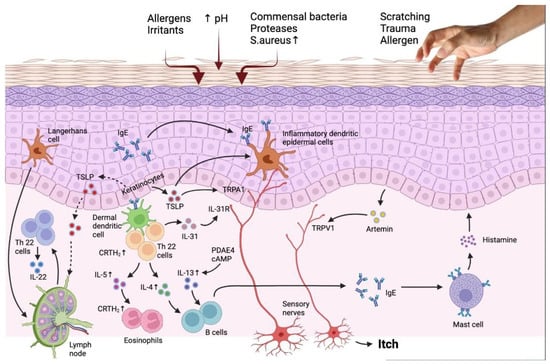
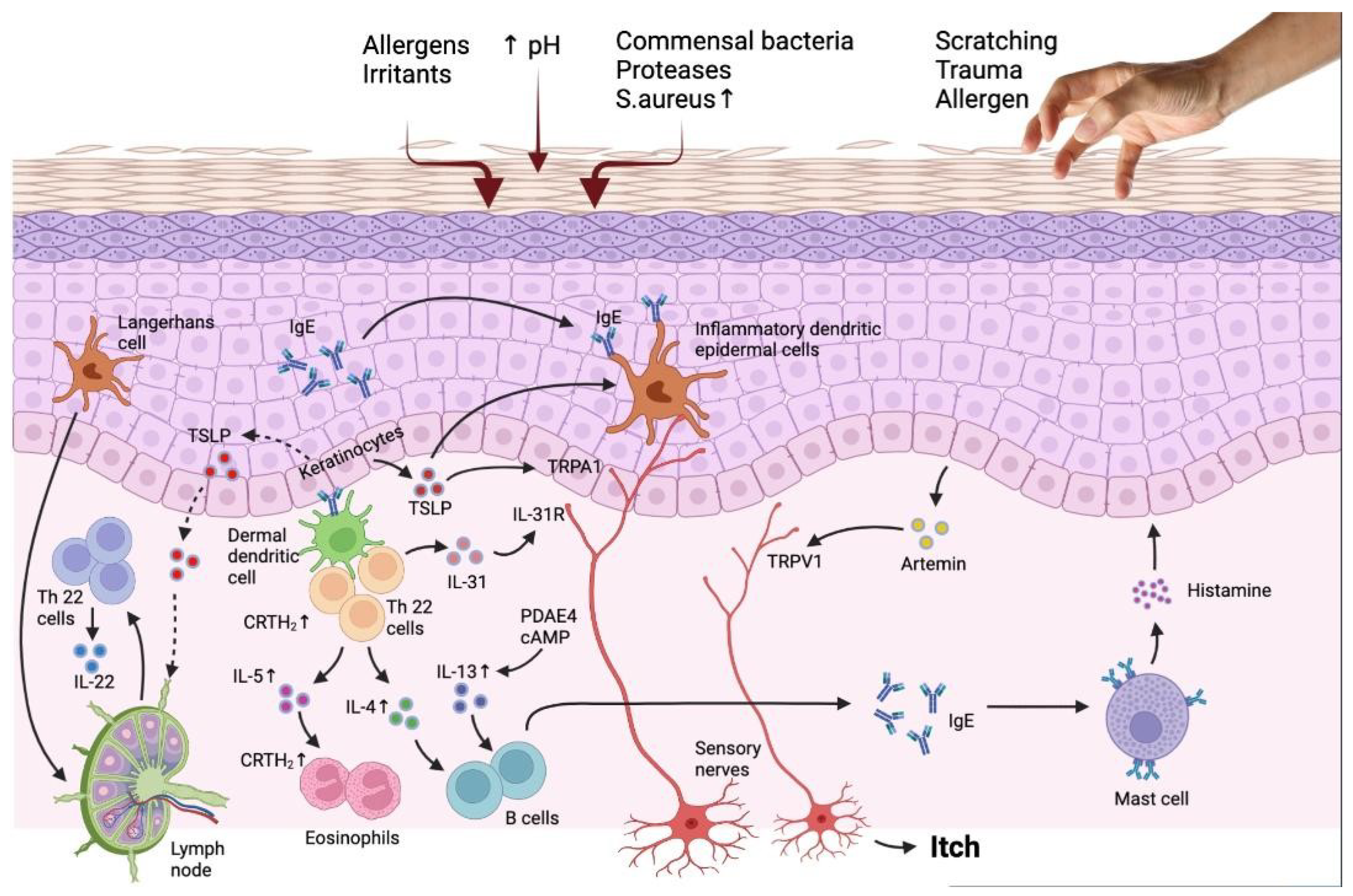
Figure 1.
The interaction of the immune system and epidermis in AD patients reveals the targets for novel treatment. Barrier flaws encourage the entry of epicutaneous antigens that interact with dermal dendritic cells and epidermal Langerhans cells, activating the immune system, especially the TH2 and TH22 signaling pathways. TH2 cell migration into the skin is facilitated by the prostaglandin D2 receptor CRTH2, which is found on a number of inflammatory cells. Peripheral eosinophils and mast cells are stimulated by TH2 cytokines, such as IL-4, IL-3, and IL-5, which also cause IgE class change. Increased PDE4 also boosts TH2 cytokine expression. IL-33, which stimulates epidermal hyperplasia and is abundant in individuals with chronic diseases, is produced by TH22 cells. The reduced production of barrier proteins and barrier impairment caused by TH2 and TH22 cytokines are also thought to enhance the risk of infection. Flares result in an increase in S aureus and a decrease in bacterial diversity. Increased IL-31, a TH2 cytokine, is linked to itching but not inflammation. It is assumed that TSLP synthesis by keratinocytes causes itch by directly activating transient receptor potential A1 (TRPA1) receptors in cutaneous sensory neurons in addition to inducing a TH2 response. AhR and H4R, are more recent targets that control IL-31 expression by being expressed on keratinocytes and TH2 cells, respectively. Adapted from: Paller et al. [35].

Table 1.
An overview of important pruritogenic mediators in atopic dermatitis (AD).
2.1. Histamine-Dependent Pruritogens
Mast cells are the main source of histamine production, but platelets, basophils, and neutrophils are all involved. Histamine receptors H1, H2, H3, and H4 act as a conduit for histamine’s actions.
In the cutaneous neuronal network, studies have differentiated the pruriceptive sensory nerves that are histamine-sensitive and histamine-insensitive [26,28]. Antihistamine medications have shown either slight or no effect on pruritus on AD. This finding suggests that, at least through the activation of H1 receptors, histamine has only a modest role in the pruritus associated with AD [26,28]. Nevertheless, histamines may still be involved in AD-related inflammation and pruritus. In an experimentally-induced pruritus study, the histamine H(4) receptor antagonists’ inhibitory effect was superior to those examined with histamine H(1) receptor antagonists [36]. However, clinical trials reported no noteworthy reduction in pruritus in patients with AD who received a selective H4 receptor antagonist [37,38].
Significantly, it has now been demonstrated that pruritogen receptors for substances generated from immune cells, such as leukotriene C4 (Cysltr2), histamine (Hrh1), IL-4 (Il4ra), IL-13 (Il13ra1), and IL-31 (Il31ra and OSMR), are expressed on the same neuronal subsets [39].
2.2. Platelet Activating Factor (PAF)
Phospholipase A2 acts on human mast cells to induce the production and secretion of platelet-activating factor (PAF). Platelets, neutrophils, monocytes, macrophages, and endothelial cells all continually produce platelet-activating factor (PAF), a phospholipid, in a very low quantity [40,41]. When there is inflammation, an allergic reaction, activation of thrombotic cascades, blood vessel dilation, platelet aggregation, or shock, its synthesis is elevated. The PAF and PAF-like phospholipids are destroyed by PAF-acetyl hydrolase (PAF-AH), which regulates their activity [42]. Preliminary evidence indicates that PAF may also be involved in human anaphylaxis, especially in the amplification of the initial response. As an example of a second-generation antihistamine agonist, rupatadine impacts the PAF and histamine pathways, which further prevents mast cell activation. The use of this dual braking is permitted in the management of chronic urticaria and allergic rhinitis [42,43,44,45]. When PAF is intradermally injected, then wheal and pruritus are evoked. This is due to histamine release by mast cells (MCs) through a neurogenic pathway as it can be blocked by the nerves [45,46].
In the study conducted by Gomułka et al. [40], circulating serum eosinophil-derived neurotoxin EDN, PAF, and vascular endothelial growth factor (VEGF) were not significantly correlated with the severity of pruritus in patients with AD. Instead, they reported that patients with AD had significantly higher serum levels of VEGF and EDN compared to the control group [40]. A study from Japan reported an improvement in total pruritus score in AD patients after administration of an H1 antihistamine, rupatadine, together with a PAF antagonist [47].
3. Histamine-Independent Pruritogens
3.1. Protease and (PARs) Protease-Activated Receptors
The homeostasis of the skin barrier depends on proteases. Abnormal protease activity contributes to the breakdown of the epidermal barrier, which then triggers the release of inflammatory cytokines by activating the associated receptors [48,49]. PARs operate as a mediator for the diverse activities of proteases. There are four PARs, or G protein-coupled receptors: PAR1, PAR2, PAR3, and PAR4. They can be activated by proteases produced both internally by immune cells and keratinocytes as well as externally by fungi, S. aureus, and dust mites, all of which are crucial AD triggers [38]. The functional role of PAR2 has been described in AD more clearly than those of the other three receptors. In the lesioned skin of AD patients, protease activity and PAR2 expression in keratinocytes and PAR+ peripheral nerves were noticeably elevated [50,51]. Specific serine and cysteine proteases such as tryptase, kallikreins (KLK), and cathepsin can activate PAR2. It has been established that KLK5 activates PAR2 to cause TSLP overexpression in keratinocytes via nuclear factor B [52]. Nevertheless, subsequent studies have demonstrated that KLK5 provoked changes in skin architecture similar to those seen in AD in a PAR2-independent way [52,53]. These studies also showed that independent of skin inflammation and PAR2, KLK7 stimulates an itch response. This shows that non-PAR2-mediated reactions have a role in the development of AD as well [54,55].
Two groups have recently shown the regulatory function of neuronal alteration by PAR2 in keratinocytes in a Grhl3PAR/+ mouse model overexpressing PAR2 in suprabasal keratinocytes. Spontaneous AD-like dermatitis and increased scratching behavior were found in these mice, and skin inflammation was exacerbated by house dust mite (HDM) protease, a major source of allergens in AD [56]. A different study, however, demonstrated that an HDM challenge is required for AD-like dermatitis and elevated scratching behavior [57]. The diverse genetic backgrounds, the age of the mice, and the living conditions could all contribute to the phenotypic discrepancies. After HDM exposure, Grhl3PAR/+ mice showed subclinical epidermal barrier damage and increased nerve fiber density. Importantly, in mice overexpressing epidermal PAR2, PAR2 expression in the DRG was increased even in the absence of HDM exposure [56]. Additionally, skin disruption in Grhl3PAR/+ mice treated with HDM increased the expression of genes linked to inflammation and pruritus in sensory neurons, including TSLPR, IL-31 receptor, and brain-derived neurotrophic factor (BDNF) [56,57]. There is evidence that there is PAR 2 neuroepidermal communication in keratinocytes. PAR2 can be found in primary sensory neurons, peripheral nerves, and keratinocytes. Different roles for PAR2 and DRG in keratinocytes may exist [58]. Primary sensory neurons also contain PAR2, in addition to keratinocytes and peripheral nerves. Some proteases have the ability to sensitize TRPV1 channels or directly activate PAR2+ sensory neurons, which may be a factor in non-histaminergic pruritus [59]. For example, it has been demonstrated that a cysteine protease, called cathepsin S, causes mice to scratch by activating PAR2 in TRPV1 sensory neurons [60]. In a mouse model of AD, pruritus and inflammation response were reduced by PZ-235, a PAR2 pepducin [61]. The use of topical E6005, a phosphodiesterase 4 inhibitor that is related to the PAR2 pathway, demonstrated an antipruritic effect [62].
3.2. Thymic Stromal Lymphopoietin (TSLP) and TSLP Receptor TSLPR
Thymic stromal lymphopoietin (TSLP) is an epithelial-cell-derived cytokine that is important in initiating allergic inflammation. There are two different isoforms of TSLP: the short- and long-form [63,64]. While the short variant is continuously expressed and is involved in homeostasis, the long form is induced during inflammation [65,66]. Allergens, bacteria, and viruses are suspected of acting as triggers to promote TSLP synthesis in inflamed tissue [67]. TSLP receptor is mainly expressed by the dendritic cells (DCs) and is a heterodimer composed of the IL-7Rα chain and the TSLP-specific receptor [68]. Raised levels of TSLP are expressed in keratinocytes of lesional skin and serum of patients with atopic dermatitis [69]. TSLP plays a major role in Th2 immune deviation via action on the DCs of the skin [70]. Recent studies have shown that TSLP can activate Langerhans cells and DCs to induce T follicular helper cells, which are linked to AD pathogenesis and severity [71,72]. It has been reported that in AD patients, CD4 + T cells express higher amounts of TSLPR [73]. Additionally, TSLP can damage the skin barrier by inhibiting the expression of filaggrin (FLG), a crucial protein for the growth and upkeep of the skin barrier via the STAT3 and ERK pathways [74]. Notably, TSLP produced by keratinocytes can promote “atopic march” (the progression of atopic disorder to the onset of allergy-induced rhinitis and asthma) and aggravate allergy-induced asthma [75].
The cytokine thymic stromal lymphopoietin (TSLP), which mediates signaling between epithelial cells and innate immune cells, is thought to be the primary cause of AD and the atopic march. It has been demonstrated that itch is promoted by direct communication between epithelial cells and cutaneous sensory neurons via TSLP [76].
One of the main mediators of serotonergic itch is the serotonin receptor HTR7, (5-Hydroxytryptamine Receptor 7). The ion channel TRPA1, transient receptor potential ankyrin 1, is encouraged to open by HTR7 activation, which in turn brings on itch-related behaviors. Moreover, HTR7 and TRPA1 are necessary for acute itch brought on by serotonin or a selective serotonin reuptake inhibitor [77].
Atopic dermatitis and other persistent itch problems in humans have long been connected to abnormal serotonin signaling [77].
Furthermore, it has been demonstrated that in atopic dermatitis, a subset of basophils stimulates sensory neurons to cause itch flare-ups that are triggered by allergens [78].
Tezepelumab is a human monoclonal immunoglobulin G2-lambda antibody (AMG 157) that binds TSLP and prevents its interaction with the TSLP receptor complex. Tezepelumab is approved by the FDA for add-on maintenance therapy in patients with severe asthma who are ≥12 years of age [79].
In a phase 2a research study (NCT02525094), 113 individuals with AD were randomly selected to receive 280 mg of subcutaneous tezepelumab or a placebo every two weeks along with class 3 topical corticosteroids (TCS). When compared to placebo with TCS, patients receiving tezepelumab showed marginally significant improvements in pruritus numeric rating scale (NRS) from baseline to week 12 (Table 2) [80].

