Abstract
Background: The aim of our study was to analyze perioperative lactate levels and their predictive value for postoperative mortality and morbidity after liver resection. Methods: The clinicopathological characteristics and outcomes of 152 patients who underwent liver resection for benign and malign diagnoses were analyzed retrospectively. Lactate concentrations at three different time points, (1) before liver resection (LAC-PRE), (2) after liver resection on day 0 (LAC-POST), and (3) on day one after the operation (LAC-POD1) were assessed regarding the prognostic value in predicting postoperative complications and mortality according to the Clavien–Dindo (CD) classification. Results: The rates of postoperative complications (CD ≥ IIIb) and mortality rates were 19.7% (N = 30) and 4.6% (N = 7), respectively. The LAC-PRE levels showed no correlation with the postoperative outcome. The ROC curve analysis showed that LCT-POST and LCT-POD1 values were moderately strong in predicting postoperative morbidity (0.681 and 0.768, respectively) and had strong predictive accuracies regarding postoperative mortality (0.800 and 0.838, respectively). The multivariate analysis revealed LAC-POST as a significant predictor of postoperative complications (CD ≥ IIIb: OR 9.28; 95% CI: 2.88–29.9; p < 0.001) and mortality (OR 11.69; 95% CI: 1.76–77.7; p = 0.011). Conclusion: Early postoperative lactate levels are a useful and easily practicable predictor of postoperative morbidity and mortality in patients after liver resection.
1. Introduction
In recent decades, the number of liver surgeries for the treatment of primary and secondary liver tumors has been steadily increasing. However, despite technical improvements in surgical techniques and anesthesiologic care, the incidence of major complications after liver resection (LR) still ranges from 19 to 23% [1,2,3], and mortality rates are approximately 6% [3,4,5,6,7]. Surgical complications such as bile leaks or intraabdominal infection and non-surgical complications such as hepatic or renal dysfunction, respiratory failure, and sepsis impact the outcome and mortality. Therefore, numerous studies have attempted to find simple parameters (i.e., hepatic steatosis, patient age, serum bilirubin) and scores to predict the patient outcome after LR [1,7,8,9,10,11]. With a suitable marker, clinicians could estimate the individual risk of postoperative complications of each patient after LR and set up an optimal patient management protocol.
In this context, the amount of lactate seems to be a potential parameter that can be determined quickly and easily in everyday clinical practice. Lactate is a byproduct of anaerobic cellular metabolism and a marker for reduced organ perfusion and end-organ damage; hence, it can be used to guide fluid therapy and to detect critically ill patients with hypoperfusion [12,13,14]. In the human body, the liver is responsible for most of the lactate clearance [15] and thus, the quality of liver parenchyma, the extent of LR, anaerobic time during vascular occlusion and surgical resection, as well as intraoperative blood loss, accompanied by hypoperfusion of the liver and hypoxia; all have a direct impact on the lactate level [16,17,18,19,20].
To date, only a few studies have investigated the role of perioperative lactate levels as predictors for the postoperative outcome after LR [17,18,19,20,21,22,23,24,25,26], and differences in study design and a lack of data regarding early postoperative lactate release make further studies necessary.
Therefore, the aim of the study was to compare lactate levels at different time points in patients undergoing various extents of LR and to determine their association with postoperative complications and mortality.
2. Patients and Methods
2.1. Data Collection and Study Population
Medical data from all adult patients (≥18 years of age) who underwent LR at the University Hospital of Leipzig between April 2016 and September 2017 were retrospectively analyzed. Patients with missing data (N = 2) or an unknown time of lactate measurement (N = 2) were excluded from the study. Data were collected from anesthesiologic and operative documentation of the patient’s history, the intraoperative procedure, histological results, the process at the intensive care unit, and the medical discharge report.
Before surgery, each case was reviewed in a multidisciplinary tumor-board meeting. Patients were not considered for surgery in case of advanced liver disease (e.g., liver cirrhosis Child–Pugh stage B or C, extensive portal vein thrombosis), poor general conditions, severe cardial or pulmonal failure. Sufficient liver parenchyma and liver function were measured by CT volumetry and the LiMAx® test [27]. If the future liver remnant was too small, patients received portal vein embolization (PVE) in order to obtain resectabilty. About one month after PVE, CT volumetry was repeated to ensure a sufficient volume increase in the future liver remnant. Prior to a major liver resection, 25–30% of the total liver volume or a future remnant liver volume of 0.8% of the patient’s body weight was considered sufficient. Furthermore, the future residual liver function—calculated from the preoperative LiMAx® test and future remnant liver volume—should be above 100 µg/h/kg [27].
