Pruritogenic Mediators and New Antipruritic Drugs in Atopic Dermatitis
Abstract
:1. Introduction
2. Pruritogenic Mediators
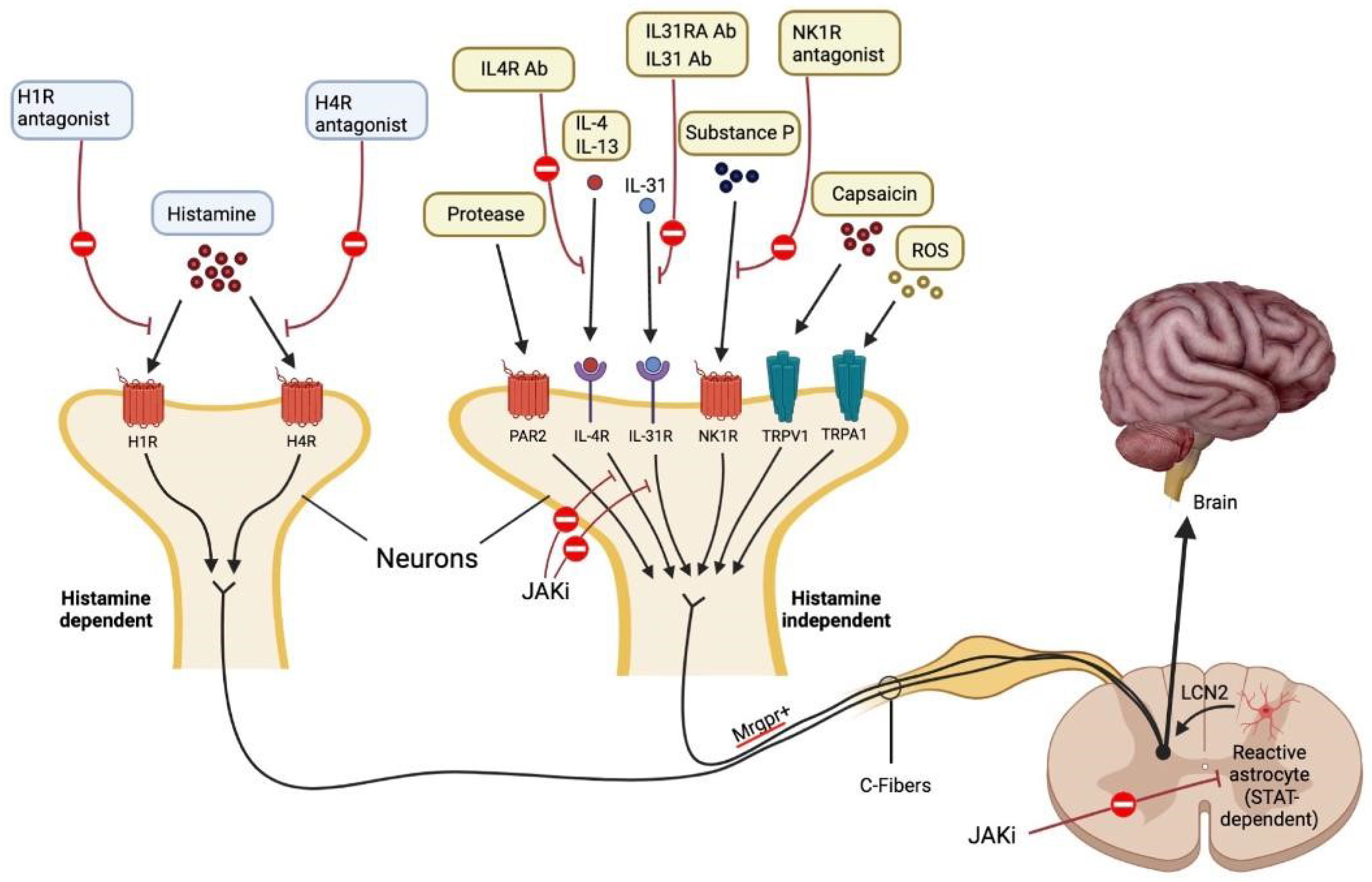
2.1. Histamine-Dependent Pruritogens
2.2. Platelet Activating Factor (PAF)
3. Histamine-Independent Pruritogens
3.1. Protease and (PARs) Protease-Activated Receptors
3.2. Thymic Stromal Lymphopoietin (TSLP) and TSLP Receptor TSLPR
3.3. IL-33
3.4. IL-4 and IL-13
3.5. IL-31
3.6. IL-6
3.7. Endothelin-1 (ET-1)
3.8. Neurotrophins (NTs)
3.9. Neuropeptides
3.10. Toll-like Receptors (TLRs)
3.11. Janus Kinase (JAK)/Signal Transducer and Activator of Transcription (STAT)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AD | Atopic dermatitis |
| IL | Interleukin |
| PAF | Platelet activating factor |
| PARs | Protease-activated receptors |
| HTR7 | 5-Hydroxytryptamine receptor 7 |
| TCS | Topical corticosteroids |
| TNF | Tumor necrosis factor alpha |
| TGF | Transforming growth factor |
| BNP | Brain natriuretic peptide |
| CGRP | Calcitonin gene-related peptide |
| TRPA1 | Transient receptor potential ankyrin 1 |
| KLK | Kallikreins |
| ET-1 | Endothelin-1 |
| BDNF | Brain-derived neurotrophic factor |
| NGF | Nerve growth factor |
| TSLP | Thymic stromal lymphopoietin |
| TLRs | Toll-like receptors |
| VEGF | Vascular endothelial growth factor |
| OSMRβ | Oncostatin M receptor beta |
| PRRs | Pattern recognition receptors |
| PAMPs | Pathogen-associated molecular patterns |
| DAMPS | Damage-associated molecular patterns |
| NTs | Neurotrophins |
| NPY | Neuropeptide Y |
| NK1R | Neurokinin 1 receptor |
| MRGPCRs | Mas-related G protein-coupled receptors |
| GRPR | Gastrin-releasing peptide |
| STAT | Signal transducer and activator of transcription |
| JAK | Janus kinase |
| TYKs | Tyrosine kinases |
| TG | Trigeminal ganglia |
| LCN | Lipocalin |
References
- Spergel, J.M. Epidemiology of Atopic Dermatitis and Atopic March in Children. Immunol. Allergy Clin. N. Am. 2010, 30, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Williams, H.; Robertson, C.; Stewart, A.; Aït-Khaled, N.; Anabwani, G.; Anderson, R.; Asher, I.; Beasley, R.; Björkstén, B.; Burr, M.; et al. Worldwide variations in the prevalence of symptoms of atopic eczema in the international study of asthma and allergies in childhood. J. Allergy Clin. Immunol. 1999, 103, 125–138. [Google Scholar] [CrossRef]
- Odhiambo, J.A.; Williams, H.C.; Clayton, T.O.; Robertson, C.F.; Asher, M.I.; ISAAC Phase Three Study Group. Glob-al variations in prevalence of eczema symptoms in children from ISAAC Phase Three. J. Allergy Clin. Immunol. 2009, 124, 1251–1258.e23. [Google Scholar] [CrossRef]
- Vinding, G.; Zarchi, K.; Ibler, K.; Miller, I.; Ellervik, C.; Jemec, G. Is Adult Atopic Eczema More Common Than We Think?—A Population-based Study in Danish Adults. Acta Derm.-Venereol. 2014, 94, 480–482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rönmark, E.; Ekerljung, L.; Lötvall, J.; Wennergren, G.; Torén, K.; Lundbäck, B. Eczema among adults: Prevalence, risk factors and relation to airway diseases. Results from a large-scale population survey in Sweden. Br. J. Dermatol. 2012, 166, 1301–1308. [Google Scholar] [CrossRef] [PubMed]
- Mortz, C.G.; Andersen, K.E.; Dellgren, C.; Barington, T.; Bindslev-Jensen, C. Atopic dermatitis from adolescence to adulthood in the TOACS cohort: Prevalence, persistence and comorbidities. Allergy 2015, 70, 836–845. [Google Scholar] [CrossRef]
- Wolkewitz, M.; Rothenbacher, D.; Löw, M.; Stegmaier, C.; Ziegler, H.; Radulescu, M.; Brenner, H.; Diepgen, T. Lifetime prevalence of self-reported atopic diseases in a population-based sample of elderly subjects: Results of the ESTHER study. Br. J. Dermatol. 2007, 156, 693–697. [Google Scholar] [CrossRef]
- Sybilski, A.J.; Raciborski, F.; Lipiec, A.; Tomaszewska, A.; Lusawa, A.; Samel-Kowalik, P.; Walkiewicz, A.; Krzych-Fałta, E.; Samoliński, B. Epidemiology of atopic dermatitis in Poland according to the Epidemiology of Allergic Disorders in Poland (ECAP) study. J. Dermatol. 2015, 42, 140–147. [Google Scholar] [CrossRef]
- Saeki, H.; Iizuka, H.; Mori, Y.; Akasaka, T.; Takagi, H.; Kitajima, Y.; Tezuka, T.; Tanaka, T.; Hide, M.; Yamamoto, S.; et al. Prevalence of atopic dermatitis in Japanese elementary schoolchildren. Br. J. Dermatol. 2005, 152, 110–114. [Google Scholar] [CrossRef]
- Sugiura, H.; Umemoto, N.; Deguchi, H.; Murata, Y.; Tanaka, K.; Sawai, T.; Omoto, M.; Uchiyama, M.; Kiriyama, T.; Uehara, M. Preva-lence of childhood and adolescent atopic dermatitis in a Japanese population: Comparison with the disease frequency examined 20 years ago. Acta Derm. Venereol. 1998, 78, 293–294. [Google Scholar] [CrossRef] [Green Version]
- Williamson, S.; Merritt, J.; De Benedetto, A. Atopic dermatitis in the elderly: A review of clinical and pathophysiological hallmarks. Br. J. Dermatol. 2019, 182, 47–54. [Google Scholar] [CrossRef]
- Gerner, T.; Haugaard, J.; Vestergaard, C.; Deleuran, M.; Jemec, G.; Mortz, C.; Agner, T.; Egeberg, A.; Skov, L.; Thyssen, J. Disease severity and trigger factors in Danish children with atopic dermatitis: A nationwide study. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 948–957. [Google Scholar] [CrossRef] [PubMed]
- Sandström, M.H.; Faergemann, J. Prognosis and prognostic factors in adult patients with atopic dermatitis: A long-term follow-up questionnaire study. Br. J. Dermatol. 2004, 150, 103. [Google Scholar] [CrossRef] [PubMed]
- Irvine, A.D.; McLean, W.I.; Leung, D.Y. Filaggrin Mutations Associated with Skin and Allergic Diseases. N. Engl. J. Med. 2011, 365, 1315–1327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van den Oord, R.A.; Sheikh, A. Filaggrin gene defects and risk of developing allergic sensitisation and allergic disorders: Sys-tematic review and meta-analysis. BMJ 2009, 339, b2433. [Google Scholar] [CrossRef] [Green Version]
- Paternoster, L.; Standl, M.; Waage, J.; Baurecht, H.; Hotze, M.; Strachan, D.; Curtin, J.; Bønnelykke, K.; Tian, C.; Takahashi, A.; et al. Multi-ancestry genome-wide association study of 21,000 cases and 95,000 controls identifies new risk loci for atopic dermatitis. Nat. Genet. 2015, 47, 1449–1456. [Google Scholar] [CrossRef]
- Elias, P.M.; Wakefield, J.S. Mechanisms of abnormal lamellar body secretion and the dysfunctional skin barrier in patients with atopic dermatitis. J. Allergy Clin. Immunol. 2014, 134, 781–791.e1. [Google Scholar] [CrossRef] [Green Version]
- Feingold, K.R.; Elias, P.M. Role of lipids in the formation and maintenance of the cutaneous permeability barrier. Biochim. Biophys. Acta (BBA)—Mol. Cell Biol. Lipids 2014, 1841, 280–294. [Google Scholar] [CrossRef]
- Cork, M.J.; Robinson, D.A.; Vasilopoulos, Y.; Ferguson, A.; Moustafa, M.; MacGowan, A.; Duff, G.W.; Ward, S.J.; Tazi-Ahnini, R. New perspectives on epidermal barrier dysfunction in atopic dermatitis: Gene–environment interactions. J. Allergy Clin. Immunol. 2006, 118, 3–21. [Google Scholar] [CrossRef]
- Tanei, R.; Hasegawa, Y. Atopic dermatitis in older adults: A viewpoint from geriatric dermatology. Geriatr. Gerontol. Int. 2016, 16, 75–86. [Google Scholar] [CrossRef]
- Mitsuyama, S.; Higuchi, T. Effectiveness of dupilumab for chronic prurigo in elderly patients with atopic dermatitis. An. Bras. Dermatol. 2023, 98, 86–89. [Google Scholar] [CrossRef] [PubMed]
- Černiauskienė, M.; Bagdonaitė, L.; Karčiauskaitė, D.; Kvedarienė, V. Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase Levels. Med. Sci. Monit. 2022, 28, e937990. [Google Scholar] [CrossRef]
- Jaworek, A.K.; Jaworek, M.; Szafraniec, K.; Pawlicka, A.; Wojas-Pelc, A. Can the blood tryptase be an indicator of the severity of atopic dermatitis? Pol. Merkur. Lekarski. 2020, 48, 162–165. [Google Scholar] [PubMed]
- Yu, L.; Li, L. Potential biomarkers of atopic dermatitis. Front. Med. 2022, 9, 1028694. [Google Scholar] [CrossRef] [PubMed]
- Yue, W.; Cheng, D.; Sun, Z.; Shen, Y.; Wang, S.; Liu, X.; Pei, X.; Deng, S.; Pan, H.; Liao, Z.; et al. Validation of diagnostic criteria for atopic dermatitis and proposal of novel diagnostic criteria for adult and elderly Chinese populations: A multicentre, prospective, clinical setting-based study. Br. J. Dermatol. 2022, 188, 420–426. [Google Scholar] [CrossRef]
- Legat, F.J. Itch in Atopic Dermatitis—What Is New? Front. Med. 2021, 8, 644760. [Google Scholar] [CrossRef] [PubMed]
- Ständer, S.; Simpson, E.L.; Guttman-Yassky, E.; Thyssen, J.P.; Kabashima, K.; Ball, S.G.; Rueda, M.J.; DeLozier, A.M.; Silverberg, J.I. Clinical Relevance of Skin Pain in Atopic Dermatitis. J. Drugs Dermatol. 2020, 19, 921–926. [Google Scholar] [CrossRef]
- Yosipovitch, G.; Berger, T.; Fassett, M. Neuroimmune interactions in chronic itch of atopic dermatitis. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 239–250. [Google Scholar] [CrossRef] [Green Version]
- Wei, W.; Anderson, P.; Gadkari, A.; Blackburn, S.; Moon, R.; Piercy, J.; Shinde, S.; Gomez, J.; Ghorayeb, E. Extent and consequences of inade quate disease control among adults with a history of moderate to severe atopic dermatitis. J. Dermatol. 2018, 45, 150–157. [Google Scholar] [CrossRef] [Green Version]