Table 2.
Summary of new atopic dermatitis treatments’ effectiveness in reducing pruritus.
3.3. IL-33
IL-33 belongs to the IL-1 cytokine family, is produced by immune, endothelial, and keratinocytes cells, and is upregulated in patients with atopic dermatitis, activating the innate immune system [104,105]. IL-33 stimulates the expression of IL-4 and IL-13 which consequently enhance the expression of IL-31, a pruritogenic interleukin [105]. Furthermore, ILC2 and IL4 are cytokines that both induce pruritus and are stimulated via basophils through the action of IL33. On the other hand, itch mediators including IL-31, substance P, and brain natriuretic peptide (BNP) can also cause the release of cytokines and chemokines that are associated with pain, inflammation, and barrier dysfunction [106,107,108]. Serum levels of IL-33 were linked with the severity of AD and were considerably elevated in AD patients compared to urticaria and psoriasis patients and healthy controls [109]. Additionally, IL-33 levels were highly linked with excoriation and xerosis scores [109]. Another study found that in patients with AD, the level of lichenification and the intensity of pruritus were both linked with the expression of IL-33 in keratinocytes [110]. In addition, IL-33 induces and maintains the Th2 inflammatory response by enhancing the TSLP-DC-OX40L axis [111]. Pruritus in atopic dermatitis is caused in large part by skin-infiltrating neutrophils. CXCL10, a ligand of the CXCR3 receptor that enhances itch by activating sensory neurons, is only produced by neutrophils, and CXCR3 antagonism reduces chronic itch [112]. In a mouse model of allergic contact dermatitis caused by poison ivy, pruritus and skin inflammation were reduced by neutralized antibodies against ST2 or IL-33. Cutaneous inflammation and scratching were both diminished by ST2 expression knockdown in dorsal root ganglia (DRG) neurons [113]. In another study, chronic itch caused by IL-33/ST2 was mediated by astrocytic JAK2-STAT3 cascades, which stimulated TNF- to sensitize gastrin-releasing peptide GRPR signaling. In a 2,4-dinitrofluorobenzene (DNFB)-induced dry skin and allergic contact dermatitis mouse model, it was shown that scratching could be reduced in ST knockout mice [114]. In a phase 2a clinical trial with a human monoclonal antibody against IL-33, etokimab (ANB020), patients with AD showed significant improvements in 5D itch measures and EASI scores [115]. In a phase 2 randomized clinical trial with astegolimumab, a fully human IgG2 IL-33 inhibitor, primary and secondary outcomes did not significantly differ between astegolimab and placebo [116].
3.4. IL-4 and IL-13
Along with neuropeptides, the production of the alarmins TSLP, IL-33, and IL-25 eventually stimulates the innate and adaptive immune systems in addition to inducing and perceiving itch [14]. The primary Th2 immune response in AD is started and subsequently propagated by this activation. The production of a number of pro-inflammatory mediators occurs next, either directly by type 2 innate lymphoid cells (ILC2) or Th2 effector lymphocytes, or indirectly by stimulating mast cells, basophils, or eosinophils. A number of these mediators can either directly or indirectly increase AD patients’ itching [117,118]. The pathogenesis of AD and pruritus in AD patients are both significantly influenced by the cytokines IL-4 and IL-13. ILC2 and Th2 cells are the main sources of these cytokines’ production and release. They have a variety of impacts on epidermal and dermal cells, as well as on sensory nerve fibers by activating certain receptors that are members of the IL-4Ra chain [117,118]. The ability of IL-4 and IL-13 to sensitize sensory nerves to itch by reducing the sensitivity thresholds to other pruritogenic stimuli, such as histamine, IL-31, and TSLP, has been demonstrated in both in vitro and in vivo studies in mice [119]. Nevertheless, additional research has demonstrated that IL-4 and IL-13 can both directly activate pruritus in mice, and that applying mixtures of these mediators even increased the induction of itch [120]. Transient receptor potential (TRP) V1 and TRPA1, which are unspecific cation channels, are carried by involved sensory nerve fibers [121]. The activation of TRPV1 and/or TRPA1 causes calcium influx after these nerves have been activated by IL-4, IL-13, or IL-31 via their particular receptors, which ultimately causes the release of action potentials via the sodium channels NaV1.7, NaV1.8, or NaV1.9. In order for pruritus to be induced or sensory nerves to be sensitized by these pruritogens, TRPV1 and TRPA1 should be present [121,122]. Due to the downregulation of crucial skin barrier proteins including filaggrin, involucrin, and loricrin, IL-4 and IL-13 also have an impact on the skin barrier and impair its functionality [117,118,119]. According to a recent study, IL-4 increased IL-31/IL-31 receptor signaling, which is crucial for the pathophysiology and transmission of pruritus in AD [123,124]. A pruritic phenotype was induced in the skin of a mouse by transgenic overexpression of IL-13 [125]. Dupilumab, a fully humanized IL-4 receptor α blocker is licensed for the management of atopic dermatitis (AD), and it has been demonstrated to decrease pruritus and the severity of AD [102,126,127].
A completely human anti-IL-13 monoclonal antibody called tralokinumab has been licensed for use in Europe and the United States to treat individuals with moderate to severe atopic dermatitis who are not satisfactorily managed by topical prescription treatments [93]. Randomized trials have assessed the efficacy of tralokinumab in pruritus in AD alone or in combination with topical corticosteroids. In a phase 2b clinical trial (NCT02347176), 204 adults were randomly assigned 1:1:1:1 to receive subcutaneous tralokinumab at doses of 45, 150, or 300 mg every two weeks for 12 weeks, or a placebo, along with concurrent topical glucocorticoids. Upon taking 45 or 300 mg of tralokinumab, participants showed improvements in pruritus Numerical Rating Scale, NRS, (7-day mean) scores from their baseline to week 12 compared to those taking a placebo [93]. A total of 1596 adult patients with atopic dermatitis who were candidates for systemic therapy participated in two identical, randomized phase 3 clinical trials (ECZTRA 1 and ECZTRA 2) and were given 300 mg of subcutaneous tralokinumab as monotherapy or a placebo every other week for 16 weeks [94]. Tralokinumab showed an noticeable reduction of pruritus and an improvement of several scale of AD severity after 16 weeks of monotherapy treatment and a constant response at week 52 [94]. However, in another clinical trial, ECZTRA 3, tralokinumab was used together with topical corticosteroids (TCS) in order to meet the primary and secondary endpoints [95]. In another study, tralokinumab plus TCS resulted in a larger percentage of patients experiencing a decrease in their worst daily pruritus NRS (weekly average) of 4 from their baseline to week 16 compared to placebo plus TCS (45.5% vs. 35.6%). However, this was not statistically significant [96]. In a retrospective study of 12 patients with severe resistant AD who were treated with tralokinumab, using the itch Numerical Rating Scale, it was shown that the severity of the itching was significantly decreased and there was also a great reduction in EASI score [128]. Both dupilumab and tralokinumab may provoke conjunctivitis as a side effect [129].
Lebrikizumab is a new monoclonal antibody that targets IL-13 which inhibits the formation of the signaling complex of the IL-13Rα1/IL-4Rα receptor in adult patients with moderate to severe atopic dermatitis [100,101,130,131].
When combined with topical corticosteroids and given subcutaneously every 4 weeks to patients with AD, the monoclonal IL-13 antibody lebrikizumab outperformed the control group. At week 20, dose-dependent responses for the percentage change from the baseline pruritus VAS were noted [100]. In a phase 2b, placebo-controlled, double-blind trial, as early as day 2 in the higher-dosage group, the humanized high-affinity IL-13 antibody lebrikizumab significantly reduced the severity of AD and the intensity of pruritus [101]. In a preclinical study regarding the role of IL-13 and its blockage in pruritus, it was shown that IL-13 is a powerful amplifier of neural responses to various itch stimuli, and it is therefore likely that the neuroimmune axis plays a role in the development of inflammatory skin diseases linked to chronic itch [132].
3.5. IL-31
IL-31 belongs to the IL-6 cytokine family [118]. IL-4, a significant atopic dermatitis mediator, stimulates IL-31 production in human lymphocytes via activating the JAK-STAT signaling pathway. IL-33, which is secreted by keratinocytes in response to injury, works in concert with IL-4 to increase the production of IL-31 via the NF-kB pathway [133,134]. A heterodimeric receptor complex made up of the oncostatin M receptor beta (OSMRβ) and the IL-31 receptor alpha (Il-31RA) is responsible for transmitting the IL-31 signal [135]. When the IL-31 receptor is activated, either directly or indirectly, at least four signaling pathways—AKT/PI3K, JAK-STAT, MAPK (ERK1/2, p38, JNK), and NF-kB—are activated in a cell-dependent manner [135,136,137,138,139]. IL-31 activity in keratinocytes is mostly regulated by STAT3 phosphorylation, while various IL-31RA isoforms may have an impact on this activity [136]. IL-31RA has a changeable domain at the end terminal that can be either long or short. The strongest STAT3 activity is induced by the long isoforms 1 and 2 [133]. Similar to neuron growth factor, IL-31 stimulates neural development in mice by phosphorylating STAT3 [42]. The OSMR subunit of the Il-31 receptor may activate the remaining signaling pathways even if the IL-31RA subunit is what causes STAT3 activation [136,140]. Independent of p38, IL-31-induced pruritus is brought on by ERK phosphorylation in the primary afferent neurons of the dorsal root ganglia. Short and long versions of IL-31RA undergo identical ERK activation when activated by IL-31 [121]. Although pruritus provoked by IL-31 may occur independently of mast cells, IL-4 causes overexpression of IL-31 in lymphocytes and mast cells [121]. When IL-31 is injected or overexpressed pruritus is provoked and the neural growth of neurons with a small diameter is selectively promoted which activates several of the same genes as a nerve growth factor [140]. In mice, calcium influx-induced pruritus is mediated through transient receptor potential cation channel 1 and transient receptor potential vanilloid 1 (TRPV1). In TRPA1-deficient mice, pruritus induced by IL-31 is significantly attenuated [121]. Studies have shown that IL-31 is overexpression in transgenic mice causes primary alopecia, severe pruritus, and peripheral lymphadenopathy independent of mast cells [135]. Upregulation of IL-31 has been shown to cause an atopic dermatitis-like phenotype in mouse models [137].
Nemolizumab is a newly discovered humanized monoclonal antibody against receptor A of IL-31 [136]. Nemolizumab may be useful in reducing atopic dermatitis-related pruritus, according to several trials. Nemolizumab displayed a significant improvement in the EASI score and pruritus at a dose of 30 mg every four weeks in a phase 2b trial when combined with TCS [97]. The efficacy of nemolizumab in reducing pruritus related to AD was validated further in a Japanese randomized trial that investigated 215 patients aged 13 years or older with AD and moderate to severe pruritus [98]. In this phase 3 trial, the Japanese AD group showed a higher reduction in pruritus score at a dose of 60 mg of nemolizumab combined with TCS every four weeks [98]. In two long-term phase III studies, in the nemolizumab group, the pruritus Visual Analogue Scale scores decreased by 66% and the EASI score by 78% [99]. The strong antipruritic effect of nemolizumab is promising [141,142].
3.6. IL-6
Interleukin (IL)-6 is a proinflammatory cytokine, that exerts a great variety of activities during inflammatory illness, host infection, oncogenesis, and hematopoiesis. It was first identified as a B cell differentiation factor. Additionally, it has anti-inflammatory properties [143,144]. The IL-6 receptor (IL-6R) is composed of two functional chains: the IL-6R alpha (IL-6Ra) and the 130 kD glycoprotein 130 (gp130), a non-ligand binding chain capable of signal transduction, which mediates gene activation and a variety of biological actions [145]. IL-6 binds to both membrane-bound and soluble receptors, by activating three distinct but interconnected signaling pathways [146,147]. Immune response, specifically T cell proliferation and differentiation, as well as B cell terminal differentiation, are regulated by IL-6. IL-6 is a key modulator of the hepatic acute phase response and also activates macrophages and osteoclasts. IL-6 promotes the production of metalloproteinase and vascular endothelial growth factor (VEGF) in conjunction with tumor necrosis factor (TNF) alpha and IL-1. Additionally, transforming growth factor (TGF) beta and IL-6 play a part in the maturation of regulatory T cells. Similar to IL-1, IL-6 is crucial for systemic and local inflammation, which is frequently accompanied by symptoms like fever, exhaustion, and anorexia as well as alterations in acute phase proteins in the plasma (e.g., C-reactive protein and fibrinogen) [146,147,148]. Mast cells (MCs) and activated T cells produce IL-6, and it has been shown that is highly expressed in the skin and T cells of AD patients [149,150,151].
Serum naturally contains IL-6 in levels of a picogram per milliliter (pg/mL), a glycoprotein from the IL-6 family that is implicated in both health and sickness. The release of IL-6 by the skeletal muscle during exercise or even following multiple injuries can cause IL-6 levels to rise in practically any inflammatory condition [152].
Tocilizumab, a human recombinant monoclonal antibody against the IL-6 receptor that is administered intravenously, was effective in three patients with severe AD but it was also related to bacterial superinfection [153]. There are reports that IL-6 is associated with prurigo nodularis [154] and calcium phosphate-induced pruritus [155].
3.7. Endothelin-1 (ET-1)
Endothelin-1 (ET-1), a 21-amino-acid peptide, is a potent direct vasoconstrictor, also known as a histamine-independent pruritogen that may stimulate the proliferation of fibroblasts and vascular smooth cells [156]. Several different cell types, including endothelial cells, and immunological cells such as dendritic cells, keratinocytes monocytes, neurons, and macrophages, express this 21-amino-acid peptide [157,158]. ET-1 mainly acts via two receptors, ETAR and ETBR. Only the ETA receptor has a role in pruritus transmission. A study showed that the neural peptidase ECE-1 controls pruritus caused by ET-1 in mice and humans, acting as a negative regulator of itch on sensory nerves [159]. Additionally, another study found increased ET-1 expression in the cutaneous lesions of AD patients [160]. Elevated serum ET-1 levels are associated with itch intensity, serum IgE levels, and the severity of AD [161]. Another research group demonstrated that the expression of ET-1 is elevated in the skin and serum of prurigo nodularis patients [162]. In a mouse-induced AD model, pruritus and dermatitis were both improved with the use of a nonselective ETAR and ETBR antagonist, bosentan [158].