- Reddy, V.B.; Iuga, A.O.; Shimada, S.G.; LaMotte, R.H.; Lerner, E.A. Cowhage-Evoked Itch Is Mediated by a Novel Cysteine Protease: A Ligand of Protease-Activated Receptors. J. Neurosci. 2008, 28, 4331–4335. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.-G.; Zhao, Z.-Q.; Meng, X.-L.; Yin, J.; Liu, X.-Y.; Chen, Z.-F. Cellular Basis of Itch Sensation. Science 2009, 325, 1531–1534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davidson, S.; Zhang, X.; Yoon, C.H.; Khasabov, S.G.; Simone, D.A.; Giesler, G.J. The Itch-Producing Agents Histamine and Cowhage Activate Separate Populations of Primate Spinothalamic Tract Neurons. J. Neurosci. 2007, 27, 10007–10014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matterne, U.; Strassner, T.; Apfelbacher, C.J.; Diepgen, T.L.; Weisshaar, E. Measuring the prevalence of chronic itch in the general population: Development and validation of a questionnaire for use in large-scale studies. Acta Derm. Venereol. 2009, 89, 250–256. [Google Scholar] [CrossRef] [Green Version]
- Mollanazar, N.K.; Koch, S.D.; Yosipovitch, G. Epidemiology of chronic pruritus: Where have we been and where are we going? Curr. Dermatol. Rep. 2015, 4, 20–29. [Google Scholar] [CrossRef]
- Paller, A.S.; Kabashima, K.; Bieber, T. Therapeutic pipeline for atopic dermatitis: End of the drought? J. Allergy Clin. Immunol. 2017, 140, 633–643. [Google Scholar] [CrossRef] [Green Version]
- Dunford, P.J.; Williams, K.N.; Desai, P.J.; Karlsson, L.; McQueen, D.; Thurmond, R.L. Histamine H4 receptor antagonists are superior to traditional antihistamines in the attenuation of experimental pruritus. J. Allergy Clin. Immunol. 2007, 119, 176–183. [Google Scholar] [CrossRef]
- Werfel, T.; Layton, G.; Yeadon, M.; Whitlock, L.; Osterloh, I.; Jimenez, P.; Liu, W.; Lynch, V.; Asher, A.; Tsianakas, A.; et al. Efficacy and safety of the histamine H4 receptor antagonist ZPL-3893787 in patients with atopic dermatitis. J. Allergy Clin. Immunol. 2019, 143, 1830–1837.e4. [Google Scholar] [CrossRef]
- Wong, L.-S.; Yen, Y.-T.; Lee, C.-H. The Implications of Pruritogens in the Pathogenesis of Atopic Dermatitis. Int. J. Mol. Sci. 2021, 22, 7227. [Google Scholar] [CrossRef]
- Kim, B.S. The translational revolution of itch. Neuron 2022, 110, 2209–2214. [Google Scholar] [CrossRef]
- Gomułka, K.; Wójcik, E.; Szepietowski, J.C. Serum Levels of Eosinophil-Derived Neurotoxin, Platelet-Activating Factor and Vascular Endothelial Growth Factor in Adult Patients with Atopic Dermatitis-A Pilot Study. Biomedicines 2022, 10, 3109. [Google Scholar] [CrossRef]
- Gomułka, K.; Mędrala, W. Serum Levels of Vascular Endothelial Growth Factor, Platelet Activating Factor and Eosinophil-Derived Neurotoxin in Chronic Spontaneous Urticaria-A Pilot Study in Adult Patients. Int. J. Mol. Sci. 2022, 23, 9631. [Google Scholar] [CrossRef] [PubMed]
- Yost, C.C.; Weyrich, A.S.; Zimmerman, G.A. The platelet activating factor (PAF) signaling cascade in systemic inflammatory responses. Biochimie 2010, 92, 692–697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mullol, J.; Bousquet, J.; Bachert, C.; Canonica, G.W.; Gimenez-Arnau, A.; Kowalski, M.L.; Martí-Guadaño, E.; Maurer, M.; Picado, C.; Scadding, G.; et al. Rupatadine in allergic rhinitis and chronic urticaria. Allergy 2008, 63, 5–28. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, T.; Rosen, J.D.; Sanders, K.M.; Yosipovitch, G. Possible roles of basophils in chronic itch. Exp. Dermatol. 2018, 28, 1373–1379. [Google Scholar] [CrossRef]
- Schauberger, E.; Peinhaupt, M.; Cazares, T.; Lindsley, A.W. Lipid Mediators of Allergic Disease: Pathways, Treatments, and Emerging Therapeutic Targets. Curr. Allergy Asthma Rep. 2016, 16, 48. [Google Scholar] [CrossRef] [Green Version]
- Dyer, K.D.; Percopo, C.M.; Xie, Z.; Yang, Z.; Kim, J.D.; Davoine, F.; Lacy, P.; Druey, K.M.; Moqbel, R.; Rosenberg, H.F. Mouse and Human Eosinophils Degranulate in Response to Platelet-Activating Factor (PAF) and LysoPAF via a PAF-Receptor–Independent Mechanism: Evidence for a Novel Receptor. J. Immunol. 2010, 184, 6327–6334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petersen, L.J.; Church, M.K.; Skov, P.S. Platelet-activating factor induces histamine release from human skin mast cells in vivo, which is reduced by local nerve blockade. J. Allergy Clin. Immunol. 1997, 99, 640–647. [Google Scholar] [CrossRef]
- Ocana, J.A.; Romer, E.; Sahu, R.; Pawelzik, S.-C.; FitzGerald, G.A.; Kaplan, M.H.; Travers, J.B. Platelet-Activating Factor–Induced Reduction in Contact Hypersensitivity Responses Is Mediated by Mast Cells via Cyclooxygenase-2–Dependent Mechanisms. J. Immunol. 2018, 200, 4004–4011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hide, M.; Suzuki, T.; Tanaka, A.; Aoki, H. Long-term safety and efficacy of rupatadine in Japanese patients with itching due to chronic spontaneous urticaria, dermatitis, or pruritus: A 12-month, multicenter, open-label clinical trial. J. Dermatol. Sci. 2019, 94, 339–345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nomura, H.; Suganuma, M.; Takeichi, T.; Kono, M.; Isokane, Y.; Sunagawa, K.; Kobashi, M.; Sugihara, S.; Kajita, A.; Miyake, T.; et al. Multifaceted Analyses of Epidermal Serine Protease Activity in Patients with Atopic Dermatitis. Int. J. Mol. Sci. 2020, 21, 913. [Google Scholar] [CrossRef] [Green Version]
- Steinhoff, M.; Neisius, U.; Ikoma, A.; Fartasch, M.; Heyer, G.; Skov, P.S.; Luger, T.A.; Schmelz, M. Proteinase-activated re-ceptor-2 mediates itch: A novel pathway for pruritus in human skin. J. Neurosci. 2003, 23, 6176–6180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Briot, A.; Deraison, C.; Lacroix, M.; Bonnart, C.; Robin, A.; Besson, C.; Dubus, P.; Hovnanian, A. Kallikrein 5 induces atopic dermatitis–like lesions through PAR2-mediated thymic stromal lymphopoietin expression in Netherton syndrome. J. Exp. Med. 2009, 206, 1135–1147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moniaga, C.S.; Jeong, S.K.; Egawa, G.; Nakajima, S.; Hara-Chikuma, M.; Jeon, J.E.; Lee, S.H.; Hibino, T.; Miyachi, Y.; Kabashima, K. Protease Activity Enhances Production of Thymic Stromal Lymphopoietin and Basophil Accumulation in Flaky Tail Mice. Am. J. Pathol. 2013, 182, 841–851. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.J.; Mack, M.R.; Oetjen, L.K.; Trier, A.M.; Council, M.L.; Pavel, A.B.; Guttman-Yassky, E.; Kim, B.S.; Liu, Q. Kallikrein 7 Promotes Atopic Dermatitis-Associated Itch Independently of Skin Inflammation. J. Investig. Dermatol. 2020, 140, 1244–1252.e4. [Google Scholar] [CrossRef]
- Zhu, Y.; Underwood, J.; Macmillan, D.; Shariff, L.; O’Shaughnessy, R.; Harper, J.I.; Pickard, C.; Friedmann, P.S.; Healy, E.; Di, W.-L. Persistent kallikrein 5 activation induces atopic dermatitis-like skin architecture independent of PAR2 activity. J. Allergy Clin. Immunol. 2017, 140, 1310–1322.e5. [Google Scholar] [CrossRef] [Green Version]
- Buhl, T.; Ikoma, A.; Kempkes, C.; Cevikbas, F.; Sulk, M.; Buddenkotte, J.; Akiyama, T.; Crumrine, D.; Camerer, E.; Carstens, E.; et al. Protease-Activated Receptor-2 Regulates Neuro-Epidermal Communication in Atopic Dermatitis. Front. Immunol. 2020, 11, 1740. [Google Scholar] [CrossRef]
- Braz, J.M.; Dembo, T.; Charruyer, A.; Ghadially, R.; Fassett, M.S.; Basbaum, A.I. Genetic priming of sensory neurons in mice that overexpress PAR2 enhances allergen responsiveness. Proc. Natl. Acad. Sci. USA 2021, 118, e2021386118. [Google Scholar] [CrossRef]
- Akiyama, T.; Carstens, M.I.; Carstens, E. Excitation of Mouse Superficial Dorsal Horn Neurons by Histamine and/or PAR-2 Agonist: Potential Role in Itch. J. Neurophysiol. 2009, 102, 2176–2183. [Google Scholar] [CrossRef]
- Amadesi, S.; Nie, J.; Vergnolle, N.; Cottrell, G.S.; Grady, E.F.; Trevisani, M.; Manni, C.; Geppetti, P.; McRoberts, J.A.; Ennes, H.; et al. Protease-Activated Receptor 2 Sensitizes the Capsaicin Receptor Transient Receptor Potential Vanilloid Receptor 1 to Induce Hyperalgesia. J. Neurosci. 2004, 24, 4300–4312. [Google Scholar] [CrossRef] [Green Version]
- Chung, K.; Pitcher, T.; Grant, A.D.; Hewitt, E.; Lindstrom, E.; Malcangio, M. Cathepsin S acts via protease-activated receptor 2 to activate sensory neurons and induce itch-like behaviour. Neurobiol. Pain 2019, 6, 100032. [Google Scholar] [CrossRef]
- Barr, T.P.; Garzia, C.; Guha, S.; Fletcher, E.K.; Nguyen, N.; Wieschhaus, A.J.; Ferrer, L.; Covic, L.; Kuliopulos, A. PAR2 Pepducin-Based Suppression of Inflammation and Itch in Atopic Dermatitis Models. J. Investig. Dermatol. 2019, 139, 412–421. [Google Scholar] [CrossRef] [Green Version]
- Andoh, T.; Kuraishi, Y. Antipruritic mechanisms of topical E6005, a phosphodiesterase 4 inhibitor: Inhibition of responses to proteinase-activated receptor 2 stimulation mediated by increase in intracellular cyclic AMP. J. Dermatol. Sci. 2014, 76, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Varricchi, G.; Pecoraro, A.; Marone, G.; Criscuolo, G.; Spadaro, G.; Genovese, A.; Marone, G. Thymic Stromal Lymphopoietin Isoforms, Inflammatory Disorders, and Cancer. Front. Immunol. 2018, 9, 1595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harada, M.; Hirota, T.; Jodo, A.I.; Doi, S.; Kameda, M.; Fujita, K.; Miyatake, A.; Enomoto, T.; Noguchi, E.; Yoshihara, S.; et al. Functional Analysis of the Thymic Stromal Lymphopoietin Variants in Human Bronchial Epithelial Cells. Am. J. Respir. Cell Mol. Biol. 2009, 40, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Takai, T.; Chen, X.; Okumura, K.; Ogawa, H. Long TSLP transcript expression and release of TSLP induced by TLR ligands and cytokines in human keratinocytes. J. Dermatol. Sci. 2012, 66, 233–237. [Google Scholar] [CrossRef]
- Dong, H.; Hu, Y.; Liu, L.; Zou, M.; Huang, C.; Luo, L.; Yu, C.; Wan, X.; Zhao, H.; Chen, J.; et al. Distinct roles of short and long thymic stromal lymphopoietin isoforms in house dust mite-induced asthmatic airway epithelial barrier disruption. Sci. Rep. 2016, 6, 39559. [Google Scholar] [CrossRef] [Green Version]
- Bjerkan, L.; Sonesson, A.; Schenck, K. Multiple Functions of the New Cytokine-Based Antimicrobial Peptide Thymic Stromal Lymphopoietin (TSLP). Pharmaceuticals 2016, 9, 41. [Google Scholar] [CrossRef]
- Lee, E.B.; Kim, K.W.; Hong, J.Y.; Jee, H.M.; Sohn, M.H.; Kim, K.-E. Increased serum thymic stromal lymphopoietin in children with atopic dermatitis. Pediatr. Allergy Immunol. 2010, 21, e457–e460. [Google Scholar] [CrossRef]
- Takai, T. TSLP Expression: Cellular Sources, Triggers, and Regulatory Mechanisms. Allergol. Int. 2012, 61, 3–17. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, N.; Hanabuchi, S.; Soumelis, V.; Yuan, W.; Ho, S.; de Waal Malefyt, R.; Liu, Y.-J. Human thymic stromal lympho-poietin promotes dendritic cell–mediated CD4+ T cell homeostatic expansion. Nat. Immunol. 2004, 5, 426–434. [Google Scholar] [CrossRef]
- Pattarini, L.; Trichot, C.; Bogiatzi, S.; Grandclaudon, M.; Meller, S.; Keuylian, Z.; Durand, M.; Volpe, E.; Madonna, S.; Cavani, A.; et al. TSLP-activated dendritic cells induce human T follicular helper cell differentiation through OX40-ligand. J. Exp. Med. 2017, 214, 1529–1546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marschall, P.; Wei, R.; Segaud, J.; Yao, W.; Hener, P.; German, B.F.; Meyer, P.; Hugel, C.; Da Silva, G.A.; Braun, R.; et al. Dual function of Langerhans cells in skin TSLP-promoted TFH differentiation in mouse atopic dermatitis. J. Allergy Clin. Immunol. 2020, 147, 1778–1794. [Google Scholar] [CrossRef] [PubMed]
- Tatsuno, K.; Fujiyama, T.; Yamaguchi, H.; Waki, M.; Tokura, Y. TSLP directly interacts with skin-homing Th2 cells highly ex-pressing its receptor to enhance IL-4 production in atopic dermatitis. J. Investig. Derm. 2015, 135, 3017–3024. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.H.; Bae, H.C.; Ko, N.Y.; Lee, S.H.; Jeong, S.H.; Lee, H.; Ryu, W.-I.; Kye, Y.C.; Son, S.W. Thymic stromal lymphopoietin downregulates filaggrin expression by signal transducer and activator of transcription 3 (STAT3) and extracellular sig-nal-regulated kinase (ERK) phosphorylation in keratinocytes. J. Allergy Clin. Immunol. 2015, 136, 205–208.e9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leyva-Castillo, J.M.; Hener, P.; Jiang, H.; Li, M. TSLP Produced by Keratinocytes Promotes Allergen Sensitization through Skin and Thereby Triggers Atopic March in Mice. J. Investig. Dermatol. 2013, 133, 154–163. [Google Scholar] [CrossRef] [Green Version]
- Wilson, S.R.; Thé, L.; Batia, L.M.; Beattie, K.; Katibah, G.E.; McClain, S.P.; Pellegrino, M.; Estandian, D.M.; Bautista, D.M. The Epithelial Cell-Derived Atopic Dermatitis Cytokine TSLP Activates Neurons to Induce Itch. Cell 2013, 155, 285–295. [Google Scholar] [CrossRef] [Green Version]
- Morita, T.; McClain, S.P.; Batia, L.M.; Pellegrino, M.; Wilson, S.R.; Kienzler, M.A.; Lyman, K.; Olsen, A.S.B.; Wong, J.F.; Stucky, C.L.; et al. HTR7 Mediates Serotonergic Acute and Chronic Itch. Neuron 2015, 87, 124–138. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Trier, A.M.; Li, F.; Kim, S.; Chen, Z.; Chai, J.N.; Mack, M.R.; Morrison, S.A.; Hamilton, J.D.; Baek, J.; et al. A basophil-neuronal axis promotes itch. Cell 2021, 184, 422–440.e17. [Google Scholar] [CrossRef]
- Gauvreau, G.M.; O’Byrne, P.M.; Boulet, L.-P.; Wang, Y.; Cockcroft, D.; Bigler, J.; FitzGerald, J.M.; Boedigheimer, M.; Davis, B.E.; Dias, C.; et al. Effects of an Anti-TSLP Antibody on Allergen-Induced Asthmatic Responses. N. Engl. J. Med. 2014, 370, 2102–2110. [Google Scholar] [CrossRef]
- Simpson, E.L.; Parnes, J.R.; She, D.; Crouch, S.; Rees, W.; Mo, M.; van der Merwe, R. Tezepelumab, an anti–thymic stromal lymphopoietin monoclonal antibody, in the treatment of moderate to severe atopic dermatitis: A randomized phase 2a clinical trial. J. Am. Acad. Dermatol. 2019, 80, 1013–1021. [Google Scholar] [CrossRef] [Green Version]
- Simpson, E.L.; Sinclair, R.; Forman, S.; Wollenberg, A.; Aschoff, R.; Cork, M.; Bieber, T.; Thyssen, J.P.; Yosipovitch, G.; Flohr, C.; et al. Efficacy and safety of abrocitinib in adults and adolescents with moderate-to-severe atopic dermatitis (JADE MONO-1): A multicentre, double-blind, randomised, placebo-controlled, phase 3 trial. Lancet 2020, 396, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Silverberg, J.I.; Simpson, E.L.; Thyssen, J.P.; Gooderham, M.; Chan, G.; Feeney, C.; Biswas, P.; Valdez, H.; DiBonaventura, M.; Nduaka, C.; et al. Efficacy and Safety of Abrocitinib in Patients with Moderate-to-Severe Atopic Dermatitis: A Randomized Clinical Trial. JAMA Dermatol. 2020, 156, 863–873. [Google Scholar] [CrossRef] [PubMed]
- Bieber, T.; Simpson, E.L.; Silverberg, J.I.; Thaçi, D.; Paul, C.; Pink, A.E.; Kataoka, Y.; Chu, C.Y.; DiBonaventura, M.; Rojo, R.; et al. Abrocitinib versus Placebo or Dupilumab for Atopic Dermatitis. N. Engl. J. Med. 2021, 384, 1101–1112. [Google Scholar] [CrossRef]
- Gooderham, M.J.; Forman, S.B.; Bissonnette, R.; Beebe, J.S.; Zhang, W.; Banfield, C.; Zhu, L.; Papacharalambous, J.; Vincent, M.S.; Peeva, E. Efficacy and Safety of Oral Janus Kinase 1 Inhibitor Abrocitinib for Patients with Atopic Dermatitis: A Phase 2 Randomized Clinical Trial. JAMA Dermatol. 2019, 155, 1371–1379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simpson, E.L.; Forman, S.; Silverberg, J.I.; Zirwas, M.; Maverakis, E.; Han, G.; Guttman-Yassky, E.; Marnell, D.; Bissonnette, R.; Waibel, J.; et al. Baricitinib in patients with moderate-to-severe atopic dermatitis: Results from a randomized monotherapy phase 3 trial in the United States and Canada (BREEZE-AD5). J. Am. Acad. Dermatol. 2021, 85, 62–70. [Google Scholar] [CrossRef]
- Guttman-Yassky, E.; Silverberg, J.I.; Nemoto, O.; Forman, S.B.; Wilke, A.; Prescilla, R.; de la Peña, A.; Nunes, F.P.; Janes, J.; Gamalo, M.; et al. Baricitinib in adult patients with moderate-to-severe atopic dermatitis: A phase 2 parallel, double-blinded, randomized placebo-controlled multiple-dose study. J. Am. Acad. Dermatol. 2019, 80, 913–921.e9. [Google Scholar] [CrossRef]
- Guttman-Yassky, E.; Thaçi, D.; Pangan, A.L.; Hong, H.C.-H.; Papp, K.A.; Reich, K.; Beck, L.A.; Mohamed, M.-E.F.; Othman, A.A.; Anderson, J.K.; et al. Upadacitinib in adults with moderate to severe atopic dermatitis: 16-week results from a randomized, placebo-controlled trial. J. Allergy Clin. Immunol. 2020, 145, 877–884. [Google Scholar] [CrossRef] [Green Version]
- Reich, K.; Teixeira, H.D.; de Bruin-Weller, M.; Bieber, T.; Soong, W.; Kabashima, K.; Werfel, T.; Zeng, J.; Huang, X.; Hu, X.; et al. Safety and efficacy of upadacitinib in combination with topical corticosteroids in adolescents and adults with moderate-to-severe atopic dermatitis (AD Up): Results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2021, 397, 2169–2181. [Google Scholar] [CrossRef]
- Nakagawa, H.; Nemoto, O.; Igarashi, A.; Saeki, H.; Kabashima, K.; Oda, M.; Nagata, T. Delgocitinib ointment in pediatric patients with atopic dermatitis: A phase 3, randomized, double-blind, vehicle-controlled study and a subsequent open-label, long-term study. J. Am. Acad. Dermatol. 2021, 85, 854–862. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, H.; Nemoto, O.; Igarashi, A.; Saeki, H.; Kaino, H.; Nagata, T. Delgocitinib ointment, a topical Janus kinase inhibitor, in adult patients with moderate to severe atopic dermatitis: A phase 3, randomized, double-blind, vehicle-controlled study and an open-label, long-term extension study. J. Am. Acad. Dermatol. 2020, 82, 823–831. [Google Scholar] [CrossRef] [Green Version]
- Bissonnette, R.; Papp, K.; Poulin, Y.; Gooderham, M.; Raman, M.; Mallbris, L.; Wang, C.; Purohit, V.; Mamolo, C.; Papacharalambous, J.; et al. Topical tofacitinib for atopic dermatitis: A phase II a randomized trial. Br. J. Dermatol. 2016, 175, 902–911. [Google Scholar] [CrossRef] [PubMed]
- Papp, K.; Szepietowski, J.C.; Kircik, L.; Toth, D.; Eichenfield, L.F.; Leung, D.Y.; Forman, S.B.; Venturanza, M.E.; Sun, K.; Kuligowski, M.E.; et al. Efficacy and safety of ruxolitinib cream for the treatment of atopic dermatitis: Results from 2 phase 3, randomized, double-blind studies. J. Am. Acad. Dermatol. 2021, 85, 863–872. [Google Scholar] [CrossRef]
- Wollenberg, A.; Howell, M.D.; Guttman-Yassky, E.; Silverberg, J.I.; Kell, C.; Ranade, K.; Moate, R.; van der Merwe, R. Treatment of atopic dermatitis with tralokinumab, an anti–IL-13 mAb. J. Allergy Clin. Immunol. 2019, 143, 135–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wollenberg, A.; Blauvelt, A.; Guttman-Yassky, E.; Worm, M.; Lynde, C.; Lacour, J.; Spelman, L.; Katoh, N.; Saeki, H.; Poulin, Y.; et al. Tralokinumab for moderate-to-severe atopic dermatitis: Results from two 52-week, randomized, double-blind, multicentre, placebo-controlled phase III trials (ECZTRA 1 and ECZTRA 2). Br. J. Dermatol. 2021, 184, 437–449. [Google Scholar] [CrossRef]
- Silverberg, J.I.; Toth, D.; Bieber, T.; Alexis, A.F.; Elewski, B.E.; Pink, A.E.; Hijnen, D.; Jensen, T.N.; Bang, B.; Olsen, C.K.; et al. Tralokinumab plus topical corticosteroids for the treatment of moderate-to-severe atopic dermatitis: Results from the dou-ble-blind, randomized, multicentre, placebo-controlled phase III ECZTRA 3 trial. Br. J. Derm. 2021, 184, 450–463. [Google Scholar] [CrossRef] [PubMed]
- Gutermuth, J.; Pink, A.; Worm, M.; Soldbro, L.; Øland, C.B.; Weidinger, S. Tralokinumab plus topical corticosteroids in adults with severe atopic dermatitis and inadequate response to or intolerance of ciclosporin A: A placebo-controlled, randomized, phase III clinical trial (ECZTRA 7). Br. J. Dermatol. 2021, 186, 440–452. [Google Scholar] [CrossRef]