3.8. Neurotrophins (NTs)
Neurotrophins (NTs) are proteins that induce the development, function, and survival of neurons. Additionally, they boost neurons’ plasticity and damage tolerance and inhibit pathways that lead to apoptosis, enabling nerve cells to survive under conditions of elevated oxidative stress. NTs include brain-derived neurotrophic factor (BDNF), nerve growth factor (NGF), NT3, and NT4/5 [163]. It has been demonstrated that serum levels of NGF are elevated in AD patients [164,165]. Given the fact that other studies have not shown any correlation between AD severity and NGF levels, this might suggest that NGF might have a local effect on AD pathophysiology [166,167]. A recent study reported raised levels of NT-4 in a group of patients with chronic kidney-induced pruritus. Additionally, there was no connection between serum NT-4 concentrations and the intensity of pruritus [168]. A topical tropomyosin-receptor kinase A (TrkA) inhibitor, pegcantratinib CT327, has been shown to significantly alleviate pruritus in psoriasis patients [169].
3.9. Neuropeptides
Neurons produce and release neuropeptides, which are chemical messengers consisting of short chains of amino acids. Normally, neuropeptides attach to G protein-coupled receptors (GPCRs) to influence the nervous system’s function and other tissues like the heart, muscles, and gut. The biggest and most diversified class of signaling molecules in the nervous system, neuropeptides, are known to number over 100. Common neuropeptides that are released by cutaneous nerve terminals are substance P (SP), vasoactive intestinal peptide (VIP), and calcitonin gene-related peptide (CGRP). In particular, SP and CGRP have a well-established role in the pathogenesis of AD [170].
The peptide neurotransmitter called substance P (SP) is released by sensory neurons. Its receptor is found in a wide variety of cell types and tissues, and it has the ability to control inflammation, cytokine release, and immune cell proliferation. It has direct antibacterial activity and shares common physical and chemical characteristics with AMPs [171]. SP signals mainly via the neurokinin 1 receptor (NK1R) [172]. Additionally, SP binds to MRGPCRs (Mas-related G protein-coupled receptors) that play an important role in pruritus signaling and nociception [173]. One study showed that AD patients have elevated serum SP levels and elevated expression of SP and NKIR+ in lesional skin [174], but a different study did not find any correlation between AD and serum SP levels [175]. One study found the use of a neurokinin receptor 1 (NKR1) antagonist, aprepitant, effective in treating pruritus [176], but another study did not show any effect as an adjuvant treatment to topical corticosteroids (TCS) [177]. In a phase 3 clinical trial (EPIONE), tradipitant (VLY-686) a new NK-1R antagonist was beneficial for treating pruritus and aiding sleep in patients with mild AD [103].
Calcitonin gene-related peptide (CGRP) is mainly found in primary afferent sensory fibers. Human CGRP comes in two different forms, CGRP- and CGRP-, with CGRP- being the main type that is present in the central and peripheral nervous systems. LCs, monocytes, and keratinocytes are a few examples of cell types that can release CGRP in addition to cutaneous nerve endings [178]. Inflammatory cytokines can be produced by keratinocytes and MC degranulation when CGRP is present [178,179]. Most significantly, CGRP steers LCs toward a Th2 pole, which promotes atopic skin disease [180]. Patients with AD have been observed to have higher levels of serum CGRP and lesioned CGRP+ nerve fibers [181,182]. CGRP hyposecretion and SP hypersecretion in the skin has been observed in an AD mouse model, [183].
In patients with AD, elevated serum levels of neuropeptide Y (NPY) [182] and increased serum VIP levels are associated with pruritus [184,185]. VIP inhibits Th1 differentiation while promoting Th2 cell survival and differentiation [186]. NPY stimulates Th2 differentiation, boosts IL-4 production, and is necessary for type 2 responses [187]. Patients with AD have been found to have increased expression of GRP in the skin and elevated serum levels of GRP, and there is a link between the intensity of their pruritus [188,189]. A study demonstrated that levels of GRP expression in the skin of AD patients were related to the severity of AD and pruritus intensity [188].
3.10. Toll-like Receptors (TLRs)
Toll-like receptors (TLRs) are transmembrane pattern recognition receptors (PRRs) that are present on and within innate immune system cells, including dendritic cells, monocytes, macrophages, epithelial cells, and neutrophils. TLRs can recognize a range of pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPS), such as proteins and nucleic acids found in microbial cell walls [190]. High susceptibility to AD is connected with TLR2 and TLR4 polymorphisms [191]. Additionally, TLR2 activation may lead to increased chemokine mRNA production, which could aid in AD development [192]. In AD patients, high expression of TLR3 in the epidermis is correlated with AD severity and hydration [193]. Additionally, TLR3 can activate a variety of pruritogens in keratinocytes such as ET-1, TSLP, and IL-33 [194,195]. In a dry-skin mouse model, the knockdown of TLR3 in dorsal root ganglia (DRG), showed exacerbation of pruritus [196]. Although TLR7 is related to non-histamine pruritus [197], imiquimod, which is a TLR7 agonist, showed a TLR7-independent and TRV1-dependent pruritus pathway [198].
A change in the diversity of the microbiota affects how AD develops and progresses, for example, a decrease in microbiome diversity is correlated with disease severity, especially in the lesional skin of AD. Current research suggests the possibility that the microbiome may influence AD itching through interactions between the gut, skin, and brain [199].
Acute itch behavior may be lessened by antibiotic-induced gut microbiota depletion, which may be related to lessened trigeminal ganglia (TG) neuronal activity [200].
3.11. Janus Kinase (JAK)/Signal Transducer and Activator of Transcription (STAT)
The Janus kinases (JAKs) are protein tyrosine kinases (TYKs) that bind to transmembrane type 1 and type 2 cytokine receptors and mediate cellular responses to numerous cytokines and growth factors; these mediators are important in immune defense and in immune-mediated disease [201]. Persistent pruritus in AD leads to scratching, which in turn triggers STAT3 activation in astrocytes of the spinal dorsal horn. Activation of lipocalin (LCN) 2 by astrocytes sensitizes a pruritus-processing neural network made up of GRPR1 spinal dorsal horn neurons, which in turn causes chronic pruritus and the cycle of itch and scratch [35,202] (Figure 2).
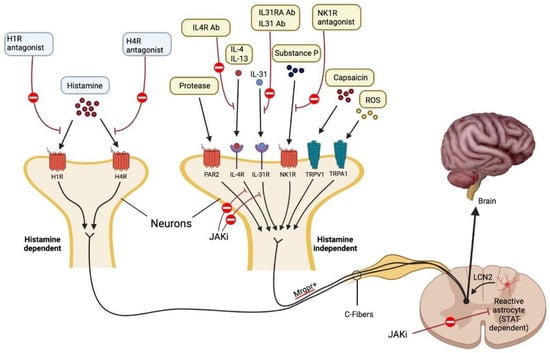
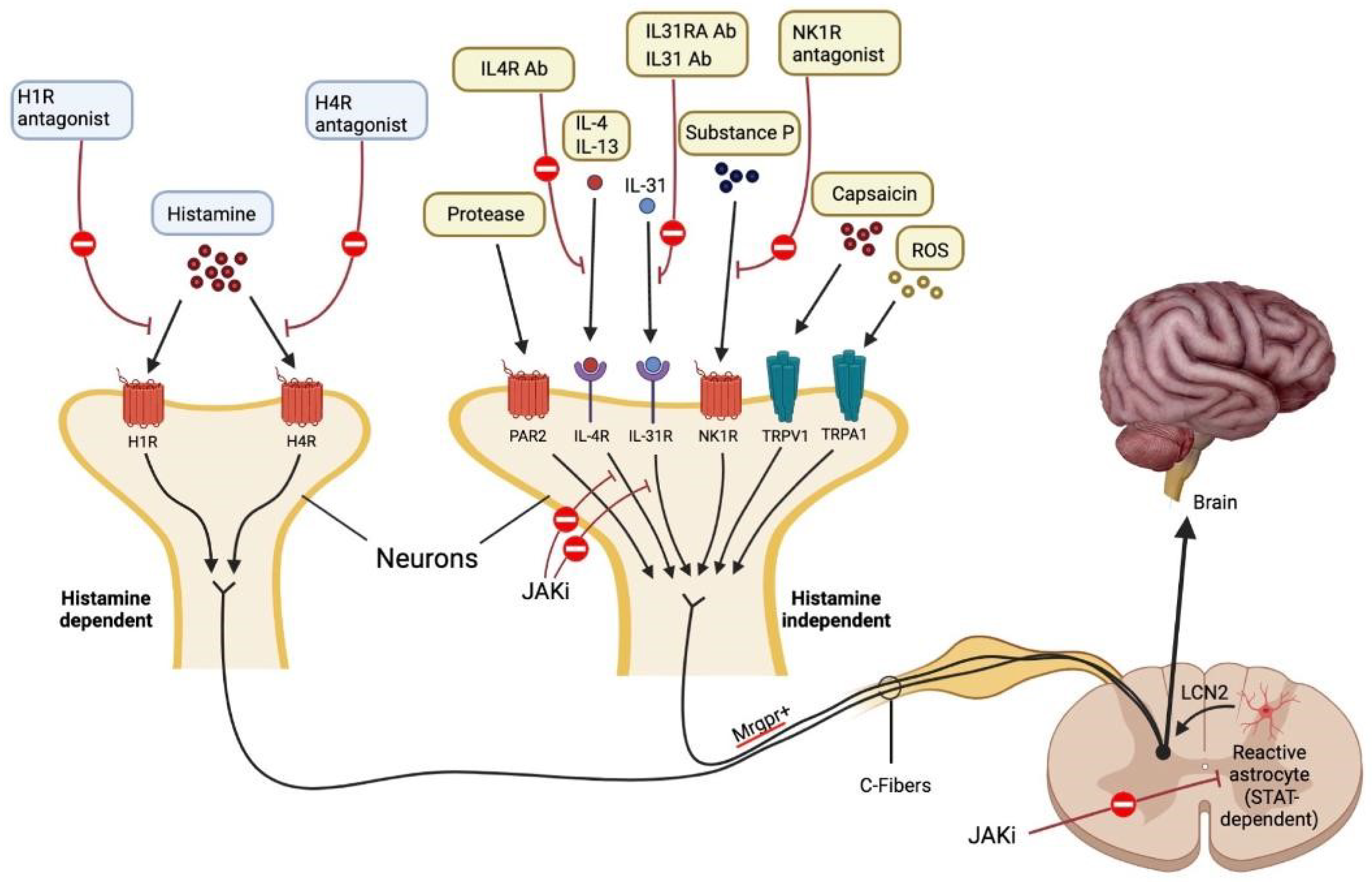
Figure 2.
Mechanisms that cause itch. Each pruritogen involved in AD has a unique receptor that causes itching. It is believed that people with AD experience pruritus through both H1R1 histamine-dependent and Mas-related G protein-coupled receptor (Mrgpr)1 histamine-independent signaling pathways. Lipocalin 2 (LCN2), which is produced by STAT3-dependent reactive astrocytes in the spinal dorsal horn and sensitizes a network of GRPR1 neurons involved in the processing of itch, causes chronic pruritus. Each antipruritic medication blocks a different pruritic route, as depicted in the diagram. JAK-STAT signaling causes IL-4, IL-13, and IL-31 to perform their specific tasks. Protease-activated receptor 2 is abbreviated as PAR2. Adapted from: Paller et al. [35].
Janus kinases (JAKs) are a family of kinases that include JAK1, JAK2, JAK3, and tyrosine kinase 2 (TYK2); JAKs mediate cytokine signaling by activating signal transducer and activator of transcription (STAT) transcription levels. The JAK/STAT pathway is activated in a variety of disease states [203].
Cytokines including IL-31, IL-4, IL-13, and TSLP are crucial for pruritus in AD transmit their signals into the cells via JAK-1 and JAK-2 [202]. In animal studies, JAK-1/2 inhibition or deletion greatly decreased the itch signals brought on by these mediators [119]. When the oral JAK 1/3 inhibitor tofacitinib greatly decreased pruritus in elderly patients with chronic pruritus of unknown cause, it was demonstrated that JAKs play a crucial role in human pruritus. In AD, inflammation and pruritus are influenced by cytokines such as IL-31, IL-4, IL-13, TSLP, and IL-5. A new therapy option is made possible through the possibility of inhibiting JAK-1 and JAK-2 with specific JAK inhibitors [119].
Abrocitinib, an oral selective JA-1 inhibitor, has been proven to significantly reduce pruritus in AD [81,82,84]. In a randomized, phase 2b, double-blinded, placebo-controlled trial in adult patients with AD, abrocitinib (200 mg, 100 mg, 30 mg, or 10 mg) or a placebo were given to participants at random once a day for 12 weeks. In comparison to the placebo group, differences in pruritus were seen in the 200 mg and 100 mg groups [84]. In a further double-blind, randomized phase 3 trial (JADE MONO-1), with patients with moderate to severe AD and more than 12 years old, abrocitinib 200 mg, abrocitinib 100 mg, or a placebo was given to patients at random (2:2:1) once a day for 12 weeks. Between the start of the treatment and week 12, a considerable, quick (i.e., within two days) reduction in the severity of pruritus and other atopic dermatitis symptoms was noticed [81]. In a phase 3 trial, in terms of itch response at week two, abrocitinib 200 mg was superior to dupilumab, but the 100 mg dose was not. For the majority of other important secondary end-point comparisons, neither abrocitinib dose substantially varied from dupilumab at week 16 [83].
Baricitinib, an oral JAK inhibitor that reversibly inhibits JAK1 and JAK2 and moderately inhibits TYK2 has been shown to reduce pruritus in AD patients [85,86].
Furthermore, upadacibitinib, an oral JAK-1 inhibitor, has effectively reduced pruritus in AD [87,88]. With each upadacitinib dosage regimen of 7.5, 15, or 30 mg once daily, a significant improvement in pruritus from the baseline to week 16 was also observed compared to the placebo in a clinical trial [87].
Topical delgocitinib is an investigational JAK inhibitor that blocks tyrosine kinase 2, JAK1, JAK2, and JAK 3. When given to adult Japanese patients with moderate to severe atopic dermatitis for up to 28 weeks, delgocitinib 0.5% ointment, a new topical Janus kinase inhibitor, improved clinical signs and symptoms with a positive safety profile [89]. Nakagawa H. et al. 2020 JAAD. At week one, the delgocitinib group had a lower pruritus Numerical Rating Scale (NRS) than the vehicle group, and this difference persisted over time in adults and children [89,204]. Tofacitinib reversibly inhibits JAK1 and JAK3 in vitro and to a lesser extent inhibits JAK2 and TYK2 [89]. Tofacitinib, by inhibition of JAK3, inhibits cytokines IL-2, IL-4, IL-7, IL-9, IL-15, and IL-21; and through JAK1 inhibition, inhibits IL-6, type 1 IFNs, and IFN-gamma. In a phase 2a, randomized trial, the effectiveness of topical tofacitinib for the treatment of atopic dermatitis was assessed. In this trial, tofacitinib 2% ointment or placebo was applied twice daily for a period of four weeks on 69 adult patients with clinically stable, mild to moderate atopic dermatitis. Significant improvements in pruritus in the tofacitinib group were observed by day two [91,205].