- Silverberg, J.I.; Pinter, A.; Pulka, G.; Poulin, Y.; Bouaziz, J.-D.; Wollenberg, A.; Murrell, D.F.; Alexis, A.; Lindsey, L.; Ahmad, F.; et al. Phase 2B randomized study of nemolizumab in adults with moderate-to-severe atopic dermatitis and severe pruritus. J. Allergy Clin. Immunol. 2020, 145, 173–182. [Google Scholar] [CrossRef] [Green Version]
- Kabashima, K.; Matsumura, T.; Komazaki, H.; Kawashima, M. Trial of Nemolizumab and Topical Agents for Atopic Dermatitis with Pruritus. N. Engl. J. Med. 2020, 383, 141–150. [Google Scholar] [CrossRef]
- Kabashima, K.; Matsumura, T.; Komazaki, H.; Kawashima, M.; for the Nemolizumab JP01 and JP02 Study Group. Nemolizumab plus topical agents in patients with atopic dermatitis (AD) and moderate-to-severe pruritus provide improvement in pruritus and signs of AD for up to 68 weeks: Results from two phase III, long-term studies. Br. J. Dermatol. 2021, 186, 642–651. [Google Scholar] [CrossRef]
- Simpson, E.L.; Flohr, C.; Eichenfield, L.F.; Bieber, T.; Sofen, H.; Taïeb, A.; Owen, R.; Putnam, W.; Castro, M.; DeBusk, K.; et al. Efficacy and safety of lebrikizumab (an anti-IL-13 monoclonal antibody) in adults with moderate-to-severe atopic dermatitis inadequately controlled by topical corticosteroids: A randomized, placebo-controlled phase II trial (TREBLE). J. Am. Acad. Dermatol. 2018, 78, 863–871.e11. [Google Scholar] [CrossRef] [Green Version]
- Guttman-Yassky, E.; Blauvelt, A.; Eichenfield, L.F.; Paller, A.S.; Armstrong, A.W.; Drew, J.; Gopalan, R.; Simpson, E.L. Efficacy and safety of Lebrikizumab, a high-affinity interleukin 13 inhibitor, in adults with moderate to severe atopic dermatitis. JAMA Derm. 2020, 156, 411. [Google Scholar] [CrossRef] [Green Version]
- Blauvelt, A.; de Bruin-Weller, M.; Gooderham, M.; Cather, J.C.; Weisman, J.; Pariser, D.; Simpson, E.L.; Papp, K.A.; Hong, H.C.-H.; Rubel, D.; et al. Long-term management of moderate-to-severe atopic dermatitis with dupilumab and concomitant topical corticosteroids (LIBERTY AD CHRONOS): A 1-year, randomised, double-blinded, placebo-controlled, phase 3 trial. Lancet 2017, 389, 2287–2303. [Google Scholar] [CrossRef]
- Welsh, S.E.; Xiao, C.; Kaden, A.R.; Brzezynski, J.L.; Mohrman, M.A.; Wang, J.; Smieszek, S.P.; Przychodzen, B.; Ständer, S.; Polymeropoulos, C.; et al. Neurokinin-1 receptor antagonist tradipitant has mixed effects on itch in atopic dermatitis: Results from EPIONE, a randomized clinical trial. J. Eur. Acad. Derm. Venereol. 2020, 35, e338–e340. [Google Scholar] [CrossRef] [PubMed]
- Kwatra, S.G.; Misery, L.; Clibborn, C.; Steinhoff, M. Molecular and cellular mechanisms of itch and pain in atopic dermatitis and implications for novel therapeutics. Clin. Transl. Immunol. 2022, 11, e1390. [Google Scholar] [CrossRef] [PubMed]
- Imai, Y. Interleukin-33 in atopic dermatitis. J. Dermatol. Sci. 2019, 96, 2–7. [Google Scholar] [CrossRef] [Green Version]
- Meng, J.; Moriyama, M.; Feld, M.; Buddenkotte, J.; Buhl, T.; Szöllösi, A.; Zhang, J.; Miller, P.; Ghetti, A.; Fischer, M.; et al. New mechanism underlying IL-31–induced atopic dermatitis. J. Allergy Clin. Immunol. 2018, 141, 1677–1689.e8. [Google Scholar] [CrossRef] [Green Version]
- Meng, J.; Wang, J.; Buddenkotte, J.; Buhl, T.; Steinhoff, M. Role of SNAREs in Atopic Dermatitis–Related Cytokine Secretion and Skin-Nerve Communication. J. Investig. Dermatol. 2019, 139, 2324–2333. [Google Scholar] [CrossRef]
- Steinhoff, M.; Schmelz, M.; Szabó, I.L.; Oaklander, A.L. Clinical presentation, management, and pathophysiology of neuropathic itch. Lancet Neurol. 2018, 17, 709–720. [Google Scholar] [CrossRef]
- Tamagawa-Mineoka, R.; Okuzawa, Y.; Masuda, K.; Katoh, N. Increased serum levels of interleukin 33 in patients with atopic dermatitis. J. Am. Acad. Dermatol. 2014, 70, 882–888. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, N.; Tamagawa-Mineoka, R.; Yasuike, R.; Masuda, K.; Matsunaka, H.; Murakami, Y.; Yokosawa, E.; Katoh, N. Stratum corneum interleukin-33 expressions correlate with the degree of lichenification and pruritus in atopic dermatitis lesions. Clin. Immunol. 2019, 201, 1–3. [Google Scholar] [CrossRef]
- Murakami-Satsutani, N.; Ito, T.; Nakanishi, T.; Inagaki, N.; Tanaka, A.; Vien, P.T.X.; Kibata, K.; Inaba, M.; Nomura, S. IL-33 Promotes the Induction and Maintenance of Th2 Immune Responses by Enhancing the Function of OX40 Ligand. Allergol. Int. 2014, 63, 443–455. [Google Scholar] [CrossRef] [Green Version]
- Walsh, C.M.; Hill, R.Z.; Schwendinger-Schreck, J.; Deguine, J.; Brock, E.C.; Kucirek, N.; Rifi, Z.; Wei, J.; Gronert, K.; Brem, R.B.; et al. Author response: Neutrophils promote CXCR3-dependent itch in the development of atopic dermatitis. Elife 2019, 8, e48448. [Google Scholar] [CrossRef]
- Liu, B.; Tai, Y.; Achanta, S.; Kaelberer, M.M.; Caceres, A.I.; Shao, X.; Fang, J.; Jordt, S.-E. IL-33/ST2 signaling excites sensory neurons and mediates itch response in a mouse model of poison ivy contact allergy. Proc. Natl. Acad. Sci. USA 2016, 113, E7572–E7579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, L.; Hu, X.; Yang, W.; Yasheng, H.; Liu, S.; Zhang, W.; Zhou, Y.; Cui, W.; Zhu, J.; Qiao, Z.; et al. Spinal IL-33/ST2 signaling mediates chronic itch in mice through the astrocytic JAK2-STAT3 cascade. Glia 2019, 67, 1680–1693. [Google Scholar] [CrossRef]
- Chen, Y.L.; Gutowska-Owsiak, D.; Hardman, C.S.; Westmoreland, M.; MacKenzie, T.; Cifuentes, L.; Waithe, D.; Lloyd-Lavery, A.; Marquette, A.; Londei, M.; et al. Proof-of-concept clinical trial of Etokimab shows a key role for IL-33 in atopic dermatitis pathogenesis. Sci. Transl. Med. 2019, 11, 2945. [Google Scholar] [CrossRef]
- Maurer, M.; Cheung, D.S.; Theess, W.; Yang, X.; Dolton, M.; Guttman, A.; Choy, D.F.; Dash, A.; Grimbaldeston, M.A.; Soong, W. Phase 2 randomized clinical trial of astegolimab in patients with moderate to severe atopic dermatitis. J. Allergy Clin. Immunol. 2022, 150, 1517–1524. [Google Scholar] [CrossRef]
- Weidinger, S.; Beck, L.A.; Bieber, T.; Kabashima, K.; Irvine, A.D. Atopic dermatitis. Nat. Rev. Dis. Primers. 2018, 4, 1. [Google Scholar] [CrossRef] [PubMed]
- Langan, S.M.; Irvine, A.D.; Weidinger, S. Atopic dermatitis. Lancet 2020, 396, 345–360. [Google Scholar] [CrossRef] [PubMed]
- Oetjen, L.K.; Mack, M.R.; Feng, J.; Whelan, T.M.; Niu, H.; Guo, C.J.; Chen, S.; Trier, A.M.; Xu, A.Z.; Tripathi, S.V.; et al. Sensory Neurons Co-opt Classical Immune Signaling Pathways to Mediate Chronic Itch. Cell 2017, 171, 217–228.e13. [Google Scholar] [CrossRef] [Green Version]
- Campion, M.; Smith, L.; Gatault, S.; Métais, C.; Buddenkotte, J.; Steinhoff, M. Interleukin-4 and interleukin-13 evoke scratching behaviour in mice. Exp. Dermatol. 2019, 28, 1501–1504. [Google Scholar] [CrossRef] [Green Version]
- Cevikbas, F.; Wang, X.; Akiyama, T.; Kempkes, C.; Savinko, T.; Antal, A.; Kukova, G.; Buhl, T.; Ikoma, A.; Buddenkotte, J.; et al. A sensory neuron–expressed IL-31 receptor mediates T helper cell–dependent itch: Involvement of TRPV1 and TRPA1. J. Allergy Clin. Immunol. 2014, 133, 448–460.e7. [Google Scholar] [CrossRef] [Green Version]
- Cevikbas, F.; Lerner, E.A. Physiology and Pathophysiology of Itch. Physiol. Rev. 2020, 100, 945–982. [Google Scholar] [CrossRef]
- Howell, M.D.; Kim, B.E.; Gao, P.; Grant, A.V.; Boguniewicz, M.; DeBenedetto, A.; Schneider, L.; Beck, L.A.; Barnes, K.C.; Leung, D.Y. Cytokine modulation of atopic dermatitis filaggrin skin expression. J. Allergy Clin. Immunol. 2007, 120, 150–155. [Google Scholar] [CrossRef] [Green Version]
- Miake, S.; Tsuji, G.; Takemura, M.; Hashimoto-Hachiya, A.; Vu, Y.H.; Furue, M.; Nakahara, T. IL-4 augments IL-31/IL-31 receptor alpha interaction leading to enhanced CCL 17 and CCL 22 production in dendritic cells: Implications for atopic dermatitis. Int. J. Mol. Sci. 2019, 20, 4053. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, T.; Oh, M.H.; Oh, S.Y.; Schroeder, J.T.; Glick, A.B.; Zhu, Z. Transgenic expression of interleukin-13 in the skin induces a pruritic dermatitis and skin remodeling. J. Investig. Derm. 2009, 129, 742–751. [Google Scholar] [CrossRef] [Green Version]
- Gooderham, M.J.; Hong, H.C.-H.; Eshtiaghi, P.; Papp, K.A. Dupilumab: A review of its use in the treatment of atopic dermatitis. J. Am. Acad. Derm. 2018, 78, S28–S36. [Google Scholar] [CrossRef] [PubMed]
- Simpson, E.L.; Bieber, T.; Guttman-Yassky, E.; Beck, L.A.; Blauvelt, A.; Cork, M.J.; Silverberg, J.I.; Deleuran, M.; Kataoka, Y.; Lacour, J.-P.; et al. Two Phase 3 Trials of Dupilumab versus Placebo in Atopic Dermatitis. N. Engl. J. Med. 2016, 375, 2335–2348. [Google Scholar] [CrossRef]
- Pezzolo, E.; Naldi, L. Tralokinumab in the treatment of resistant atopic dermatitis: An open-label, retrospective case series study. J. Eur. Acad. Dermatol. Venereol. 2022; Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Mickevicius, T.; Pink, A.E.; Bhogal, M.B.; O’Brart, D.M.; Robbie, S.J.M. Dupilumab-Induced, Tralokinumab-Induced, and Belantamab Mafodotin–Induced Adverse Ocular Events—Incidence, Etiology, and Management. Cornea, 2022; Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jing, D.; Cheng, J.; Chen, X.; Shen, M.; Liu, H. The efficacy and safety of IL-13 inhibitors in atopic dermatitis: A systematic review and meta-analysis. Front. Immunol. 2022, 13, 923362. [Google Scholar] [CrossRef]
- Labib, A.; Ju, T.; Yosipovitch, G. Managing Atopic Dermatitis with Lebrikizumab—The Evidence to Date. Clin. Cosmet. Investig. Dermatol. 2022, 15, 1065–1072. [Google Scholar] [CrossRef]
- Miron, Y.; Miller, P.E.; Hughes, C.; Indersmitten, T.; Lerner, E.A.; Cevikbas, F. Mechanistic insights into the antipruritic effects of lebrikizumab, an anti–IL-13 mAb. J. Allergy Clin. Immunol. 2022, 150, 690–700. [Google Scholar] [CrossRef]