A topical version of the drug ruxolitinib, which is a strong and specific inhibitor of Janus kinase JAK1 and JAK2, is available as ruxolitinib cream 1.5% (OPZELURATM) that has been used topically in atopic dermatitis [206]. Ruxolitinib cream 1.5%, when used twice daily for eight weeks, significantly reduced indices of disease severity, pruritus, and sleep disturbance in two identically constructed, international, phase 3 studies in patients with mild to severe AD aged 12 years [92,206,207].
4. Conclusions
The most distressing symptom of AD, regardless of its severity level, is chronic pruritus. It affects patients’ quality of life significantly and adversely affects their wellness. Our findings suggest that one of the major players in the pathophysiology of AD is pruritogen. The neurocutaneous interaction in chronic pruritic skin disorders is currently being partly elucidated thanks to significant research on the pathophysiology of pruritus. This aids the development of AD-specific treatments. The development of biologics that target IL-4, IL-13, IL-31, and other molecules has produced promising results. The development of new and efficient medicines for atopic dermatitis (AD) may provide a great variety of options for treatment for AD patients. Additionally, new AD therapies will allow us to adapt our treatment plan to our AD patients’ current and future needs.
Author Contributions
Conceptualization, D.K., S.G., G.E. and K.K.; methodology, D.K. and K.K.; writing—original draft preparation, D.K., G.E. and K.K.; writing—review and editing, S.G., G.E. and K.K.; supervision, S.G., G.E. and K.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| AD | Atopic dermatitis |
| IL | Interleukin |
| PAF | Platelet activating factor |
| PARs | Protease-activated receptors |
| HTR7 | 5-Hydroxytryptamine receptor 7 |
| TCS | Topical corticosteroids |
| TNF | Tumor necrosis factor alpha |
| TGF | Transforming growth factor |
| BNP | Brain natriuretic peptide |
| CGRP | Calcitonin gene-related peptide |
| TRPA1 | Transient receptor potential ankyrin 1 |
| KLK | Kallikreins |
| ET-1 | Endothelin-1 |
| BDNF | Brain-derived neurotrophic factor |
| NGF | Nerve growth factor |
| TSLP | Thymic stromal lymphopoietin |
| TLRs | Toll-like receptors |
| VEGF | Vascular endothelial growth factor |
| OSMRβ | Oncostatin M receptor beta |
| PRRs | Pattern recognition receptors |
| PAMPs | Pathogen-associated molecular patterns |
| DAMPS | Damage-associated molecular patterns |
| NTs | Neurotrophins |
| NPY | Neuropeptide Y |
| NK1R | Neurokinin 1 receptor |
| MRGPCRs | Mas-related G protein-coupled receptors |
| GRPR | Gastrin-releasing peptide |
| STAT | Signal transducer and activator of transcription |
| JAK | Janus kinase |
| TYKs | Tyrosine kinases |
| TG | Trigeminal ganglia |
| LCN | Lipocalin |
References
- Spergel, J.M. Epidemiology of Atopic Dermatitis and Atopic March in Children. Immunol. Allergy Clin. N. Am. 2010, 30, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Williams, H.; Robertson, C.; Stewart, A.; Aït-Khaled, N.; Anabwani, G.; Anderson, R.; Asher, I.; Beasley, R.; Björkstén, B.; Burr, M.; et al. Worldwide variations in the prevalence of symptoms of atopic eczema in the international study of asthma and allergies in childhood. J. Allergy Clin. Immunol. 1999, 103, 125–138. [Google Scholar] [CrossRef]
- Odhiambo, J.A.; Williams, H.C.; Clayton, T.O.; Robertson, C.F.; Asher, M.I.; ISAAC Phase Three Study Group. Glob-al variations in prevalence of eczema symptoms in children from ISAAC Phase Three. J. Allergy Clin. Immunol. 2009, 124, 1251–1258.e23. [Google Scholar] [CrossRef]
- Vinding, G.; Zarchi, K.; Ibler, K.; Miller, I.; Ellervik, C.; Jemec, G. Is Adult Atopic Eczema More Common Than We Think?—A Population-based Study in Danish Adults. Acta Derm.-Venereol. 2014, 94, 480–482. [Google Scholar] [CrossRef] [PubMed]
- Rönmark, E.; Ekerljung, L.; Lötvall, J.; Wennergren, G.; Torén, K.; Lundbäck, B. Eczema among adults: Prevalence, risk factors and relation to airway diseases. Results from a large-scale population survey in Sweden. Br. J. Dermatol. 2012, 166, 1301–1308. [Google Scholar] [CrossRef] [PubMed]
- Mortz, C.G.; Andersen, K.E.; Dellgren, C.; Barington, T.; Bindslev-Jensen, C. Atopic dermatitis from adolescence to adulthood in the TOACS cohort: Prevalence, persistence and comorbidities. Allergy 2015, 70, 836–845. [Google Scholar] [CrossRef]
- Wolkewitz, M.; Rothenbacher, D.; Löw, M.; Stegmaier, C.; Ziegler, H.; Radulescu, M.; Brenner, H.; Diepgen, T. Lifetime prevalence of self-reported atopic diseases in a population-based sample of elderly subjects: Results of the ESTHER study. Br. J. Dermatol. 2007, 156, 693–697. [Google Scholar] [CrossRef]
- Sybilski, A.J.; Raciborski, F.; Lipiec, A.; Tomaszewska, A.; Lusawa, A.; Samel-Kowalik, P.; Walkiewicz, A.; Krzych-Fałta, E.; Samoliński, B. Epidemiology of atopic dermatitis in Poland according to the Epidemiology of Allergic Disorders in Poland (ECAP) study. J. Dermatol. 2015, 42, 140–147. [Google Scholar] [CrossRef]
- Saeki, H.; Iizuka, H.; Mori, Y.; Akasaka, T.; Takagi, H.; Kitajima, Y.; Tezuka, T.; Tanaka, T.; Hide, M.; Yamamoto, S.; et al. Prevalence of atopic dermatitis in Japanese elementary schoolchildren. Br. J. Dermatol. 2005, 152, 110–114. [Google Scholar] [CrossRef]
- Sugiura, H.; Umemoto, N.; Deguchi, H.; Murata, Y.; Tanaka, K.; Sawai, T.; Omoto, M.; Uchiyama, M.; Kiriyama, T.; Uehara, M. Preva-lence of childhood and adolescent atopic dermatitis in a Japanese population: Comparison with the disease frequency examined 20 years ago. Acta Derm. Venereol. 1998, 78, 293–294. [Google Scholar] [CrossRef]
- Williamson, S.; Merritt, J.; De Benedetto, A. Atopic dermatitis in the elderly: A review of clinical and pathophysiological hallmarks. Br. J. Dermatol. 2019, 182, 47–54. [Google Scholar] [CrossRef]
- Gerner, T.; Haugaard, J.; Vestergaard, C.; Deleuran, M.; Jemec, G.; Mortz, C.; Agner, T.; Egeberg, A.; Skov, L.; Thyssen, J. Disease severity and trigger factors in Danish children with atopic dermatitis: A nationwide study. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 948–957. [Google Scholar] [CrossRef] [PubMed]
- Sandström, M.H.; Faergemann, J. Prognosis and prognostic factors in adult patients with atopic dermatitis: A long-term follow-up questionnaire study. Br. J. Dermatol. 2004, 150, 103. [Google Scholar] [CrossRef] [PubMed]
- Irvine, A.D.; McLean, W.I.; Leung, D.Y. Filaggrin Mutations Associated with Skin and Allergic Diseases. N. Engl. J. Med. 2011, 365, 1315–1327. [Google Scholar] [CrossRef] [PubMed]
- van den Oord, R.A.; Sheikh, A. Filaggrin gene defects and risk of developing allergic sensitisation and allergic disorders: Sys-tematic review and meta-analysis. BMJ 2009, 339, b2433. [Google Scholar] [CrossRef]
- Paternoster, L.; Standl, M.; Waage, J.; Baurecht, H.; Hotze, M.; Strachan, D.; Curtin, J.; Bønnelykke, K.; Tian, C.; Takahashi, A.; et al. Multi-ancestry genome-wide association study of 21,000 cases and 95,000 controls identifies new risk loci for atopic dermatitis. Nat. Genet. 2015, 47, 1449–1456. [Google Scholar] [CrossRef]
- Elias, P.M.; Wakefield, J.S. Mechanisms of abnormal lamellar body secretion and the dysfunctional skin barrier in patients with atopic dermatitis. J. Allergy Clin. Immunol. 2014, 134, 781–791.e1. [Google Scholar] [CrossRef]
- Feingold, K.R.; Elias, P.M. Role of lipids in the formation and maintenance of the cutaneous permeability barrier. Biochim. Biophys. Acta (BBA)—Mol. Cell Biol. Lipids 2014, 1841, 280–294. [Google Scholar] [CrossRef]
- Cork, M.J.; Robinson, D.A.; Vasilopoulos, Y.; Ferguson, A.; Moustafa, M.; MacGowan, A.; Duff, G.W.; Ward, S.J.; Tazi-Ahnini, R. New perspectives on epidermal barrier dysfunction in atopic dermatitis: Gene–environment interactions. J. Allergy Clin. Immunol. 2006, 118, 3–21. [Google Scholar] [CrossRef]
- Tanei, R.; Hasegawa, Y. Atopic dermatitis in older adults: A viewpoint from geriatric dermatology. Geriatr. Gerontol. Int. 2016, 16, 75–86. [Google Scholar] [CrossRef]
- Mitsuyama, S.; Higuchi, T. Effectiveness of dupilumab for chronic prurigo in elderly patients with atopic dermatitis. An. Bras. Dermatol. 2023, 98, 86–89. [Google Scholar] [CrossRef] [PubMed]
- Černiauskienė, M.; Bagdonaitė, L.; Karčiauskaitė, D.; Kvedarienė, V. Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase Levels. Med. Sci. Monit. 2022, 28, e937990. [Google Scholar] [CrossRef]
- Jaworek, A.K.; Jaworek, M.; Szafraniec, K.; Pawlicka, A.; Wojas-Pelc, A. Can the blood tryptase be an indicator of the severity of atopic dermatitis? Pol. Merkur. Lekarski. 2020, 48, 162–165. [Google Scholar] [PubMed]
- Yu, L.; Li, L. Potential biomarkers of atopic dermatitis. Front. Med. 2022, 9, 1028694. [Google Scholar] [CrossRef] [PubMed]
- Yue, W.; Cheng, D.; Sun, Z.; Shen, Y.; Wang, S.; Liu, X.; Pei, X.; Deng, S.; Pan, H.; Liao, Z.; et al. Validation of diagnostic criteria for atopic dermatitis and proposal of novel diagnostic criteria for adult and elderly Chinese populations: A multicentre, prospective, clinical setting-based study. Br. J. Dermatol. 2022, 188, 420–426. [Google Scholar] [CrossRef]
- Legat, F.J. Itch in Atopic Dermatitis—What Is New? Front. Med. 2021, 8, 644760. [Google Scholar] [CrossRef] [PubMed]
- Ständer, S.; Simpson, E.L.; Guttman-Yassky, E.; Thyssen, J.P.; Kabashima, K.; Ball, S.G.; Rueda, M.J.; DeLozier, A.M.; Silverberg, J.I. Clinical Relevance of Skin Pain in Atopic Dermatitis. J. Drugs Dermatol. 2020, 19, 921–926. [Google Scholar] [CrossRef]
- Yosipovitch, G.; Berger, T.; Fassett, M. Neuroimmune interactions in chronic itch of atopic dermatitis. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 239–250. [Google Scholar] [CrossRef]
- Wei, W.; Anderson, P.; Gadkari, A.; Blackburn, S.; Moon, R.; Piercy, J.; Shinde, S.; Gomez, J.; Ghorayeb, E. Extent and consequences of inade quate disease control among adults with a history of moderate to severe atopic dermatitis. J. Dermatol. 2018, 45, 150–157. [Google Scholar] [CrossRef]
- Reddy, V.B.; Iuga, A.O.; Shimada, S.G.; LaMotte, R.H.; Lerner, E.A. Cowhage-Evoked Itch Is Mediated by a Novel Cysteine Protease: A Ligand of Protease-Activated Receptors. J. Neurosci. 2008, 28, 4331–4335. [Google Scholar] [CrossRef]
- Sun, Y.-G.; Zhao, Z.-Q.; Meng, X.-L.; Yin, J.; Liu, X.-Y.; Chen, Z.-F. Cellular Basis of Itch Sensation. Science 2009, 325, 1531–1534. [Google Scholar] [CrossRef] [PubMed]
- Davidson, S.; Zhang, X.; Yoon, C.H.; Khasabov, S.G.; Simone, D.A.; Giesler, G.J. The Itch-Producing Agents Histamine and Cowhage Activate Separate Populations of Primate Spinothalamic Tract Neurons. J. Neurosci. 2007, 27, 10007–10014. [Google Scholar] [CrossRef] [PubMed]
- Matterne, U.; Strassner, T.; Apfelbacher, C.J.; Diepgen, T.L.; Weisshaar, E. Measuring the prevalence of chronic itch in the general population: Development and validation of a questionnaire for use in large-scale studies. Acta Derm. Venereol. 2009, 89, 250–256. [Google Scholar] [CrossRef]
- Mollanazar, N.K.; Koch, S.D.; Yosipovitch, G. Epidemiology of chronic pruritus: Where have we been and where are we going? Curr. Dermatol. Rep. 2015, 4, 20–29. [Google Scholar] [CrossRef]
- Paller, A.S.; Kabashima, K.; Bieber, T. Therapeutic pipeline for atopic dermatitis: End of the drought? J. Allergy Clin. Immunol. 2017, 140, 633–643. [Google Scholar] [CrossRef]
- Dunford, P.J.; Williams, K.N.; Desai, P.J.; Karlsson, L.; McQueen, D.; Thurmond, R.L. Histamine H4 receptor antagonists are superior to traditional antihistamines in the attenuation of experimental pruritus. J. Allergy Clin. Immunol. 2007, 119, 176–183. [Google Scholar] [CrossRef]
- Werfel, T.; Layton, G.; Yeadon, M.; Whitlock, L.; Osterloh, I.; Jimenez, P.; Liu, W.; Lynch, V.; Asher, A.; Tsianakas, A.; et al. Efficacy and safety of the histamine H4 receptor antagonist ZPL-3893787 in patients with atopic dermatitis. J. Allergy Clin. Immunol. 2019, 143, 1830–1837.e4. [Google Scholar] [CrossRef]
- Wong, L.-S.; Yen, Y.-T.; Lee, C.-H. The Implications of Pruritogens in the Pathogenesis of Atopic Dermatitis. Int. J. Mol. Sci. 2021, 22, 7227. [Google Scholar] [CrossRef]
- Kim, B.S. The translational revolution of itch. Neuron 2022, 110, 2209–2214. [Google Scholar] [CrossRef]
- Gomułka, K.; Wójcik, E.; Szepietowski, J.C. Serum Levels of Eosinophil-Derived Neurotoxin, Platelet-Activating Factor and Vascular Endothelial Growth Factor in Adult Patients with Atopic Dermatitis-A Pilot Study. Biomedicines 2022, 10, 3109. [Google Scholar] [CrossRef]
- Gomułka, K.; Mędrala, W. Serum Levels of Vascular Endothelial Growth Factor, Platelet Activating Factor and Eosinophil-Derived Neurotoxin in Chronic Spontaneous Urticaria-A Pilot Study in Adult Patients. Int. J. Mol. Sci. 2022, 23, 9631. [Google Scholar] [CrossRef] [PubMed]
- Yost, C.C.; Weyrich, A.S.; Zimmerman, G.A. The platelet activating factor (PAF) signaling cascade in systemic inflammatory responses. Biochimie 2010, 92, 692–697. [Google Scholar] [CrossRef] [PubMed]
- Mullol, J.; Bousquet, J.; Bachert, C.; Canonica, G.W.; Gimenez-Arnau, A.; Kowalski, M.L.; Martí-Guadaño, E.; Maurer, M.; Picado, C.; Scadding, G.; et al. Rupatadine in allergic rhinitis and chronic urticaria. Allergy 2008, 63, 5–28. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, T.; Rosen, J.D.; Sanders, K.M.; Yosipovitch, G. Possible roles of basophils in chronic itch. Exp. Dermatol. 2018, 28, 1373–1379. [Google Scholar] [CrossRef]
- Schauberger, E.; Peinhaupt, M.; Cazares, T.; Lindsley, A.W. Lipid Mediators of Allergic Disease: Pathways, Treatments, and Emerging Therapeutic Targets. Curr. Allergy Asthma Rep. 2016, 16, 48. [Google Scholar] [CrossRef]
- Dyer, K.D.; Percopo, C.M.; Xie, Z.; Yang, Z.; Kim, J.D.; Davoine, F.; Lacy, P.; Druey, K.M.; Moqbel, R.; Rosenberg, H.F. Mouse and Human Eosinophils Degranulate in Response to Platelet-Activating Factor (PAF) and LysoPAF via a PAF-Receptor–Independent Mechanism: Evidence for a Novel Receptor. J. Immunol. 2010, 184, 6327–6334. [Google Scholar] [CrossRef] [PubMed]
- Petersen, L.J.; Church, M.K.; Skov, P.S. Platelet-activating factor induces histamine release from human skin mast cells in vivo, which is reduced by local nerve blockade. J. Allergy Clin. Immunol. 1997, 99, 640–647. [Google Scholar] [CrossRef]
- Ocana, J.A.; Romer, E.; Sahu, R.; Pawelzik, S.-C.; FitzGerald, G.A.; Kaplan, M.H.; Travers, J.B. Platelet-Activating Factor–Induced Reduction in Contact Hypersensitivity Responses Is Mediated by Mast Cells via Cyclooxygenase-2–Dependent Mechanisms. J. Immunol. 2018, 200, 4004–4011. [Google Scholar] [CrossRef] [PubMed]
- Hide, M.; Suzuki, T.; Tanaka, A.; Aoki, H. Long-term safety and efficacy of rupatadine in Japanese patients with itching due to chronic spontaneous urticaria, dermatitis, or pruritus: A 12-month, multicenter, open-label clinical trial. J. Dermatol. Sci. 2019, 94, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Nomura, H.; Suganuma, M.; Takeichi, T.; Kono, M.; Isokane, Y.; Sunagawa, K.; Kobashi, M.; Sugihara, S.; Kajita, A.; Miyake, T.; et al. Multifaceted Analyses of Epidermal Serine Protease Activity in Patients with Atopic Dermatitis. Int. J. Mol. Sci. 2020, 21, 913. [Google Scholar] [CrossRef]
- Steinhoff, M.; Neisius, U.; Ikoma, A.; Fartasch, M.; Heyer, G.; Skov, P.S.; Luger, T.A.; Schmelz, M. Proteinase-activated re-ceptor-2 mediates itch: A novel pathway for pruritus in human skin. J. Neurosci. 2003, 23, 6176–6180. [Google Scholar] [CrossRef] [PubMed]
- Briot, A.; Deraison, C.; Lacroix, M.; Bonnart, C.; Robin, A.; Besson, C.; Dubus, P.; Hovnanian, A. Kallikrein 5 induces atopic dermatitis–like lesions through PAR2-mediated thymic stromal lymphopoietin expression in Netherton syndrome. J. Exp. Med. 2009, 206, 1135–1147. [Google Scholar] [CrossRef] [PubMed]
- Moniaga, C.S.; Jeong, S.K.; Egawa, G.; Nakajima, S.; Hara-Chikuma, M.; Jeon, J.E.; Lee, S.H.; Hibino, T.; Miyachi, Y.; Kabashima, K. Protease Activity Enhances Production of Thymic Stromal Lymphopoietin and Basophil Accumulation in Flaky Tail Mice. Am. J. Pathol. 2013, 182, 841–851. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.J.; Mack, M.R.; Oetjen, L.K.; Trier, A.M.; Council, M.L.; Pavel, A.B.; Guttman-Yassky, E.; Kim, B.S.; Liu, Q. Kallikrein 7 Promotes Atopic Dermatitis-Associated Itch Independently of Skin Inflammation. J. Investig. Dermatol. 2020, 140, 1244–1252.e4. [Google Scholar] [CrossRef]
- Zhu, Y.; Underwood, J.; Macmillan, D.; Shariff, L.; O’Shaughnessy, R.; Harper, J.I.; Pickard, C.; Friedmann, P.S.; Healy, E.; Di, W.-L. Persistent kallikrein 5 activation induces atopic dermatitis-like skin architecture independent of PAR2 activity. J. Allergy Clin. Immunol. 2017, 140, 1310–1322.e5. [Google Scholar] [CrossRef]
- Buhl, T.; Ikoma, A.; Kempkes, C.; Cevikbas, F.; Sulk, M.; Buddenkotte, J.; Akiyama, T.; Crumrine, D.; Camerer, E.; Carstens, E.; et al. Protease-Activated Receptor-2 Regulates Neuro-Epidermal Communication in Atopic Dermatitis. Front. Immunol. 2020, 11, 1740. [Google Scholar] [CrossRef]
- Braz, J.M.; Dembo, T.; Charruyer, A.; Ghadially, R.; Fassett, M.S.; Basbaum, A.I. Genetic priming of sensory neurons in mice that overexpress PAR2 enhances allergen responsiveness. Proc. Natl. Acad. Sci. USA 2021, 118, e2021386118. [Google Scholar] [CrossRef]
- Akiyama, T.; Carstens, M.I.; Carstens, E. Excitation of Mouse Superficial Dorsal Horn Neurons by Histamine and/or PAR-2 Agonist: Potential Role in Itch. J. Neurophysiol. 2009, 102, 2176–2183. [Google Scholar] [CrossRef]
- Amadesi, S.; Nie, J.; Vergnolle, N.; Cottrell, G.S.; Grady, E.F.; Trevisani, M.; Manni, C.; Geppetti, P.; McRoberts, J.A.; Ennes, H.; et al. Protease-Activated Receptor 2 Sensitizes the Capsaicin Receptor Transient Receptor Potential Vanilloid Receptor 1 to Induce Hyperalgesia. J. Neurosci. 2004, 24, 4300–4312. [Google Scholar] [CrossRef]
- Chung, K.; Pitcher, T.; Grant, A.D.; Hewitt, E.; Lindstrom, E.; Malcangio, M. Cathepsin S acts via protease-activated receptor 2 to activate sensory neurons and induce itch-like behaviour. Neurobiol. Pain 2019, 6, 100032. [Google Scholar] [CrossRef]
- Barr, T.P.; Garzia, C.; Guha, S.; Fletcher, E.K.; Nguyen, N.; Wieschhaus, A.J.; Ferrer, L.; Covic, L.; Kuliopulos, A. PAR2 Pepducin-Based Suppression of Inflammation and Itch in Atopic Dermatitis Models. J. Investig. Dermatol. 2019, 139, 412–421. [Google Scholar] [CrossRef]
- Andoh, T.; Kuraishi, Y. Antipruritic mechanisms of topical E6005, a phosphodiesterase 4 inhibitor: Inhibition of responses to proteinase-activated receptor 2 stimulation mediated by increase in intracellular cyclic AMP. J. Dermatol. Sci. 2014, 76, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Varricchi, G.; Pecoraro, A.; Marone, G.; Criscuolo, G.; Spadaro, G.; Genovese, A.; Marone, G. Thymic Stromal Lymphopoietin Isoforms, Inflammatory Disorders, and Cancer. Front. Immunol. 2018, 9, 1595. [Google Scholar] [CrossRef] [PubMed]
- Harada, M.; Hirota, T.; Jodo, A.I.; Doi, S.; Kameda, M.; Fujita, K.; Miyatake, A.; Enomoto, T.; Noguchi, E.; Yoshihara, S.; et al. Functional Analysis of the Thymic Stromal Lymphopoietin Variants in Human Bronchial Epithelial Cells. Am. J. Respir. Cell Mol. Biol. 2009, 40, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Takai, T.; Chen, X.; Okumura, K.; Ogawa, H. Long TSLP transcript expression and release of TSLP induced by TLR ligands and cytokines in human keratinocytes. J. Dermatol. Sci. 2012, 66, 233–237. [Google Scholar] [CrossRef]
- Dong, H.; Hu, Y.; Liu, L.; Zou, M.; Huang, C.; Luo, L.; Yu, C.; Wan, X.; Zhao, H.; Chen, J.; et al. Distinct roles of short and long thymic stromal lymphopoietin isoforms in house dust mite-induced asthmatic airway epithelial barrier disruption. Sci. Rep. 2016, 6, 39559. [Google Scholar] [CrossRef]
- Bjerkan, L.; Sonesson, A.; Schenck, K. Multiple Functions of the New Cytokine-Based Antimicrobial Peptide Thymic Stromal Lymphopoietin (TSLP). Pharmaceuticals 2016, 9, 41. [Google Scholar] [CrossRef]
- Lee, E.B.; Kim, K.W.; Hong, J.Y.; Jee, H.M.; Sohn, M.H.; Kim, K.-E. Increased serum thymic stromal lymphopoietin in children with atopic dermatitis. Pediatr. Allergy Immunol. 2010, 21, e457–e460. [Google Scholar] [CrossRef]
- Takai, T. TSLP Expression: Cellular Sources, Triggers, and Regulatory Mechanisms. Allergol. Int. 2012, 61, 3–17. [Google Scholar] [CrossRef]
- Watanabe, N.; Hanabuchi, S.; Soumelis, V.; Yuan, W.; Ho, S.; de Waal Malefyt, R.; Liu, Y.-J. Human thymic stromal lympho-poietin promotes dendritic cell–mediated CD4+ T cell homeostatic expansion. Nat. Immunol. 2004, 5, 426–434. [Google Scholar] [CrossRef]
- Pattarini, L.; Trichot, C.; Bogiatzi, S.; Grandclaudon, M.; Meller, S.; Keuylian, Z.; Durand, M.; Volpe, E.; Madonna, S.; Cavani, A.; et al. TSLP-activated dendritic cells induce human T follicular helper cell differentiation through OX40-ligand. J. Exp. Med. 2017, 214, 1529–1546. [Google Scholar] [CrossRef] [PubMed]
- Marschall, P.; Wei, R.; Segaud, J.; Yao, W.; Hener, P.; German, B.F.; Meyer, P.; Hugel, C.; Da Silva, G.A.; Braun, R.; et al. Dual function of Langerhans cells in skin TSLP-promoted TFH differentiation in mouse atopic dermatitis. J. Allergy Clin. Immunol. 2020, 147, 1778–1794. [Google Scholar] [CrossRef] [PubMed]
- Tatsuno, K.; Fujiyama, T.; Yamaguchi, H.; Waki, M.; Tokura, Y. TSLP directly interacts with skin-homing Th2 cells highly ex-pressing its receptor to enhance IL-4 production in atopic dermatitis. J. Investig. Derm. 2015, 135, 3017–3024. [Google Scholar] [CrossRef]
- Kim, J.H.; Bae, H.C.; Ko, N.Y.; Lee, S.H.; Jeong, S.H.; Lee, H.; Ryu, W.-I.; Kye, Y.C.; Son, S.W. Thymic stromal lymphopoietin downregulates filaggrin expression by signal transducer and activator of transcription 3 (STAT3) and extracellular sig-nal-regulated kinase (ERK) phosphorylation in keratinocytes. J. Allergy Clin. Immunol. 2015, 136, 205–208.e9. [Google Scholar] [CrossRef] [PubMed]
- Leyva-Castillo, J.M.; Hener, P.; Jiang, H.; Li, M. TSLP Produced by Keratinocytes Promotes Allergen Sensitization through Skin and Thereby Triggers Atopic March in Mice. J. Investig. Dermatol. 2013, 133, 154–163. [Google Scholar] [CrossRef]
- Wilson, S.R.; Thé, L.; Batia, L.M.; Beattie, K.; Katibah, G.E.; McClain, S.P.; Pellegrino, M.; Estandian, D.M.; Bautista, D.M. The Epithelial Cell-Derived Atopic Dermatitis Cytokine TSLP Activates Neurons to Induce Itch. Cell 2013, 155, 285–295. [Google Scholar] [CrossRef]
- Morita, T.; McClain, S.P.; Batia, L.M.; Pellegrino, M.; Wilson, S.R.; Kienzler, M.A.; Lyman, K.; Olsen, A.S.B.; Wong, J.F.; Stucky, C.L.; et al. HTR7 Mediates Serotonergic Acute and Chronic Itch. Neuron 2015, 87, 124–138. [Google Scholar] [CrossRef]
- Wang, F.; Trier, A.M.; Li, F.; Kim, S.; Chen, Z.; Chai, J.N.; Mack, M.R.; Morrison, S.A.; Hamilton, J.D.; Baek, J.; et al. A basophil-neuronal axis promotes itch. Cell 2021, 184, 422–440.e17. [Google Scholar] [CrossRef]