- Maier, E.; Mittermeir, M.; Ess, S.; Neuper, T.; Schmiedlechner, A.; Duschl, A.; Horejs-Hoeck, J. Prerequisites for Functional Interleukin 31 Signaling and Its Feedback Regulation by Suppressor of Cytokine Signaling 3 (SOCS3). J. Biol. Chem. 2015, 290, 24747–24759. [Google Scholar] [CrossRef] [Green Version]
- Park, K.; Park, J.-H.; Yang, W.-J.; Lee, J.-J.; Song, M.-J.; Kim, H.-P. Transcriptional activation of theIL31gene by NFAT and STAT6. J. Leukoc. Biol. 2012, 91, 245–257. [Google Scholar] [CrossRef]
- Dillon, S.R.; Sprecher, C.; Hammond, A.; Bilsborough, J.; Rosenfeld-Franklin, M.; Presnell, S.R.; Haugen, H.S.; Maurer, M.; Harder, B.; Johnston, J.; et al. Interleukin 31, a cytokine produced by activated T cells, induces dermatitis in mice. Nat. Immunol. 2004, 5, 752–760. [Google Scholar] [CrossRef]
- Saleem, M.D.; Oussedik, E.; D’Amber, V.; Feldman, S.R. Interleukin-31 pathway and its role in atopic dermatitis: A systematic review. J. Dermatol. Treat. 2017, 28, 591–599. [Google Scholar] [CrossRef]
- Tatu, A.L.; Nadasdy, T.; Arbune, A.; Chioncel, V.; Bobeica, C.; Niculet, E.; Iancu, A.V.; Dumitru, C.; Popa, V.T.; Kluger, N.; et al. Interrelationship and Sequencing of Interleukins4, 13, 31, and 33—An Integrated Systematic Review: Dermatological and Multidisciplinary Perspectives. J. Inflamm. Res. 2022, 15, 5163–5184. [Google Scholar] [CrossRef] [PubMed]
- Diveu, C.; Lelièvre, E.; Perret, D.; Lak-Hal, A.-H.L.; Froger, J.; Guillet, C.; Chevalier, S.; Rousseau, F.; Wesa, A.; Preisser, L.; et al. GPL, a Novel Cytokine Receptor Related to GP130 and Leukemia Inhibitory Factor Receptor. J. Biol. Chem. 2003, 278, 49850–49859. [Google Scholar] [CrossRef] [Green Version]
- Diveu, C.; Lak-Hal, A.-H.L.; Froger, J.; Ravon, E.; Grimaud, L.; Barbier, F.; Hermann, J.; Gascan, H.; Chevalier, S. Predominant expression of the long isoform of GP130-like (GPL) receptor is required for interleukin-31 signaling. Eur. Cytokine Netw. 2004, 15, 291–302. [Google Scholar] [PubMed]
- Feld, M.; Garcia, R.; Buddenkotte, J.; Katayama, S.; Lewis, K.; Muirhead, G.; Hevezi, P.; Plesser, K.; Schrumpf, H.; Krjutskov, K.; et al. The pruritus- and TH2-associated cytokine IL-31 promotes growth of sensory nerves. J. Allergy Clin. Immunol. 2016, 138, 500–508.e24. [Google Scholar] [CrossRef] [Green Version]
- Liang, J.; Hu, F.; Dan, M.; Sang, Y.; Abulikemu, K.; Wang, Q.; Hong, Y.; Kang, X. Safety and Efficacy of Nemolizumab for Atopic Dermatitis with Pruritus: A Systematic Review and Meta-Regression Analysis of Randomized Controlled Trials. Front. Immunol. 2022, 13, 825312. [Google Scholar] [CrossRef]
- Tan, X.L.; Thomas, B.R.; Tan, Y.J.; O’Toole, E.A. Effects of systemic therapies on pruritus in adults with atopic dermatitis: A systematic review and meta-analysis. Clin. Exp. Dermatol. 2022, 47, 658–666. [Google Scholar] [CrossRef] [PubMed]
- Akira, S.; Taga, T.; Kishimoto, T. Interleukin-6 in Biology and Medicine. Adv Immunol. 1993, 54, 1–78. [Google Scholar] [CrossRef]
- Choy, E.H.; De Benedetti, F.; Takeuchi, T.; Hashizume, M.; John, M.R.; Kishimoto, T. Translating IL-6 biology into effective treatments. Nat. Rev. Rheumatol. 2020, 16, 335–345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirano, T. Interleukin 6 and its Receptor: Ten Years Later. Int. Rev. Immunol. 1998, 16, 249–284. [Google Scholar] [CrossRef]
- Avci, A.B.; Feist, E.; Burmester, G.R. Targeting IL-6 or IL-6 Receptor in Rheumatoid Arthritis: What’s the Difference? BioDrugs 2018, 32, 531–546. [Google Scholar] [CrossRef] [PubMed]
- Heink, S.; Yogev, N.; Garbers, C.; Herwerth, M.; Aly, L.; Gasperi, C.; Husterer, V.; Croxford, A.L.; Möller-Hackbarth, K.; Bartsch, H.S.; et al. Trans-presentation of IL-6 by dendritic cells is required for the priming of pathogenic TH17 cells. Nat. Immunol. 2017, 18, 74–85. [Google Scholar] [CrossRef] [Green Version]
- Okuda, Y. Review of tocilizumab in the treatment of rheumatoid arthritis. Biol. Targets Ther. 2008, 2, 75–82. [Google Scholar] [CrossRef] [Green Version]
- Toshitani, A.; Ansel, J.C.; Chan, S.C.; Li, S.H.; Hanifin, J.M. Increased interleukin 6 production by T cells derived from pa-tients with atopic dermatitis. J. Investig. Derm. 1993, 100, 293–304. [Google Scholar]
- Conti, P.; Kempuraj, D.; Di Gioacchino, M.; Boucher, W.; Letourneau, R.; Kandere, K.; Barbacane, R.C.; Reale, M.; Felaco, M.; Frydas, S.; et al. Interleukin-6 and mast cells. Allergy Asthma Proc. 2002, 23, 331–335. [Google Scholar]
- Fedenko, E.S.; Elisyutina, O.G.; Filimonova, T.M.; Boldyreva, M.N.; Burmenskaya, O.V.; Rebrova, O.Y.; Yarilin, A.A.; Khaitov, R.M. Cytokine gene expression in the skin and peripheral blood of atopic dermatitis patients and healthy individuals. Self/Nonself 2011, 2, 120–124. [Google Scholar] [CrossRef] [Green Version]
- Niculet, E.; Chioncel, V.; Elisei, A.M.; Miulescu, M.; Buzia, O.D.; Nwabudike, L.C.; Craescu, M.; Draganescu, M.; Bujoreanu, F.; Marinescu, E.; et al. Multifactorial expression of IL-6 with update on COVID-19 and the therapeutic strategies of its blockade (Review). Exp. Ther. Med. 2021, 21, 263. [Google Scholar] [CrossRef]
- Navarini, A.A.; French, L.E.; Hofbauer, G.F. Interrupting IL-6–receptor signaling improves atopic dermatitis but associates with bacterial superinfection. J. Allergy Clin. Immunol. 2011, 128, 1128–1130. [Google Scholar] [CrossRef]
- Konda, D.; Chandrashekar, L.; Rajappa, M.; Kattimani, S.; Thappa, D.M.; Ananthanarayanan, P.H. Serotonin and interleukin-6: Association with pruritus severity, sleep quality and depression severity in prurigo nodularis. Asian J. Psychiatr. 2015, 17, 24–28. [Google Scholar] [CrossRef]
- Keshari, S.; Sipayung, A.D.; Hsieh, C.C.; Su, L.J.; Chiang, Y.R.; Chang, H.C.; Yang, W.C.; Chuang, T.H.; Chen, C.L.; Huang, C.M. IL-6/p-BTK/P-ERK signaling mediates calcium phosphate-induced pruritus. FASEB J. 2019, 33, 12036–12046. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakahara, T.; Kido-Nakahara, M.; Ohno, F.; Ulzii, D.; Chiba, T.; Tsuji, G.; Furue, M. The pruritogenic mediator endothelin-1 shifts the dendritic cell-T-cell response toward Th17/Th1 polarization. Allergy 2018, 73, 511–515. [Google Scholar] [CrossRef]
- Gomes, L.O.; Hara, D.B.; Rae, G.A. Endothelin-1 induces itch and pain in the mouse cheek model. Life Sci. 2012, 91, 628–633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kido-Nakahara, M.; Wang, B.; Ohno, F.; Tsuji, G.; Ulzii, D.; Takemura, M.; Furue, M.; Nakahara, T. Inhibition of mite-induced dermatitis, pruritus, and nerve sprouting in mice by the endothelin receptor antagonist bosentan. Allergy 2021, 76, 291–301. [Google Scholar] [CrossRef]
- Kido-Nakahara, M.; Buddenkotte, J.; Kempkes, C.; Ikoma, A.; Cevikbas, F.; Akiyama, T.; Nunes, F.; Seeliger, S.; Hasdemir, B.; Mess, C.; et al. Neural peptidase endothelin-converting enzyme 1 regulates endothelin 1–induced pruritus. J. Clin. Investig. 2014, 124, 2683–2695. [Google Scholar] [CrossRef] [Green Version]
- Aktar, M.K.; Kido-Nakahara, M.; Furue, M.; Nakahara, T. Mutual upregulation of endothelin-1 and IL-25 in atopic dermatitis. Allergy 2015, 70, 846–854. [Google Scholar] [CrossRef] [PubMed]
- Tsybikov, N.N.; Petrisheva, I.V.; Kuznik, B.I.; Magen, E. Plasma endothelin-1 levels during exacerbation of atopic dermatitis. Allergy Asthma Proc. 2015, 36, 320–324. [Google Scholar] [CrossRef] [PubMed]
- Wong, L.-S.; Yen, Y.-T.; Lin, S.-H.; Lee, C.-H. IL-17A Induces Endothelin-1 Expression through p38 Pathway in Prurigo Nodularis. J. Investig. Dermatol. 2020, 140, 702–706.e2. [Google Scholar] [CrossRef] [PubMed]
- Nockher, W.A.; Renz, H. Neurotrophins in allergic diseases: From neuronal growth factors to intercellular signaling molecules. J. Allergy Clin. Immunol. 2006, 117, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Dou, Y.-C.; Hagströmer, L.; Emtestam, L.; Johansson, O. Increased nerve growth factor and its receptors in atopic dermatitis: An immunohistochemical study. Arch. Dermatol. Res. 2006, 298, 31–37. [Google Scholar] [CrossRef]
- Toyoda, M.; Nakamura, M.; Makino, T.; Hino, T.; Kagoura, M.; Morohashi, M. Nerve growth factor and substance P are useful plasma markers of disease activity in atopic dermatitis. Br. J. Derm. 2002, 147, 71–79. [Google Scholar] [CrossRef]
- Papoiu, A.D.; Wang, H.; Nattkemper, L.; Tey, H.L.; Ishiuji, Y.; Chan, Y.-H.; Schmelz, M.; Yosipovitch, G. A study of serum concentrations and dermal levels of NGF in atopic dermatitis and healthy subjects. Neuropeptides 2011, 45, 417–422. [Google Scholar] [CrossRef] [Green Version]
- Schulte-Herbrüggen, O.; Fölster-Holst, R.; von Elstermann, M.; Augustin, M.; Hellweg, R. Clinical Relevance of Nerve Growth Factor Serum Levels in Patients with Atopic Dermatitis and Psoriasis. Int. Arch. Allergy Immunol. 2007, 144, 211–216. [Google Scholar] [CrossRef]
- Wala-Zielińska, K.; Świerczyńska-Mróz, K.; Krajewski, P.K.; Nowicka-Suszko, D.; Krajewska, M.; Szepietowski, J.C. Elevated Level of Serum Neurotrophin-4, but Not of Brain-Derived Neurotrophic Factor, in Patients with Chronic Kidney Disease-Associated Pruritus. J. Clin. Med. 2022, 11, 6292. [Google Scholar] [CrossRef]
- Roblin, D.; Yosipovitch, G.; Boyce, B.; Robinson, J.; Sandy, J.; Mainero, V.; Wickramasinghe, R.; Anand, U.; Anand, P. Topical TrkA kinase inhibitor CT327 is an effective, novel therapy for the treatment of pruritus due to psoriasis: Results from experimentalstudies, and efficacy and safety of CT327 in a phase 2b clinical trial in patients with psoriasis. Acta Derm. Venereol. 2015, 95, 542–548. [Google Scholar] [CrossRef] [Green Version]