- Gauvreau, G.M.; O’Byrne, P.M.; Boulet, L.-P.; Wang, Y.; Cockcroft, D.; Bigler, J.; FitzGerald, J.M.; Boedigheimer, M.; Davis, B.E.; Dias, C.; et al. Effects of an Anti-TSLP Antibody on Allergen-Induced Asthmatic Responses. N. Engl. J. Med. 2014, 370, 2102–2110. [Google Scholar] [CrossRef]
- Simpson, E.L.; Parnes, J.R.; She, D.; Crouch, S.; Rees, W.; Mo, M.; van der Merwe, R. Tezepelumab, an anti–thymic stromal lymphopoietin monoclonal antibody, in the treatment of moderate to severe atopic dermatitis: A randomized phase 2a clinical trial. J. Am. Acad. Dermatol. 2019, 80, 1013–1021. [Google Scholar] [CrossRef]
- Simpson, E.L.; Sinclair, R.; Forman, S.; Wollenberg, A.; Aschoff, R.; Cork, M.; Bieber, T.; Thyssen, J.P.; Yosipovitch, G.; Flohr, C.; et al. Efficacy and safety of abrocitinib in adults and adolescents with moderate-to-severe atopic dermatitis (JADE MONO-1): A multicentre, double-blind, randomised, placebo-controlled, phase 3 trial. Lancet 2020, 396, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Silverberg, J.I.; Simpson, E.L.; Thyssen, J.P.; Gooderham, M.; Chan, G.; Feeney, C.; Biswas, P.; Valdez, H.; DiBonaventura, M.; Nduaka, C.; et al. Efficacy and Safety of Abrocitinib in Patients with Moderate-to-Severe Atopic Dermatitis: A Randomized Clinical Trial. JAMA Dermatol. 2020, 156, 863–873. [Google Scholar] [CrossRef] [PubMed]
- Bieber, T.; Simpson, E.L.; Silverberg, J.I.; Thaçi, D.; Paul, C.; Pink, A.E.; Kataoka, Y.; Chu, C.Y.; DiBonaventura, M.; Rojo, R.; et al. Abrocitinib versus Placebo or Dupilumab for Atopic Dermatitis. N. Engl. J. Med. 2021, 384, 1101–1112. [Google Scholar] [CrossRef]
- Gooderham, M.J.; Forman, S.B.; Bissonnette, R.; Beebe, J.S.; Zhang, W.; Banfield, C.; Zhu, L.; Papacharalambous, J.; Vincent, M.S.; Peeva, E. Efficacy and Safety of Oral Janus Kinase 1 Inhibitor Abrocitinib for Patients with Atopic Dermatitis: A Phase 2 Randomized Clinical Trial. JAMA Dermatol. 2019, 155, 1371–1379. [Google Scholar] [CrossRef] [PubMed]
- Simpson, E.L.; Forman, S.; Silverberg, J.I.; Zirwas, M.; Maverakis, E.; Han, G.; Guttman-Yassky, E.; Marnell, D.; Bissonnette, R.; Waibel, J.; et al. Baricitinib in patients with moderate-to-severe atopic dermatitis: Results from a randomized monotherapy phase 3 trial in the United States and Canada (BREEZE-AD5). J. Am. Acad. Dermatol. 2021, 85, 62–70. [Google Scholar] [CrossRef]
- Guttman-Yassky, E.; Silverberg, J.I.; Nemoto, O.; Forman, S.B.; Wilke, A.; Prescilla, R.; de la Peña, A.; Nunes, F.P.; Janes, J.; Gamalo, M.; et al. Baricitinib in adult patients with moderate-to-severe atopic dermatitis: A phase 2 parallel, double-blinded, randomized placebo-controlled multiple-dose study. J. Am. Acad. Dermatol. 2019, 80, 913–921.e9. [Google Scholar] [CrossRef]
- Guttman-Yassky, E.; Thaçi, D.; Pangan, A.L.; Hong, H.C.-H.; Papp, K.A.; Reich, K.; Beck, L.A.; Mohamed, M.-E.F.; Othman, A.A.; Anderson, J.K.; et al. Upadacitinib in adults with moderate to severe atopic dermatitis: 16-week results from a randomized, placebo-controlled trial. J. Allergy Clin. Immunol. 2020, 145, 877–884. [Google Scholar] [CrossRef]
- Reich, K.; Teixeira, H.D.; de Bruin-Weller, M.; Bieber, T.; Soong, W.; Kabashima, K.; Werfel, T.; Zeng, J.; Huang, X.; Hu, X.; et al. Safety and efficacy of upadacitinib in combination with topical corticosteroids in adolescents and adults with moderate-to-severe atopic dermatitis (AD Up): Results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2021, 397, 2169–2181. [Google Scholar] [CrossRef]
- Nakagawa, H.; Nemoto, O.; Igarashi, A.; Saeki, H.; Kabashima, K.; Oda, M.; Nagata, T. Delgocitinib ointment in pediatric patients with atopic dermatitis: A phase 3, randomized, double-blind, vehicle-controlled study and a subsequent open-label, long-term study. J. Am. Acad. Dermatol. 2021, 85, 854–862. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, H.; Nemoto, O.; Igarashi, A.; Saeki, H.; Kaino, H.; Nagata, T. Delgocitinib ointment, a topical Janus kinase inhibitor, in adult patients with moderate to severe atopic dermatitis: A phase 3, randomized, double-blind, vehicle-controlled study and an open-label, long-term extension study. J. Am. Acad. Dermatol. 2020, 82, 823–831. [Google Scholar] [CrossRef]
- Bissonnette, R.; Papp, K.; Poulin, Y.; Gooderham, M.; Raman, M.; Mallbris, L.; Wang, C.; Purohit, V.; Mamolo, C.; Papacharalambous, J.; et al. Topical tofacitinib for atopic dermatitis: A phase II a randomized trial. Br. J. Dermatol. 2016, 175, 902–911. [Google Scholar] [CrossRef] [PubMed]
- Papp, K.; Szepietowski, J.C.; Kircik, L.; Toth, D.; Eichenfield, L.F.; Leung, D.Y.; Forman, S.B.; Venturanza, M.E.; Sun, K.; Kuligowski, M.E.; et al. Efficacy and safety of ruxolitinib cream for the treatment of atopic dermatitis: Results from 2 phase 3, randomized, double-blind studies. J. Am. Acad. Dermatol. 2021, 85, 863–872. [Google Scholar] [CrossRef]
- Wollenberg, A.; Howell, M.D.; Guttman-Yassky, E.; Silverberg, J.I.; Kell, C.; Ranade, K.; Moate, R.; van der Merwe, R. Treatment of atopic dermatitis with tralokinumab, an anti–IL-13 mAb. J. Allergy Clin. Immunol. 2019, 143, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Wollenberg, A.; Blauvelt, A.; Guttman-Yassky, E.; Worm, M.; Lynde, C.; Lacour, J.; Spelman, L.; Katoh, N.; Saeki, H.; Poulin, Y.; et al. Tralokinumab for moderate-to-severe atopic dermatitis: Results from two 52-week, randomized, double-blind, multicentre, placebo-controlled phase III trials (ECZTRA 1 and ECZTRA 2). Br. J. Dermatol. 2021, 184, 437–449. [Google Scholar] [CrossRef]
- Silverberg, J.I.; Toth, D.; Bieber, T.; Alexis, A.F.; Elewski, B.E.; Pink, A.E.; Hijnen, D.; Jensen, T.N.; Bang, B.; Olsen, C.K.; et al. Tralokinumab plus topical corticosteroids for the treatment of moderate-to-severe atopic dermatitis: Results from the dou-ble-blind, randomized, multicentre, placebo-controlled phase III ECZTRA 3 trial. Br. J. Derm. 2021, 184, 450–463. [Google Scholar] [CrossRef] [PubMed]
- Gutermuth, J.; Pink, A.; Worm, M.; Soldbro, L.; Øland, C.B.; Weidinger, S. Tralokinumab plus topical corticosteroids in adults with severe atopic dermatitis and inadequate response to or intolerance of ciclosporin A: A placebo-controlled, randomized, phase III clinical trial (ECZTRA 7). Br. J. Dermatol. 2021, 186, 440–452. [Google Scholar] [CrossRef]
- Silverberg, J.I.; Pinter, A.; Pulka, G.; Poulin, Y.; Bouaziz, J.-D.; Wollenberg, A.; Murrell, D.F.; Alexis, A.; Lindsey, L.; Ahmad, F.; et al. Phase 2B randomized study of nemolizumab in adults with moderate-to-severe atopic dermatitis and severe pruritus. J. Allergy Clin. Immunol. 2020, 145, 173–182. [Google Scholar] [CrossRef]
- Kabashima, K.; Matsumura, T.; Komazaki, H.; Kawashima, M. Trial of Nemolizumab and Topical Agents for Atopic Dermatitis with Pruritus. N. Engl. J. Med. 2020, 383, 141–150. [Google Scholar] [CrossRef]
- Kabashima, K.; Matsumura, T.; Komazaki, H.; Kawashima, M.; for the Nemolizumab JP01 and JP02 Study Group. Nemolizumab plus topical agents in patients with atopic dermatitis (AD) and moderate-to-severe pruritus provide improvement in pruritus and signs of AD for up to 68 weeks: Results from two phase III, long-term studies. Br. J. Dermatol. 2021, 186, 642–651. [Google Scholar] [CrossRef]
- Simpson, E.L.; Flohr, C.; Eichenfield, L.F.; Bieber, T.; Sofen, H.; Taïeb, A.; Owen, R.; Putnam, W.; Castro, M.; DeBusk, K.; et al. Efficacy and safety of lebrikizumab (an anti-IL-13 monoclonal antibody) in adults with moderate-to-severe atopic dermatitis inadequately controlled by topical corticosteroids: A randomized, placebo-controlled phase II trial (TREBLE). J. Am. Acad. Dermatol. 2018, 78, 863–871.e11. [Google Scholar] [CrossRef]
- Guttman-Yassky, E.; Blauvelt, A.; Eichenfield, L.F.; Paller, A.S.; Armstrong, A.W.; Drew, J.; Gopalan, R.; Simpson, E.L. Efficacy and safety of Lebrikizumab, a high-affinity interleukin 13 inhibitor, in adults with moderate to severe atopic dermatitis. JAMA Derm. 2020, 156, 411. [Google Scholar] [CrossRef]
- Blauvelt, A.; de Bruin-Weller, M.; Gooderham, M.; Cather, J.C.; Weisman, J.; Pariser, D.; Simpson, E.L.; Papp, K.A.; Hong, H.C.-H.; Rubel, D.; et al. Long-term management of moderate-to-severe atopic dermatitis with dupilumab and concomitant topical corticosteroids (LIBERTY AD CHRONOS): A 1-year, randomised, double-blinded, placebo-controlled, phase 3 trial. Lancet 2017, 389, 2287–2303. [Google Scholar] [CrossRef]
- Welsh, S.E.; Xiao, C.; Kaden, A.R.; Brzezynski, J.L.; Mohrman, M.A.; Wang, J.; Smieszek, S.P.; Przychodzen, B.; Ständer, S.; Polymeropoulos, C.; et al. Neurokinin-1 receptor antagonist tradipitant has mixed effects on itch in atopic dermatitis: Results from EPIONE, a randomized clinical trial. J. Eur. Acad. Derm. Venereol. 2020, 35, e338–e340. [Google Scholar] [CrossRef] [PubMed]
- Kwatra, S.G.; Misery, L.; Clibborn, C.; Steinhoff, M. Molecular and cellular mechanisms of itch and pain in atopic dermatitis and implications for novel therapeutics. Clin. Transl. Immunol. 2022, 11, e1390. [Google Scholar] [CrossRef] [PubMed]
- Imai, Y. Interleukin-33 in atopic dermatitis. J. Dermatol. Sci. 2019, 96, 2–7. [Google Scholar] [CrossRef]
- Meng, J.; Moriyama, M.; Feld, M.; Buddenkotte, J.; Buhl, T.; Szöllösi, A.; Zhang, J.; Miller, P.; Ghetti, A.; Fischer, M.; et al. New mechanism underlying IL-31–induced atopic dermatitis. J. Allergy Clin. Immunol. 2018, 141, 1677–1689.e8. [Google Scholar] [CrossRef]
- Meng, J.; Wang, J.; Buddenkotte, J.; Buhl, T.; Steinhoff, M. Role of SNAREs in Atopic Dermatitis–Related Cytokine Secretion and Skin-Nerve Communication. J. Investig. Dermatol. 2019, 139, 2324–2333. [Google Scholar] [CrossRef]
- Steinhoff, M.; Schmelz, M.; Szabó, I.L.; Oaklander, A.L. Clinical presentation, management, and pathophysiology of neuropathic itch. Lancet Neurol. 2018, 17, 709–720. [Google Scholar] [CrossRef]
- Tamagawa-Mineoka, R.; Okuzawa, Y.; Masuda, K.; Katoh, N. Increased serum levels of interleukin 33 in patients with atopic dermatitis. J. Am. Acad. Dermatol. 2014, 70, 882–888. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, N.; Tamagawa-Mineoka, R.; Yasuike, R.; Masuda, K.; Matsunaka, H.; Murakami, Y.; Yokosawa, E.; Katoh, N. Stratum corneum interleukin-33 expressions correlate with the degree of lichenification and pruritus in atopic dermatitis lesions. Clin. Immunol. 2019, 201, 1–3. [Google Scholar] [CrossRef]
- Murakami-Satsutani, N.; Ito, T.; Nakanishi, T.; Inagaki, N.; Tanaka, A.; Vien, P.T.X.; Kibata, K.; Inaba, M.; Nomura, S. IL-33 Promotes the Induction and Maintenance of Th2 Immune Responses by Enhancing the Function of OX40 Ligand. Allergol. Int. 2014, 63, 443–455. [Google Scholar] [CrossRef]
- Walsh, C.M.; Hill, R.Z.; Schwendinger-Schreck, J.; Deguine, J.; Brock, E.C.; Kucirek, N.; Rifi, Z.; Wei, J.; Gronert, K.; Brem, R.B.; et al. Author response: Neutrophils promote CXCR3-dependent itch in the development of atopic dermatitis. Elife 2019, 8, e48448. [Google Scholar] [CrossRef]
- Liu, B.; Tai, Y.; Achanta, S.; Kaelberer, M.M.; Caceres, A.I.; Shao, X.; Fang, J.; Jordt, S.-E. IL-33/ST2 signaling excites sensory neurons and mediates itch response in a mouse model of poison ivy contact allergy. Proc. Natl. Acad. Sci. USA 2016, 113, E7572–E7579. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Hu, X.; Yang, W.; Yasheng, H.; Liu, S.; Zhang, W.; Zhou, Y.; Cui, W.; Zhu, J.; Qiao, Z.; et al. Spinal IL-33/ST2 signaling mediates chronic itch in mice through the astrocytic JAK2-STAT3 cascade. Glia 2019, 67, 1680–1693. [Google Scholar] [CrossRef]
- Chen, Y.L.; Gutowska-Owsiak, D.; Hardman, C.S.; Westmoreland, M.; MacKenzie, T.; Cifuentes, L.; Waithe, D.; Lloyd-Lavery, A.; Marquette, A.; Londei, M.; et al. Proof-of-concept clinical trial of Etokimab shows a key role for IL-33 in atopic dermatitis pathogenesis. Sci. Transl. Med. 2019, 11, 2945. [Google Scholar] [CrossRef]
- Maurer, M.; Cheung, D.S.; Theess, W.; Yang, X.; Dolton, M.; Guttman, A.; Choy, D.F.; Dash, A.; Grimbaldeston, M.A.; Soong, W. Phase 2 randomized clinical trial of astegolimab in patients with moderate to severe atopic dermatitis. J. Allergy Clin. Immunol. 2022, 150, 1517–1524. [Google Scholar] [CrossRef]
- Weidinger, S.; Beck, L.A.; Bieber, T.; Kabashima, K.; Irvine, A.D. Atopic dermatitis. Nat. Rev. Dis. Primers. 2018, 4, 1. [Google Scholar] [CrossRef] [PubMed]
- Langan, S.M.; Irvine, A.D.; Weidinger, S. Atopic dermatitis. Lancet 2020, 396, 345–360. [Google Scholar] [CrossRef] [PubMed]
- Oetjen, L.K.; Mack, M.R.; Feng, J.; Whelan, T.M.; Niu, H.; Guo, C.J.; Chen, S.; Trier, A.M.; Xu, A.Z.; Tripathi, S.V.; et al. Sensory Neurons Co-opt Classical Immune Signaling Pathways to Mediate Chronic Itch. Cell 2017, 171, 217–228.e13. [Google Scholar] [CrossRef]
- Campion, M.; Smith, L.; Gatault, S.; Métais, C.; Buddenkotte, J.; Steinhoff, M. Interleukin-4 and interleukin-13 evoke scratching behaviour in mice. Exp. Dermatol. 2019, 28, 1501–1504. [Google Scholar] [CrossRef]
- Cevikbas, F.; Wang, X.; Akiyama, T.; Kempkes, C.; Savinko, T.; Antal, A.; Kukova, G.; Buhl, T.; Ikoma, A.; Buddenkotte, J.; et al. A sensory neuron–expressed IL-31 receptor mediates T helper cell–dependent itch: Involvement of TRPV1 and TRPA1. J. Allergy Clin. Immunol. 2014, 133, 448–460.e7. [Google Scholar] [CrossRef]
- Cevikbas, F.; Lerner, E.A. Physiology and Pathophysiology of Itch. Physiol. Rev. 2020, 100, 945–982. [Google Scholar] [CrossRef]
- Howell, M.D.; Kim, B.E.; Gao, P.; Grant, A.V.; Boguniewicz, M.; DeBenedetto, A.; Schneider, L.; Beck, L.A.; Barnes, K.C.; Leung, D.Y. Cytokine modulation of atopic dermatitis filaggrin skin expression. J. Allergy Clin. Immunol. 2007, 120, 150–155. [Google Scholar] [CrossRef]
- Miake, S.; Tsuji, G.; Takemura, M.; Hashimoto-Hachiya, A.; Vu, Y.H.; Furue, M.; Nakahara, T. IL-4 augments IL-31/IL-31 receptor alpha interaction leading to enhanced CCL 17 and CCL 22 production in dendritic cells: Implications for atopic dermatitis. Int. J. Mol. Sci. 2019, 20, 4053. [Google Scholar] [CrossRef] [PubMed]
- Zheng, T.; Oh, M.H.; Oh, S.Y.; Schroeder, J.T.; Glick, A.B.; Zhu, Z. Transgenic expression of interleukin-13 in the skin induces a pruritic dermatitis and skin remodeling. J. Investig. Derm. 2009, 129, 742–751. [Google Scholar] [CrossRef]
- Gooderham, M.J.; Hong, H.C.-H.; Eshtiaghi, P.; Papp, K.A. Dupilumab: A review of its use in the treatment of atopic dermatitis. J. Am. Acad. Derm. 2018, 78, S28–S36. [Google Scholar] [CrossRef] [PubMed]
- Simpson, E.L.; Bieber, T.; Guttman-Yassky, E.; Beck, L.A.; Blauvelt, A.; Cork, M.J.; Silverberg, J.I.; Deleuran, M.; Kataoka, Y.; Lacour, J.-P.; et al. Two Phase 3 Trials of Dupilumab versus Placebo in Atopic Dermatitis. N. Engl. J. Med. 2016, 375, 2335–2348. [Google Scholar] [CrossRef]
- Pezzolo, E.; Naldi, L. Tralokinumab in the treatment of resistant atopic dermatitis: An open-label, retrospective case series study. J. Eur. Acad. Dermatol. Venereol. 2022; Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Mickevicius, T.; Pink, A.E.; Bhogal, M.B.; O’Brart, D.M.; Robbie, S.J.M. Dupilumab-Induced, Tralokinumab-Induced, and Belantamab Mafodotin–Induced Adverse Ocular Events—Incidence, Etiology, and Management. Cornea, 2022; Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jing, D.; Cheng, J.; Chen, X.; Shen, M.; Liu, H. The efficacy and safety of IL-13 inhibitors in atopic dermatitis: A systematic review and meta-analysis. Front. Immunol. 2022, 13, 923362. [Google Scholar] [CrossRef]
- Labib, A.; Ju, T.; Yosipovitch, G. Managing Atopic Dermatitis with Lebrikizumab—The Evidence to Date. Clin. Cosmet. Investig. Dermatol. 2022, 15, 1065–1072. [Google Scholar] [CrossRef]
- Miron, Y.; Miller, P.E.; Hughes, C.; Indersmitten, T.; Lerner, E.A.; Cevikbas, F. Mechanistic insights into the antipruritic effects of lebrikizumab, an anti–IL-13 mAb. J. Allergy Clin. Immunol. 2022, 150, 690–700. [Google Scholar] [CrossRef]
- Maier, E.; Mittermeir, M.; Ess, S.; Neuper, T.; Schmiedlechner, A.; Duschl, A.; Horejs-Hoeck, J. Prerequisites for Functional Interleukin 31 Signaling and Its Feedback Regulation by Suppressor of Cytokine Signaling 3 (SOCS3). J. Biol. Chem. 2015, 290, 24747–24759. [Google Scholar] [CrossRef]
- Park, K.; Park, J.-H.; Yang, W.-J.; Lee, J.-J.; Song, M.-J.; Kim, H.-P. Transcriptional activation of theIL31gene by NFAT and STAT6. J. Leukoc. Biol. 2012, 91, 245–257. [Google Scholar] [CrossRef]
- Dillon, S.R.; Sprecher, C.; Hammond, A.; Bilsborough, J.; Rosenfeld-Franklin, M.; Presnell, S.R.; Haugen, H.S.; Maurer, M.; Harder, B.; Johnston, J.; et al. Interleukin 31, a cytokine produced by activated T cells, induces dermatitis in mice. Nat. Immunol. 2004, 5, 752–760. [Google Scholar] [CrossRef]
- Saleem, M.D.; Oussedik, E.; D’Amber, V.; Feldman, S.R. Interleukin-31 pathway and its role in atopic dermatitis: A systematic review. J. Dermatol. Treat. 2017, 28, 591–599. [Google Scholar] [CrossRef]
- Tatu, A.L.; Nadasdy, T.; Arbune, A.; Chioncel, V.; Bobeica, C.; Niculet, E.; Iancu, A.V.; Dumitru, C.; Popa, V.T.; Kluger, N.; et al. Interrelationship and Sequencing of Interleukins4, 13, 31, and 33—An Integrated Systematic Review: Dermatological and Multidisciplinary Perspectives. J. Inflamm. Res. 2022, 15, 5163–5184. [Google Scholar] [CrossRef] [PubMed]
- Diveu, C.; Lelièvre, E.; Perret, D.; Lak-Hal, A.-H.L.; Froger, J.; Guillet, C.; Chevalier, S.; Rousseau, F.; Wesa, A.; Preisser, L.; et al. GPL, a Novel Cytokine Receptor Related to GP130 and Leukemia Inhibitory Factor Receptor. J. Biol. Chem. 2003, 278, 49850–49859. [Google Scholar] [CrossRef]
- Diveu, C.; Lak-Hal, A.-H.L.; Froger, J.; Ravon, E.; Grimaud, L.; Barbier, F.; Hermann, J.; Gascan, H.; Chevalier, S. Predominant expression of the long isoform of GP130-like (GPL) receptor is required for interleukin-31 signaling. Eur. Cytokine Netw. 2004, 15, 291–302. [Google Scholar] [PubMed]
- Feld, M.; Garcia, R.; Buddenkotte, J.; Katayama, S.; Lewis, K.; Muirhead, G.; Hevezi, P.; Plesser, K.; Schrumpf, H.; Krjutskov, K.; et al. The pruritus- and TH2-associated cytokine IL-31 promotes growth of sensory nerves. J. Allergy Clin. Immunol. 2016, 138, 500–508.e24. [Google Scholar] [CrossRef]
- Liang, J.; Hu, F.; Dan, M.; Sang, Y.; Abulikemu, K.; Wang, Q.; Hong, Y.; Kang, X. Safety and Efficacy of Nemolizumab for Atopic Dermatitis with Pruritus: A Systematic Review and Meta-Regression Analysis of Randomized Controlled Trials. Front. Immunol. 2022, 13, 825312. [Google Scholar] [CrossRef]
- Tan, X.L.; Thomas, B.R.; Tan, Y.J.; O’Toole, E.A. Effects of systemic therapies on pruritus in adults with atopic dermatitis: A systematic review and meta-analysis. Clin. Exp. Dermatol. 2022, 47, 658–666. [Google Scholar] [CrossRef] [PubMed]
- Akira, S.; Taga, T.; Kishimoto, T. Interleukin-6 in Biology and Medicine. Adv Immunol. 1993, 54, 1–78. [Google Scholar] [CrossRef]
- Choy, E.H.; De Benedetti, F.; Takeuchi, T.; Hashizume, M.; John, M.R.; Kishimoto, T. Translating IL-6 biology into effective treatments. Nat. Rev. Rheumatol. 2020, 16, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Hirano, T. Interleukin 6 and its Receptor: Ten Years Later. Int. Rev. Immunol. 1998, 16, 249–284. [Google Scholar] [CrossRef]
- Avci, A.B.; Feist, E.; Burmester, G.R. Targeting IL-6 or IL-6 Receptor in Rheumatoid Arthritis: What’s the Difference? BioDrugs 2018, 32, 531–546. [Google Scholar] [CrossRef] [PubMed]
- Heink, S.; Yogev, N.; Garbers, C.; Herwerth, M.; Aly, L.; Gasperi, C.; Husterer, V.; Croxford, A.L.; Möller-Hackbarth, K.; Bartsch, H.S.; et al. Trans-presentation of IL-6 by dendritic cells is required for the priming of pathogenic TH17 cells. Nat. Immunol. 2017, 18, 74–85. [Google Scholar] [CrossRef]
- Okuda, Y. Review of tocilizumab in the treatment of rheumatoid arthritis. Biol. Targets Ther. 2008, 2, 75–82. [Google Scholar] [CrossRef]
- Toshitani, A.; Ansel, J.C.; Chan, S.C.; Li, S.H.; Hanifin, J.M. Increased interleukin 6 production by T cells derived from pa-tients with atopic dermatitis. J. Investig. Derm. 1993, 100, 293–304. [Google Scholar]
- Conti, P.; Kempuraj, D.; Di Gioacchino, M.; Boucher, W.; Letourneau, R.; Kandere, K.; Barbacane, R.C.; Reale, M.; Felaco, M.; Frydas, S.; et al. Interleukin-6 and mast cells. Allergy Asthma Proc. 2002, 23, 331–335. [Google Scholar]
- Fedenko, E.S.; Elisyutina, O.G.; Filimonova, T.M.; Boldyreva, M.N.; Burmenskaya, O.V.; Rebrova, O.Y.; Yarilin, A.A.; Khaitov, R.M. Cytokine gene expression in the skin and peripheral blood of atopic dermatitis patients and healthy individuals. Self/Nonself 2011, 2, 120–124. [Google Scholar] [CrossRef]
- Niculet, E.; Chioncel, V.; Elisei, A.M.; Miulescu, M.; Buzia, O.D.; Nwabudike, L.C.; Craescu, M.; Draganescu, M.; Bujoreanu, F.; Marinescu, E.; et al. Multifactorial expression of IL-6 with update on COVID-19 and the therapeutic strategies of its blockade (Review). Exp. Ther. Med. 2021, 21, 263. [Google Scholar] [CrossRef]
- Navarini, A.A.; French, L.E.; Hofbauer, G.F. Interrupting IL-6–receptor signaling improves atopic dermatitis but associates with bacterial superinfection. J. Allergy Clin. Immunol. 2011, 128, 1128–1130. [Google Scholar] [CrossRef]
- Konda, D.; Chandrashekar, L.; Rajappa, M.; Kattimani, S.; Thappa, D.M.; Ananthanarayanan, P.H. Serotonin and interleukin-6: Association with pruritus severity, sleep quality and depression severity in prurigo nodularis. Asian J. Psychiatr. 2015, 17, 24–28. [Google Scholar] [CrossRef]
- Keshari, S.; Sipayung, A.D.; Hsieh, C.C.; Su, L.J.; Chiang, Y.R.; Chang, H.C.; Yang, W.C.; Chuang, T.H.; Chen, C.L.; Huang, C.M. IL-6/p-BTK/P-ERK signaling mediates calcium phosphate-induced pruritus. FASEB J. 2019, 33, 12036–12046. [Google Scholar] [CrossRef] [PubMed]
- Nakahara, T.; Kido-Nakahara, M.; Ohno, F.; Ulzii, D.; Chiba, T.; Tsuji, G.; Furue, M. The pruritogenic mediator endothelin-1 shifts the dendritic cell-T-cell response toward Th17/Th1 polarization. Allergy 2018, 73, 511–515. [Google Scholar] [CrossRef]
- Gomes, L.O.; Hara, D.B.; Rae, G.A. Endothelin-1 induces itch and pain in the mouse cheek model. Life Sci. 2012, 91, 628–633. [Google Scholar] [CrossRef] [PubMed]
- Kido-Nakahara, M.; Wang, B.; Ohno, F.; Tsuji, G.; Ulzii, D.; Takemura, M.; Furue, M.; Nakahara, T. Inhibition of mite-induced dermatitis, pruritus, and nerve sprouting in mice by the endothelin receptor antagonist bosentan. Allergy 2021, 76, 291–301. [Google Scholar] [CrossRef]
- Kido-Nakahara, M.; Buddenkotte, J.; Kempkes, C.; Ikoma, A.; Cevikbas, F.; Akiyama, T.; Nunes, F.; Seeliger, S.; Hasdemir, B.; Mess, C.; et al. Neural peptidase endothelin-converting enzyme 1 regulates endothelin 1–induced pruritus. J. Clin. Investig. 2014, 124, 2683–2695. [Google Scholar] [CrossRef]
- Aktar, M.K.; Kido-Nakahara, M.; Furue, M.; Nakahara, T. Mutual upregulation of endothelin-1 and IL-25 in atopic dermatitis. Allergy 2015, 70, 846–854. [Google Scholar] [CrossRef] [PubMed]