- Kabata, H.; Artis, D. Neuro-immune crosstalk and allergic inflammation. J. Clin. Investig. 2019, 129, 1475–1482. [Google Scholar] [CrossRef] [Green Version]
- Suvas, S. Role of Substance P Neuropeptide in Inflammation, Wound Healing, and Tissue Homeostasis. J. Immunol. 2017, 199, 1543–1552. [Google Scholar] [CrossRef] [Green Version]
- Mashaghi, A.; Marmalidou, A.; Tehrani, M.; Grace, P.M.; Pothoulakis, C.; Dana, R. Neuropeptide Substance P and the Immune Response. Cell Mol. Life Sci. 2016, 73, 4249–4264. [Google Scholar] [CrossRef] [Green Version]
- Meixiong, J.; Dong, X. Mas-Related G Protein–Coupled Receptors and the Biology of Itch Sensation. Annu. Rev. Genet. 2017, 51, 105–121. [Google Scholar] [CrossRef]
- Lönndahl, L.; Rasul, A.; Lonne-Rahm, S.-B.; Holst, M.; Johansson, B.; El-Nour, H.; Djurfeldt, D.R.; Nordlind, K. Tachykinin upregulation in atopic dermatitis. Immunopharmacol. Immunotoxicol. 2019, 41, 117–122. [Google Scholar] [CrossRef]
- Paramita, D.A.; Nasution, K.; Lubis, N.Z. Relationship of Substance P with the Degree of Atopic Dermatitis Severity. Clin. Cosmet. Investig. Dermatol. 2021, 14, 551–555. [Google Scholar] [CrossRef] [PubMed]
- Ständer, S.; Siepmann, D.; Herrgott, I.; Sunderkötter, C.; Luger, T.A. Targeting the Neurokinin Receptor 1 with Aprepitant: A Novel Antipruritic Strategy. PLoS ONE 2010, 5, e10968. [Google Scholar] [CrossRef]
- Lönndahl, L.; Holst, M.; Bradley, M.; Killasli, H.; Heilborn, J.; Hall, M.; Theodorsson, E.; Holmberg, J.; Nordlind, K. Sub-stance P antagonist aprepitant shows no additive effect compared with standardized topical treatment alone in patients with atopic dermatitis. Acta. Derm. Venereol. 2018, 98, 324–328. [Google Scholar] [CrossRef] [Green Version]
- Granstein, R.D.; Wagner, J.A.; Stohl, L.L.; Ding, W. Calcitonin gene-related peptide: Key regulator of cutaneous immunity. Acta Physiol. 2015, 213, 586–594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, X.; Wang, L.; Clark, J.D.; Kingery, W.S. Keratinocytes express cytokines and nerve growth factor in response to neuropeptide activation of the ERK1/2 and JNK MAPK transcription pathways. Regul. Pept. 2013, 186, 92–103. [Google Scholar] [CrossRef] [Green Version]
- Ding, W.; Stohl, L.L.; Wagner, J.A.; Granstein, R.D. Calcitonin Gene-Related Peptide Biases Langerhans Cells toward Th2-Type Immunity. J. Immunol. 2008, 181, 6020–6026. [Google Scholar] [CrossRef] [Green Version]
- Järvikallio, A.; Harvima, I.T.; Naukkarinen, A. Mast cells, nerves and neuropeptides in atopic dermatitis and nummular eczema. Arch. Derm. Res. 2003, 295, 2–7. [Google Scholar] [CrossRef]
- Hodeib, A.; EI-Samad, Z.A.; Hanafy, H.; EI-Latief, A.A.; EI-Bendary, A.; Abu-Raya, A. Nerve growth factor, neuropeptides and cutaneous nerves in atopic dermatitis. Indian J. Derm. 2010, 55, 135–139. [Google Scholar] [PubMed]
- Katsuno, M.; Aihara, M.; Kojima, M.; Osuna, H.; Hosoi, J.; Nakamura, M.; Toyoda, M.; Matsuda, H.; Ikezawa, Z. Neuropeptides concentrations in the skin of a murine (NC/Nga mice) model of atopic dermatitis. J. Derm. Sci. 2003, 33, 55–65. [Google Scholar] [CrossRef]
- Umemoto, N.; Kakurai, M.; Okazaki, H.; Kiyosawa, T.; Demitsu, T.; Nakagawa, H. Serum levels of vasoactive intestinal peptide are elevated in patients with atopic dermatitis. J. Derm. Sci. 2003, 31, 161–164. [Google Scholar] [CrossRef]
- Teresiak-Mikołajczak, E.; Czarnecka-Operacz, M.; Jenerowicz, D.; Silny, W. Neurogenic markers of the inflammatory process in atopic dermatitis: Relation to the severity and pruritus. Adv. Dermatol. Allergol. 2013, 5, 286–292. [Google Scholar] [CrossRef]
- Ganea, D.; Hooper, K.M.; Kong, W. The neuropeptide vasoactive intestinal peptide: Direct effects on immune cells and involvement in inflammatory and autoimmune diseases. Acta Physiol. 2015, 213, 442–452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oda, N.; Miyahara, N.; Taniguchi, A.; Morichika, D.; Senoo, S.; Fujii, U.; Itano, J.; Gion, Y.; Kiura, K.; Kanehiro, A.; et al. Requirement for neuropeptide Y in the development of type 2 responses and allergen-induced airway hyperresponsiveness and inflammation. Am. J. Physiol. Lung Cell Mol. Physiol. 2019, 316, L407–L417. [Google Scholar] [CrossRef] [Green Version]
- Lou, H.; Lu, J.; Choi, E.B.; Oh, M.H.; Jeong, M.; Barmettler, S.; Zhu, Z.; Zheng, T. Expression of IL-22 in the Skin Causes Th2-Biased Immunity, Epidermal Barrier Dysfunction, and Pruritus via Stimulating Epithelial Th2 Cytokines and the GRP Pathway. J. Immunol. 2017, 198, 2543–2555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tirado-Sánchez, A.; Bonifaz, A.; Ponce-Olivera, R. Serum gastrin-releasing peptide levels correlate with disease severity and pruritus in patients with atopic dermatitis. Br. J. Dermatol. 2015, 173, 298–300. [Google Scholar] [CrossRef]
- Sun, L.; Liu, W.; Zhang, L.-J. The Role of Toll-Like Receptors in Skin Host Defense, Psoriasis, and Atopic Dermatitis. J. Immunol. Res. 2019, 2019, 1824624. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, H.-C.; Feng, C.; Yan, M. Analysis of the Association of Polymorphisms rs5743708 in TLR2 and rs4986790 in TLR4 with Atopic Dermatitis Risk. Immunol. Investig. 2018, 48, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Lin, D.; Cai, X.; Cui, D.; Fang, R.; Zhang, W.; Yu, B.; Wang, X. Enhancement of Chemokine mRNA Expression by Toll-Like Receptor 2 Stimulation in Human Peripheral Blood Mononuclear Cells of Patients with Atopic Dermatitis. BioMed Res. Int. 2020, 2020, 1497175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakamura, N.; Tamagawa-Mineoka, R.; Ueta, M.; Konishi, E.; Yasuike, R.; Masuda, K.; Matsunaka, H.; Murakami, Y.; Yokosawa, E.; Katoh, N. Stratum corneum Toll-like receptor 3 expressions correlate with the severity of atopic dermatitis lesions. J. Dermatol. Sci. 2019, 94, 354–357. [Google Scholar] [CrossRef] [Green Version]
- Szöllősi, A.G.; McDonald, I.; Szabó, I.L.; Meng, J.; Bogaard, E.V.D.; Steinhoff, M. TLR3 in Chronic Human Itch: A Keratinocyte-Associated Mechanism of Peripheral Itch Sensitization. J. Investig. Dermatol. 2019, 139, 2393–2396.e6. [Google Scholar] [CrossRef] [Green Version]
- Yasuike, R.; Tamagawa-Mineoka, R.; Ueta, M.; Nakamura, N.; Kinoshita, S.; Katoh, N. The role of toll-like receptor 3 in chronic contact hypersensitivity induced by repeated elicitation. J. Dermatol. Sci. 2017, 88, 184–191. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.; Berta, T.; Xu, Z.-Z.; Park, C.-K.; Zhang, L.; Lü, N.; Liu, Q.; Liu, Y.; Gao, Y.-J.; Liu, Y.-C.; et al. TLR3 deficiency impairs spinal cord synaptic transmission, central sensitization, and pruritus in mice. J. Clin. Investig. 2012, 122, 2195–2207. [Google Scholar] [CrossRef]
- Liu, T.; Xu, Z.-Z.; Park, C.-K.; Berta, T.; Ji, R.-R. Toll-like receptor 7 mediates pruritus. Nat. Neurosci. 2010, 13, 1460–1462. [Google Scholar] [CrossRef]
- Kim, S.; Park, G.; Kim, D.; Lee, J.; Min, H.; Wall, E.; Lee, C.J.; Simon, M.I.; Lee, S.J.; Han, S.K. Analysis of cellular and behavioral responses to imiquimod reveals a unique itch pathway in transient receptor potential vanilloid 1 (TRPV1)-expressing neurons. Proc. Natl. Acad. Sci. USA 2011, 108, 3371–3376. [Google Scholar] [CrossRef] [Green Version]
- Moniaga, C.S.; Tominaga, M.; Takamori, K. An Altered Skin and Gut Microbiota Are Involved in the Modulation of Itch in Atopic Dermatitis. Cells 2022, 11, 3930. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, T.; Niu, J.; Xiao, J.; Zhang, M.; Zhang, R.; Chen, D.; Shi, Y.; Zhang, X.; Hu, X.; et al. Inhibitory effects of antibiotic-induced gut microbiota depletion on acute itch behavior in mice. Brain Res. Bull. 2022, 190, 50–61. [Google Scholar] [CrossRef]
- Ghoreschi, K.; Laurence, A.; O’Shea, J.J. Janus kinases in immune cell signaling. Immunol. Rev. 2009, 228, 273–287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shiratori-Hayashi, M.; Koga, K.; Tozaki-Saitoh, H.; Kohro, Y.; Toyonaga, H.; Yamaguchi, C.; Hasegawa, A.; Nakahara, T.; Hachisuka, J.; Akira, S.; et al. STAT3-dependent reactive astrogliosis in the spinal dorsal horn underlies chronic itch. Nat. Med. 2015, 21, 927–931. [Google Scholar] [CrossRef] [PubMed]
- Villarino, A.V.; Kanno, Y.; O’Shea, J.J. Mechanisms and consequences of Jak–STAT signaling in the immune system. Nat. Immunol. 2017, 18, 374–384. [Google Scholar] [CrossRef]
- Karaman, M.W.; Herrgard, S.; Treiber, D.K.; Gallant, P.; Atteridge, C.E.; Campbell, B.T.; Chan, K.W.; Ciceri, P.; Davis, M.I.; Edeen, P.T.; et al. A quantitative analysis of kinase inhibitor selectivity. Nat. Biotechnol. 2008, 26, 127–132. [Google Scholar] [CrossRef]
- Sadeghi, S.; Mohandesi, N.A. Efficacy and safety of topical JAK inhibitors in the treatment of atopic dermatitis in paediatrics and adults: A systematic review. Exp. Dermatol. 2023; Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Hoy, S.M. Ruxolitinib Cream 1.5%: A Review in Mild to Moderate Atopic Dermatitis. Am. J. Clin. Dermatol. 2023, 24, 143–151. [Google Scholar] [CrossRef]
- Roy, Y.R.-L.; Ficheux, A.-S.; Misery, L.; Brenaut, E. Efficacy of topical and systemic treatments for atopic dermatitis on pruritus: A systematic literature review and meta-analysis. Front. Med. 2022, 9, 1079323. [Google Scholar] [CrossRef]
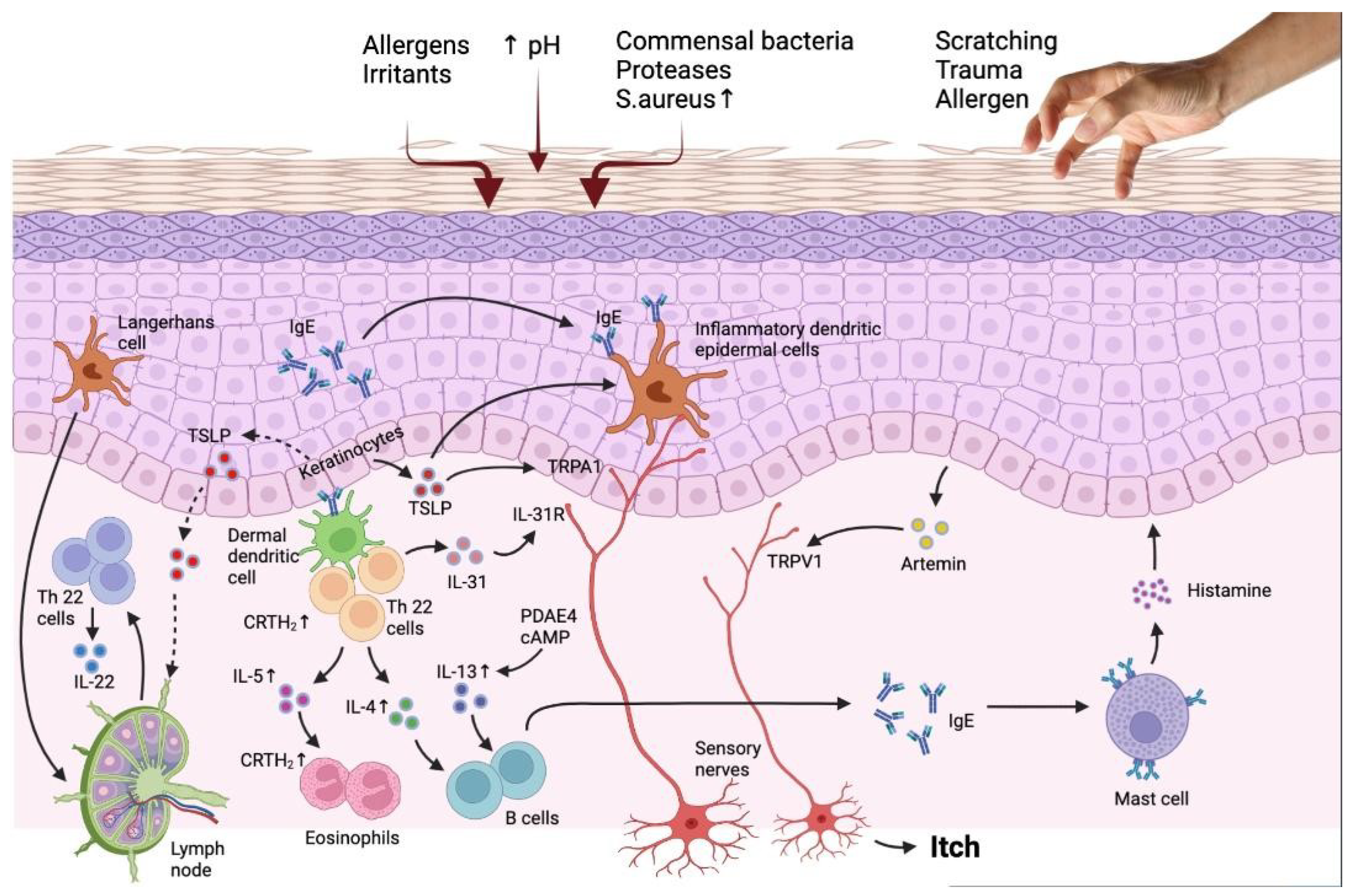
| Mediator | Origin | Overall Mechanism | Receptor |
|---|---|---|---|
| Histamine | Mast cells (MCs), basophils | Histamine provokes pruritus through H1R and H4R. | It has many activities via four receptors (H1R–H4R). |