- Tsybikov, N.N.; Petrisheva, I.V.; Kuznik, B.I.; Magen, E. Plasma endothelin-1 levels during exacerbation of atopic dermatitis. Allergy Asthma Proc. 2015, 36, 320–324. [Google Scholar] [CrossRef] [PubMed]
- Wong, L.-S.; Yen, Y.-T.; Lin, S.-H.; Lee, C.-H. IL-17A Induces Endothelin-1 Expression through p38 Pathway in Prurigo Nodularis. J. Investig. Dermatol. 2020, 140, 702–706.e2. [Google Scholar] [CrossRef] [PubMed]
- Nockher, W.A.; Renz, H. Neurotrophins in allergic diseases: From neuronal growth factors to intercellular signaling molecules. J. Allergy Clin. Immunol. 2006, 117, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Dou, Y.-C.; Hagströmer, L.; Emtestam, L.; Johansson, O. Increased nerve growth factor and its receptors in atopic dermatitis: An immunohistochemical study. Arch. Dermatol. Res. 2006, 298, 31–37. [Google Scholar] [CrossRef]
- Toyoda, M.; Nakamura, M.; Makino, T.; Hino, T.; Kagoura, M.; Morohashi, M. Nerve growth factor and substance P are useful plasma markers of disease activity in atopic dermatitis. Br. J. Derm. 2002, 147, 71–79. [Google Scholar] [CrossRef]
- Papoiu, A.D.; Wang, H.; Nattkemper, L.; Tey, H.L.; Ishiuji, Y.; Chan, Y.-H.; Schmelz, M.; Yosipovitch, G. A study of serum concentrations and dermal levels of NGF in atopic dermatitis and healthy subjects. Neuropeptides 2011, 45, 417–422. [Google Scholar] [CrossRef]
- Schulte-Herbrüggen, O.; Fölster-Holst, R.; von Elstermann, M.; Augustin, M.; Hellweg, R. Clinical Relevance of Nerve Growth Factor Serum Levels in Patients with Atopic Dermatitis and Psoriasis. Int. Arch. Allergy Immunol. 2007, 144, 211–216. [Google Scholar] [CrossRef]
- Wala-Zielińska, K.; Świerczyńska-Mróz, K.; Krajewski, P.K.; Nowicka-Suszko, D.; Krajewska, M.; Szepietowski, J.C. Elevated Level of Serum Neurotrophin-4, but Not of Brain-Derived Neurotrophic Factor, in Patients with Chronic Kidney Disease-Associated Pruritus. J. Clin. Med. 2022, 11, 6292. [Google Scholar] [CrossRef]
- Roblin, D.; Yosipovitch, G.; Boyce, B.; Robinson, J.; Sandy, J.; Mainero, V.; Wickramasinghe, R.; Anand, U.; Anand, P. Topical TrkA kinase inhibitor CT327 is an effective, novel therapy for the treatment of pruritus due to psoriasis: Results from experimentalstudies, and efficacy and safety of CT327 in a phase 2b clinical trial in patients with psoriasis. Acta Derm. Venereol. 2015, 95, 542–548. [Google Scholar] [CrossRef]
- Kabata, H.; Artis, D. Neuro-immune crosstalk and allergic inflammation. J. Clin. Investig. 2019, 129, 1475–1482. [Google Scholar] [CrossRef]
- Suvas, S. Role of Substance P Neuropeptide in Inflammation, Wound Healing, and Tissue Homeostasis. J. Immunol. 2017, 199, 1543–1552. [Google Scholar] [CrossRef]
- Mashaghi, A.; Marmalidou, A.; Tehrani, M.; Grace, P.M.; Pothoulakis, C.; Dana, R. Neuropeptide Substance P and the Immune Response. Cell Mol. Life Sci. 2016, 73, 4249–4264. [Google Scholar] [CrossRef]
- Meixiong, J.; Dong, X. Mas-Related G Protein–Coupled Receptors and the Biology of Itch Sensation. Annu. Rev. Genet. 2017, 51, 105–121. [Google Scholar] [CrossRef]
- Lönndahl, L.; Rasul, A.; Lonne-Rahm, S.-B.; Holst, M.; Johansson, B.; El-Nour, H.; Djurfeldt, D.R.; Nordlind, K. Tachykinin upregulation in atopic dermatitis. Immunopharmacol. Immunotoxicol. 2019, 41, 117–122. [Google Scholar] [CrossRef]
- Paramita, D.A.; Nasution, K.; Lubis, N.Z. Relationship of Substance P with the Degree of Atopic Dermatitis Severity. Clin. Cosmet. Investig. Dermatol. 2021, 14, 551–555. [Google Scholar] [CrossRef] [PubMed]
- Ständer, S.; Siepmann, D.; Herrgott, I.; Sunderkötter, C.; Luger, T.A. Targeting the Neurokinin Receptor 1 with Aprepitant: A Novel Antipruritic Strategy. PLoS ONE 2010, 5, e10968. [Google Scholar] [CrossRef]
- Lönndahl, L.; Holst, M.; Bradley, M.; Killasli, H.; Heilborn, J.; Hall, M.; Theodorsson, E.; Holmberg, J.; Nordlind, K. Sub-stance P antagonist aprepitant shows no additive effect compared with standardized topical treatment alone in patients with atopic dermatitis. Acta. Derm. Venereol. 2018, 98, 324–328. [Google Scholar] [CrossRef]
- Granstein, R.D.; Wagner, J.A.; Stohl, L.L.; Ding, W. Calcitonin gene-related peptide: Key regulator of cutaneous immunity. Acta Physiol. 2015, 213, 586–594. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Wang, L.; Clark, J.D.; Kingery, W.S. Keratinocytes express cytokines and nerve growth factor in response to neuropeptide activation of the ERK1/2 and JNK MAPK transcription pathways. Regul. Pept. 2013, 186, 92–103. [Google Scholar] [CrossRef]
- Ding, W.; Stohl, L.L.; Wagner, J.A.; Granstein, R.D. Calcitonin Gene-Related Peptide Biases Langerhans Cells toward Th2-Type Immunity. J. Immunol. 2008, 181, 6020–6026. [Google Scholar] [CrossRef]
- Järvikallio, A.; Harvima, I.T.; Naukkarinen, A. Mast cells, nerves and neuropeptides in atopic dermatitis and nummular eczema. Arch. Derm. Res. 2003, 295, 2–7. [Google Scholar] [CrossRef]
- Hodeib, A.; EI-Samad, Z.A.; Hanafy, H.; EI-Latief, A.A.; EI-Bendary, A.; Abu-Raya, A. Nerve growth factor, neuropeptides and cutaneous nerves in atopic dermatitis. Indian J. Derm. 2010, 55, 135–139. [Google Scholar] [PubMed]
- Katsuno, M.; Aihara, M.; Kojima, M.; Osuna, H.; Hosoi, J.; Nakamura, M.; Toyoda, M.; Matsuda, H.; Ikezawa, Z. Neuropeptides concentrations in the skin of a murine (NC/Nga mice) model of atopic dermatitis. J. Derm. Sci. 2003, 33, 55–65. [Google Scholar] [CrossRef]
- Umemoto, N.; Kakurai, M.; Okazaki, H.; Kiyosawa, T.; Demitsu, T.; Nakagawa, H. Serum levels of vasoactive intestinal peptide are elevated in patients with atopic dermatitis. J. Derm. Sci. 2003, 31, 161–164. [Google Scholar] [CrossRef]
- Teresiak-Mikołajczak, E.; Czarnecka-Operacz, M.; Jenerowicz, D.; Silny, W. Neurogenic markers of the inflammatory process in atopic dermatitis: Relation to the severity and pruritus. Adv. Dermatol. Allergol. 2013, 5, 286–292. [Google Scholar] [CrossRef]
- Ganea, D.; Hooper, K.M.; Kong, W. The neuropeptide vasoactive intestinal peptide: Direct effects on immune cells and involvement in inflammatory and autoimmune diseases. Acta Physiol. 2015, 213, 442–452. [Google Scholar] [CrossRef] [PubMed]
- Oda, N.; Miyahara, N.; Taniguchi, A.; Morichika, D.; Senoo, S.; Fujii, U.; Itano, J.; Gion, Y.; Kiura, K.; Kanehiro, A.; et al. Requirement for neuropeptide Y in the development of type 2 responses and allergen-induced airway hyperresponsiveness and inflammation. Am. J. Physiol. Lung Cell Mol. Physiol. 2019, 316, L407–L417. [Google Scholar] [CrossRef]
- Lou, H.; Lu, J.; Choi, E.B.; Oh, M.H.; Jeong, M.; Barmettler, S.; Zhu, Z.; Zheng, T. Expression of IL-22 in the Skin Causes Th2-Biased Immunity, Epidermal Barrier Dysfunction, and Pruritus via Stimulating Epithelial Th2 Cytokines and the GRP Pathway. J. Immunol. 2017, 198, 2543–2555. [Google Scholar] [CrossRef] [PubMed]
- Tirado-Sánchez, A.; Bonifaz, A.; Ponce-Olivera, R. Serum gastrin-releasing peptide levels correlate with disease severity and pruritus in patients with atopic dermatitis. Br. J. Dermatol. 2015, 173, 298–300. [Google Scholar] [CrossRef]
- Sun, L.; Liu, W.; Zhang, L.-J. The Role of Toll-Like Receptors in Skin Host Defense, Psoriasis, and Atopic Dermatitis. J. Immunol. Res. 2019, 2019, 1824624. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, H.-C.; Feng, C.; Yan, M. Analysis of the Association of Polymorphisms rs5743708 in TLR2 and rs4986790 in TLR4 with Atopic Dermatitis Risk. Immunol. Investig. 2018, 48, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Lin, D.; Cai, X.; Cui, D.; Fang, R.; Zhang, W.; Yu, B.; Wang, X. Enhancement of Chemokine mRNA Expression by Toll-Like Receptor 2 Stimulation in Human Peripheral Blood Mononuclear Cells of Patients with Atopic Dermatitis. BioMed Res. Int. 2020, 2020, 1497175. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, N.; Tamagawa-Mineoka, R.; Ueta, M.; Konishi, E.; Yasuike, R.; Masuda, K.; Matsunaka, H.; Murakami, Y.; Yokosawa, E.; Katoh, N. Stratum corneum Toll-like receptor 3 expressions correlate with the severity of atopic dermatitis lesions. J. Dermatol. Sci. 2019, 94, 354–357. [Google Scholar] [CrossRef]
- Szöllősi, A.G.; McDonald, I.; Szabó, I.L.; Meng, J.; Bogaard, E.V.D.; Steinhoff, M. TLR3 in Chronic Human Itch: A Keratinocyte-Associated Mechanism of Peripheral Itch Sensitization. J. Investig. Dermatol. 2019, 139, 2393–2396.e6. [Google Scholar] [CrossRef]
- Yasuike, R.; Tamagawa-Mineoka, R.; Ueta, M.; Nakamura, N.; Kinoshita, S.; Katoh, N. The role of toll-like receptor 3 in chronic contact hypersensitivity induced by repeated elicitation. J. Dermatol. Sci. 2017, 88, 184–191. [Google Scholar] [CrossRef]
- Liu, T.; Berta, T.; Xu, Z.-Z.; Park, C.-K.; Zhang, L.; Lü, N.; Liu, Q.; Liu, Y.; Gao, Y.-J.; Liu, Y.-C.; et al. TLR3 deficiency impairs spinal cord synaptic transmission, central sensitization, and pruritus in mice. J. Clin. Investig. 2012, 122, 2195–2207. [Google Scholar] [CrossRef]
- Liu, T.; Xu, Z.-Z.; Park, C.-K.; Berta, T.; Ji, R.-R. Toll-like receptor 7 mediates pruritus. Nat. Neurosci. 2010, 13, 1460–1462. [Google Scholar] [CrossRef]
- Kim, S.; Park, G.; Kim, D.; Lee, J.; Min, H.; Wall, E.; Lee, C.J.; Simon, M.I.; Lee, S.J.; Han, S.K. Analysis of cellular and behavioral responses to imiquimod reveals a unique itch pathway in transient receptor potential vanilloid 1 (TRPV1)-expressing neurons. Proc. Natl. Acad. Sci. USA 2011, 108, 3371–3376. [Google Scholar] [CrossRef]
- Moniaga, C.S.; Tominaga, M.; Takamori, K. An Altered Skin and Gut Microbiota Are Involved in the Modulation of Itch in Atopic Dermatitis. Cells 2022, 11, 3930. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, T.; Niu, J.; Xiao, J.; Zhang, M.; Zhang, R.; Chen, D.; Shi, Y.; Zhang, X.; Hu, X.; et al. Inhibitory effects of antibiotic-induced gut microbiota depletion on acute itch behavior in mice. Brain Res. Bull. 2022, 190, 50–61. [Google Scholar] [CrossRef]
- Ghoreschi, K.; Laurence, A.; O’Shea, J.J. Janus kinases in immune cell signaling. Immunol. Rev. 2009, 228, 273–287. [Google Scholar] [CrossRef] [PubMed]
- Shiratori-Hayashi, M.; Koga, K.; Tozaki-Saitoh, H.; Kohro, Y.; Toyonaga, H.; Yamaguchi, C.; Hasegawa, A.; Nakahara, T.; Hachisuka, J.; Akira, S.; et al. STAT3-dependent reactive astrogliosis in the spinal dorsal horn underlies chronic itch. Nat. Med. 2015, 21, 927–931. [Google Scholar] [CrossRef] [PubMed]
- Villarino, A.V.; Kanno, Y.; O’Shea, J.J. Mechanisms and consequences of Jak–STAT signaling in the immune system. Nat. Immunol. 2017, 18, 374–384. [Google Scholar] [CrossRef]
- Karaman, M.W.; Herrgard, S.; Treiber, D.K.; Gallant, P.; Atteridge, C.E.; Campbell, B.T.; Chan, K.W.; Ciceri, P.; Davis, M.I.; Edeen, P.T.; et al. A quantitative analysis of kinase inhibitor selectivity. Nat. Biotechnol. 2008, 26, 127–132. [Google Scholar] [CrossRef]
- Sadeghi, S.; Mohandesi, N.A. Efficacy and safety of topical JAK inhibitors in the treatment of atopic dermatitis in paediatrics and adults: A systematic review. Exp. Dermatol. 2023; Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Hoy, S.M. Ruxolitinib Cream 1.5%: A Review in Mild to Moderate Atopic Dermatitis. Am. J. Clin. Dermatol. 2023, 24, 143–151. [Google Scholar] [CrossRef]
- Roy, Y.R.-L.; Ficheux, A.-S.; Misery, L.; Brenaut, E. Efficacy of topical and systemic treatments for atopic dermatitis on pruritus: A systematic literature review and meta-analysis. Front. Med. 2022, 9, 1079323. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).