| Platelet Activating Factor (PAF) | MCs, eosinophils, basophils, neutrophils and epithelial cells | PAF boosts the immune response by causing immune cells like eosinophils and MCs to degranulate, and trigger chemotaxis and adhesion. PAF injection intradermally might cause wheal and irritation. | Platelet-activating factor receptor (PAF-R) |
| Protease and (PARs) Protease-Activated Receptors | Keratinocytes and immune cells | Through neurons that express BLT1, the activation of PAR-2 on keratinocytes causes the release of LTB4, which causes itching. | PAR1, PAR2, PAR3, and PAR4. In AD, the functional roles of PAR2 have been characterized in greater detail. |
| Thymic Stromal Lymphopoietin (TSLP) | TSLP is a cytokine produced by keratinocytes. | Raised levels of TSLP are expressed in keratinocytes of lesional skin and serum of patients with atopic dermatitis. | IL-7Rα, TSLPR |
| IL-33 | It functions as an alarmin and is swiftly released from the keratinocyte nucleus in response to pathogen provocation or epidermal barrier disruption. | IL-33 communicates via IL-33 Receptor (ST2) | ST2 |
| IL-4 | CD4+ T cells, basophils, eosinophils | Enhances IL-31/IL-31 receptor α signaling. It amplifies Th2 inflammation and increases the production of IgE. | IL-13 receptor α1 chain, IL-13 receptor α2 chain |
| IL-13 | CD4+ T cells, basophils, eosinophils | The ability IL-13 IS to sensitize sensory nerves to itch by reducing the sensitivity thresholds to other pruritogenic stimuli | IL-4 receptor type II (IL-4RII) |
| IL-31 | Th2 cells | Signals via IL-31RA, Interleukin-31 receptor α chain and OSM Receptor (OSMR) β chain. | Oncostatin M receptor beta (OSMRβ) and the IL-31 receptor alpha (Il-31RA |
| IL-6 | It is released by activated T cells and mast cells (MCs). | Mast cells (MCs) and activated T cells produce IL-6 and it has been shown that is highly expressed in the skin and T cells of AD patients. | IL-6R alpha (IL-6Ra) and the 130 kD glycoprotein 130 (gp130) non-ligand binding chain |
| Endothelin-1 (ET-1) | It is produced by vascular endothelial cells. | Serum ET-1 levels are elevated and associated with itch intensity, serum IgE levels, and the severity of AD. | ETAR, ETBR |
| Neurotrophins (NTs) | Keratinocytes in the skin | Hyperinnervation, peripheral sensitization, and pruritus in atopic dermatitis. | TrkA, TrkB, and TrkC (tyrosine kinases) |
| Drug | Therapeutic Target | Mode of Administration | Type of Study | Dosing and Intervention | Duration in Weeks | Number of Participants (Medication/Placebo) | Age of Participants | Outcome Measure | Effect on Pruritus |
|---|---|---|---|---|---|---|---|---|---|
| Abrocitinib | JAK1 selective inhibitor | oral | |||||||
| Simpson E.L. et al. Lancet 2020 [81] | JAK1 selective inhibitor | A multicenter, double-blind, randomized phase 3 trial (JADE MONO-1) | Abrocitinib 200 mg and 100 mg per os daily monotherapy and placebo | 12 week study | Total 387 participants, abrocitinib 100 mg daily (n = 156), abrocitinib 200 mg (n = 154), placebo (n = 77) | Adolescents and adults | Peak Pruritus Numerical Rating Scale [PP-NRS] score, ranges from 0 to 10 | PP-NRS response at week 8 Abrocitinib 100 mg: 50/147 (34%). Percentage difference compared to placebo (95% CI) 20·0 (7·4 to 32·7), abrocitinib 200 mg: 88/147 (60%), percentage difference compared to placebo (95% CI) 45·3 (32·7 to 57·8), a significant, rapid (i.e., within 2 days) reduction in pruritus severity and other atopic dermatitis symptoms were also observed between treatment initiation and week 12. The median time to PPNRS response was 84⋯0 days (IQR 10⋯0—not evaluable [NE]) in the abrocitinib 100 mg group, 14 · 0 days (6⋯0–84⋯0) in the abrocitinib 200 mg group, and 92⋯0 days (29⋯0—NE) in the placebo group. | |
| Silverberg J.I., JAMA Dermatol. 2020 [82] | Phase 3, double-blinded, placebo-controlled, parallel-group randomized clinical trial | Abrocitinib 200 mg and 100 mg per os daily monotherapy and placebo | 12-week study | 391 in total, placebo (n = 78), abrocitinib 100 mg (n = 158), abrocitinib 200 mg (n = 155) | 12 years old and older, adolescents and adults | PP-NRS, Peak Pruritus Numerical Rating Scale, ranges from 0 to 10; PSAAD, Pruritus and Symptoms Assessment for Atopic Dermatitis | Response was achieved in the 200 mg group by 35.3% of patients at week 2, 52.8% at week 4, and 55.3% at week 12 and in the 100-mg group by 23.1% at week 2, 33.4% at week 4, and 45.2%at week 12 compared to 11.5% of the placebo group at week 12. | ||
| Bieber T., N. Engl. J. Med., 2021 [83] | Phase 3, double-blind trial | (2:2:2:1 ratio) Abrocitinib 200 mg or 100 mg orally once daily, dupilumab 300 mg subcutaneously every other week (after a loading dose of 600 mg), or placebo plus topical therapy. | 12-week study | 838 in total, abrocitinib 200 mg (n = 226), abrocitinib 100 mg (n = 238), dupilumab (n = 243), placebo (n = 131) | 18 years of age or older, adult patients | PP-NRS | Abrocitinib 200 mg dose, but not the abrocitinib 100 mg dose, was superior to dupilumab with respect to itch response at week 2. | ||
| Gooderham M.J. et al. JAMA Dermatol. 2019 [84] | Phase 2b, randomized, double-blinded, placebo-controlled, parallel-group trial | (1:1:1:1:1) participants received abrocitinib (200 mg, 100 mg, 30 mg, or 10 mg) or placebo once daily | 12-week study | In total = 267 participated, but 263 were included in the full analysis. Placebo (n = 55), abrocitinib 10 mg (n = 49), abrocitinib 30 mg (n = 50), abrocitinib 100 mg (n = 55), abrocitinib (n = 54) | 18 to 75 years, adults | Pruritus NRS score (0–10) | At week 12, significant reductions in pruritus NRS scores were observed in the 200 mg (LSM difference from the placebo, –25.4%; p = 0.003) and 100 mg (−20.7%; p = 0.02) groups compared with placebo. Odds ratio abrocitinib 200 mg: 5.11 (2.43 to 10.77), abrocitinib 100 mg: 2.84 (1.40 to 5.76). | ||
| Baricitinib | Janus kinase 1/Janus kinase 2 inhibitor | Oral | |||||||
| Simpson E.L. et al. JAAD, 2021 [85] | Janus kinase 1/Janus kinase 2 inhibitor | Phase 3 trial (BREEZE-AD5/NCT03435081) | 1:1:1 once-daily placebo or baricitinib 1 mg or 2 mg. | 16-week study | 440 | Adults | Itch Numerical Rating Scale (NRS) | The proportion achieving 4-point improvement on the itch, NRS was 6% for placebo, 16% for baricitinib 1 mg, and 25% for baricitinib. 2 mg (p < 0.001) | |
| Guttman-Yassky E. et al. JAAD 2019 [86] | Phase 2, randomized, double-blind, placebo-controlled study | Placebo plus topical corticosteroids (TCS), baricitinib 2 mg plus TCS, baricitinib 4 mg plus TCS | 16-week study | 124 in total, placebo plus topical corticosteroids (TCS) n = 49, baricitinib 2 mg plus TCS (n = 37), baricitinib 4 mg plus TCS (n = 38) | Adults | NRS, SCORAD patient-reported items of pruritus | Baricitinib plus TCS showed early and significant reduction in cutaneous inflammation and pruritus. | ||
| Upadacitinib | Janus kinase (JAK)1-selective inhibitor | Oral | |||||||
| Guttman-Yassky E. et al. 2020 JACI [87] | Phase 2b, double-blind, placebo-controlled, parallel-group, dose-ranging portion | 1:1:1:1, placebo or extended-release upadacitinib (manufactured by the study sponsor) 7.5, 15, or 30 mg once daily (QD) by mouth | 16-week study | 167 patients were randomized, placebo treated (n = 40), upadacitinib 7.5 mg (n = 42), upadacitinib 15 mg (n = 42), upadacitinib 30 mg (n = 42), | Adults, 18 to 75 years | NRS (0–10) | Each upadacitinib dose level was significantly superior to the placebo. Patient assessment of pruritus (improvement in NRS and achievement of NRS reduction > 4) at week 16. | ||
| Reich K. et al. Lancet 2021 [88] | Randomized, double-blind, placebo-controlled, phase 3 trial (AD Up) | (1:1:1) to receive upadacitinib 15 mg, upadacitinib 30 mg, or placebo once daily, all in combination with topical corticosteroids (TCS) | 16-week study | 785 adults plus 116 adolescents were randomized, upadacitinib 15 mg + TCS (n = 300), upadacitinib 30 mg + TCS (n = 297), placebo + TCS (n = 280 completed of initial 304) | Adolescents and adults, age above 12 years old | Weekly average worst pruritus Numerical Rating Scale score, NRS (0–10) | Significant reduction in pruritus in both upadacitinib groups. | ||
| Delgocitinib | All JAKS | Topical use, delgocitinib 0.25% and 0.5% ointment | |||||||
| Nakagawa H. et al. 2020 JAAD [89] | Delgocitinib 0.5% ointment | 2:1 ratio to delgocitinib 0.5% ointment or vehicle ointment | 2:1 ratio to delgocitinib 0.5% ointment or vehicle ointment | 1st part: 4 weeks, 2nd part: 24 weeks | 158 patients delgocitinib (n = 106), vehicle (n = 52) | 16 years or older | Pruritus NRS scores across parts 1 and 2. | Pruritus NRS scores were significantly improved in the delgocitinib group compared with those in the vehicle group. The pruritus NRS score in the delgocitinib group was lower than in the vehicle group at week 1, which was maintained over time. | |
| Nakagawa H. et al., 2021, JAAD [90] | In part 1, delgocitinib 0.25% ointment. In part 2, delgocitinib ointment at a concentration of 0.5%. | 1:1 double-blind study | Part 1 of this study was a 4-week double-blind period in which Japanese patients ages 2 through 15 years were randomized in a 1:1 ratio to delgocitinib 0.25% ointment or vehicle ointment. | 137 in total, delgocitinib (n = 69), vehicle (n = 68) | Children, patients ages 2 through 15 years | NRS | Improvement in pruritus scores for the delgocitinib group | ||
| Tofacitinib | JAK 1/3 | Topical | |||||||
| Bissonnette R. et al., 2016, BJD [91] | Topical | 4-week, phase IIa, randomized, double-blind, vehicle-controlled study (NCT02001181) | 1:1 to 2% tofacitinib or vehicle ointment twice daily | 4-week study | Tofacitinib 2% bd (n = 35), vehicle (n = 34) | Adults | Percentage change from baseline (CFB) in patient-reported pruritus | Significant improvements in pruritus were observed by day 2. | |
| Ruxolitinib (RUX) | JAK 1/2 | Topical 0.75% RUX cream and 1.5% RUX cream | |||||||
| Papp K, et al., 2021, JAAD [92] | Two phase 3 studies | 1:1:1 vehicle, 0.75% RUX cream and 1.5% RUX cream | 8-week study | TRuE-AD1: 631 patients were randomized (vehicle, n = 126; 0.75% RUX, n = 252; 1.5% RUX, n = 253). In total, 558 (88.4%) completed the 8-week study TRuE-AD2 comprised 618 randomized patients (vehicle, n = 124; 0.75% RUX, n = 248; 1.5% RUX, n = 246). In total, 561 (90.8%) patients, respectively, completed the 8-week study. | Patients aged 12 years or older | NRS (0–10) | Significant itch reductions versus vehicle were reported within 12 hours of first application of 1.5% RUX (p < 0.05). | ||
| Tralokinumab | IL-13 | Subcutaneous (sc) injection | |||||||
| Wollenberg A. et al., 2019, JACI [93] | Phase 2b study (NCT02347176) https://clinicaltrials.gov/ct2/show/NCT02347176 (accessed on 2 February 2023) | 1:1:1:1 to receive subcutaneous tralokinumab 45, 150, or 300 mg, or placebo, every 2 weeks for 12 weeks with concomitant topical glucocorticoids. | 12-week study | 204 adults, placebo (n = 51), tralokinumab 45 mg (n = 50), tralokinumab 150 mg (n = 51), tralokinumab 300 mg (n = 52) | Adults | Pruritus Numerical Rating Scale (7-day mean) scores P-NRS (0–10) | Participants demonstrated improvements from baseline to week 12 in pruritus Numerical Rating Scale (7-day mean) scores versus those receiving placebo when receiving 45 or 300 mg of tralokinumab. These improvements were observed from week 1 onward for all tralokinumab doses and maintained beyond week 12. | ||
| Wollenberg A. et al., 2020, BJD [94] | Phase III trials, ECZTRA 1 and ECZTRA 2 | 3:1 to subcutaneous tralokinumab 300 mg, after a 600-mg loading dose on day 0, or a placebo every other week for 16 weeks. After a 16-week initial treatment period, tralokinumab-treated patients who achieved the prespecified criteria for clinical response were rerandomized 2:2:1 to tralokinumab 300 mg every 2 weeks (Q2W) or every 4 weeks (Q4W), or placebo for a 36-week maintenance treatment period. | 16 weeks initially, 52-week study | ECZTRA 1: placebo (n = 199), tralokinumab patients (n = 603), ECZTRA 2: placebo (n = 201), tralokinumab patients (n = 593) | Adults | P-NRS (0–10) | Early improvements in pruritus were observed. | ||
| Silverberg, J.I. et al. Br. J. Derm. 2021 [95] | Phase III trial | 2:1 to subcutaneous tralokinumab 300 mg or placebo every 2 weeks (Q2W) with TCS. At week 16 tralokinumab patients were rerandomized 1:1 to tralokinumab Q2W or every 4 weeks (Q4W), with TCS as needed, for another 16 weeks. | 16-week study | All participants = 380, placebo Q2W + TCS (n = 127), tralokinumab Q2W + TCS (n = 253) | ≥18 years of age adults | Worst daily pruritus Numerical Rating Scale (NRS) | Greater reduction in weekly average of worst daily pruritus was observed in the tralokinumab arm. | ||
| Gutermuth J. et al. Br. J. Dermatol. 2022 [96] | Parallel, randomized, double-blind, placebo-controlled, phase III trial | 1:1 to subcutaneous tralokinumab 300 mg or placebo every 2 weeks plus TCS as needed | 26-week study | 277 patients | Adults | Worst daily pruritus Numerical Rating Scale (NRS) | Improvement in pruritus in patients treated with tralokinumab. | ||
| Nemolizumab | IL-31 receptor α subunit | Subcutaneous (sc) injection | |||||||
| Silverberg J.I. et al. JACI, 2020 [97] | Phase 2B randomized study | Nemolizumab (10, 30, and 90 mg) subcutaneous injections every 4 weeks versus placebo, with topical corticosteroids (TCS) | 24-week study | Total = 226, Placebo n = 57, nemolizumab 10 mg n = 55, nemolizumab 30 mg n = 57, nemolizumab 90 mg n = 57 | Adults | Weekly average pruritus NRS score (0–10) | All doses of nemolizumab were associated with a rapid decrease in pruritus scores, with statistically significant differences from placebo starting as early as week 1. By week 2, scores with all nemolizumab doses were greater than those with placebo (p < 0.001). | ||
| Kabashima K. et al. NEJM, 2020 [98] | Double-blind, phase 3 trial | 2:1 ratio to receive subcutaneous nemolizumab (60 mg) or placebo every 4 weeks until week 16, with concomitant topical agents. | 16-week study | In total 215 patients, nemolizumab n = 143, placebo n = 72 | Aged 13 years or older, a body weight of 30.0 to 120.0 kg | Pruritus VAS score (0–100), NRS (0–10), itch score (0–4) | At week 16, the least-squares mean percent change from baseline in the pruritus VAS score (primary end point) was −42.8% in the nemolizumab group and −21.4% in the placebo group. | ||
| Kabashima K. et al. BJD, 2022 [99] | Two long-term phase III studies | Nemolizumab 60 mg every 4 weeks (Q4W) was administered subcutaneously, concomitantly with topical treatments. Study-JP01 patients received double-blind nemolizumab or placebo for 16 weeks, and then entered a 52-week extension period in which all patients received nemolizumab (nemolizumab/nemolizumab and placebo/nemolizumab groups). Study-JP02 patients received nemolizumab for 52 weeks. Both studies included an 8-week follow-up period. | 16 and 54 weeks | Study-JP01 nemolizumab/nemolizumab and placebo/nemolizumab, and Study-JP02 nemolizumab groups comprised 143, 72 and 88 patients, respectively. | aged ≥ 13 years, with a bodyweight of ≥30_0 kg | Pruritus VAS score (range 0–100), five-level itch scale (range 0–4), the pruritus Numerical Rating Scale (NRS, range 0–10), | Improvement in pruritus in patients treated with nemolizumab. | ||
| Tezepelumab | anti-thymic stromal lymphopoietin monoclonal antibody, TSLP | Subcutaneous injection | |||||||
| Simpson EL. et al., 2019, JAAD [80] | Phase 2a study (NCT02525094) | 1:1 to subcutaneous tezepelumab 280 mg or placebo plus TCS every 2 weeks | 16-week study | 111 patients in total, placebo plus TCS = 56 patients, tezepelumab plus TCS = 55 patients | Adults, 18 to 75 years of age | Pruritus Numerical Rating and 5-D itch scales | No statistically significant improvement. Peak pruritus NRS scores were numerically lower for tezepelumab plus TCS-treated patients at week 12 but did not reach nominal significance | ||
| Lebrikizumab | IL-13 | Subcutaneous injection | |||||||
| Simpson E.L. et al. JAAD, 2018 [100] | Phase 2 study (TREBLE) | 1:1:1:1 to receive lebrikizumab 125 mg single dose at baseline, 250 mg single dose at baseline, 125 mg once every 4 weeks, or placebo every 4 weeks plus TCS for 12 weeks | 12-week study | 212 patients in total, lebrikizumab 125 mg single dose, n = 52, lebrikizumab 250 mg single dose, n = 53, lebrikizumab 125 mg Q4W, n = 51, placebo, n = 53 | Adults, 18–75 years | Pruritus Visual Analog Scale (VAS) score | Improvement on pruritus in patients receiving lebrikizumab. | ||
| Guttman-Yassky E. et al. JAMA Dermatol. 2020 [101] | Phase 2b study | 280 patients randomized to placebo (n = 52) or to lebrikizumab at doses of 125 mg every 4 weeks (n = 73), 250 mg every 4 weeks (n = 80), or 250 mg every 2 weeks (n = 75). | 16-week study | 280 patients placebo (n = 52), lebrikizumab at doses of 125 mg (n = 73), 250 mg every 4 weeks (n = 80), or 250 mg every 2 weeks (n = 75). | Adults 18 years or older | Pruritus Numerical Rating Scale (NRS) score | Lebrikizumab improved pruritus in a dose-dependent manner vs placebo during 16 weeks of treatment. | ||
| Dupilumab | IL-4Rα | Subcutaneous injection | |||||||
| Blauvelt A. et al. Lancet 2017 [102] | Phase 3 trial | Patients were assigned randomly to 3:1:3. In total, 740 patients were enrolled; 319 received dupilumab qw plus topical corticosteroids, 106 received dupilumab q2w plus topical corticosteroids, and 315 received placebo plus topical corticosteroids. | 16-week study | In total, 740 patients were enrolled; 319 received dupilumab qw plus topical corticosteroids, 106 received dupilumab q2w plus topical corticosteroids, and 315 received placebo plus topical corticosteroids. | Adult patients, aged 18 years old or older | NRS | Patients receiving dupilumab plus TCS had a greater improvement in peak NRS pruritus than those receiving placebo. | ||
| Tradipitant (VLY-686) | a novel Neurokinin (NK)-1 receptor antagonist | Oral systemic medication | |||||||
| Welsh S.E. et al. JEADV 2021 [103] | A phase 3, randomized, placebo-controlled trial (EPIONE) | 8-week study | 375 patients, tradipitant (n = 188) or placebo (n = 187) | WI-NRS: Worst Itch Numeric Rating Scale | EPIONE did not meet its primary endpoint of reduction in pruritus. However, robust antipruritic effect was observed in patients with mild lesion severity. Tradipitant treatment resulted in a clinically meaningful reduction in patient-reported worst itch. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koumaki, D.; Gregoriou, S.; Evangelou, G.; Krasagakis, K. Pruritogenic Mediators and New Antipruritic Drugs in Atopic Dermatitis. J. Clin. Med. 2023, 12, 2091. https://doi.org/10.3390/jcm12062091
Koumaki D, Gregoriou S, Evangelou G, Krasagakis K. Pruritogenic Mediators and New Antipruritic Drugs in Atopic Dermatitis. Journal of Clinical Medicine. 2023; 12(6):2091. https://doi.org/10.3390/jcm12062091
Chicago/Turabian StyleKoumaki, Dimitra, Stamatios Gregoriou, George Evangelou, and Konstantinos Krasagakis. 2023. "Pruritogenic Mediators and New Antipruritic Drugs in Atopic Dermatitis" Journal of Clinical Medicine 12, no. 6: 2091. https://doi.org/10.3390/jcm12062091