The medical data analysis comprised patient demographics (age, gender, and body mass index [BMI, weight in kg/height in m2]), preoperative performance status according to the ASA (American Society of Anesthesiology) classes I to III, and intraoperative surgical data (operation time, blood loss, transfusion requirement [defined as units of red blood cells, albumin, or fresh frozen plasma substitution], Pringle maneuver, bile duct drain [T-drain] placement, the extent of resection, and lymphadenectomy), histopathological findings and post-operative data (length of hospital stay, postoperative complications during the hospital stay). The extent of resection was described according to the Brisbane classification [28] as being a major (exceeding three segments) or minor (less than four segments or atypical resections) LR. Accordingly, major LRs included right or left hemihepatectomy or right or left trisectorectomy (extended hemihepatectomies). In addition to these classic approaches to resection, a considerable number of patients underwent additional procedures such as vascular or biliary resection and resection of adjacent structures. These approaches were classified as “complex resection”. Lymphatic dissection during surgery always included the lymph nodes of the hepatoduodenal ligament and those at the common hepatic artery. In very few cases the retropancreatic and celiac lymph nodes were also excised.
During open liver resection (OLR), parenchymal transection was performed with the Cavitron Ultrasonic Surgical Aspirator (CUSA) and while smaller vascular and biliary structures were divided between titanium clips, larger structures were ligated. During laparoscopic liver resection (LLR), ultrasonic shears (Harmonic ACE, Ethicon®) were used as a mainstay of tissue dissection and parenchymal transection. A laparoscopic CUSA was only used during right or extended right hemi-hepatectomies for exposure of the middle hepatic vein and its tributaries and for intrahepatic exposure of the right bile duct. Additional hemostasis was performed by bipolar forceps and irrigation. In specific cases, such as resections of superior segments 7–8, a hand-assisted laparoscopic approach was applied. For the vascular inflow control, a tourniquet was placed around the hepatoduodenal ligament and temporarily closed during parenchymal resection when necessary (Pringle maneuver). All patients with LRs received at least overnight intensive care and were transferred to the normal ward at the earliest on the day after surgery.
The study was approved by the ethics committee of the University of Leipzig, number 142/18-EK, and the study protocol was performed in accordance with the relevant guidelines. Informed consent was waived by the ethics committee of the University of Leipzig due to the retrospective nature of the study.
2.2. Outcome Measures
Post-operative complications occurring in the first three months after LR were analyzed. The complications included delayed wound healing, wound infection, bleeding, development of hematoma and lymphoceles, pneumonia, unplanned intubation, pulmonary embolism, >48 h ventilator requirement, pneumothorax, renal failure, postoperative liver failure, ascites, bile leakage, stroke or cerebral vascular accident, cardiac arrest, myocardial infarction, deep venous thrombosis, or systematic sepsis. Post-hepatectomy liver failure was defined as a deterioration of the liver functions indicated by laboratory values, such as an increased INR and concomitant hyperbilirubinemia on or after postoperative day five [29], or clinical symptoms such as hepatic encephalopathy. The Clavien–Dindo (CD) classification was used for grading postoperative complications [30]. Major morbidity was defined as being CD IIIb or greater (CD ≥ IIIb), which requires surgical, endoscopic, or radiologic intervention under general anesthesia. Mortality was defined as any death occurring within 90 days following the date of surgery or in-hospital mortality.
The lactate concentrations were determined from blood samples taken before liver resection (LAC-PRE), after liver resection on day 0 (LAC-POST), and on day one day after the operation (LAC-POD1). The limit value for lactate is <2.0 mmol/L. Other laboratory values included in the analysis are preoperative bilirubin, base excess, and creatinine.
2.3. Statistical Analysis
For comparison between the groups, the appropriate statistical significance test, including the Student’s t-test, the chi-squared test, analysis of variance (ANOVA), the Kruskal–Wallis test, and the Wilcoxon–Mann–Whitney test were used. Receiver operator characteristic (ROC) curves were generated to determine the optimal diagnostic criterion threshold for predicting postoperative outcomes. A ROC curve displayed the false positive rate on the x-axis (specificity), and the true positive rate on the y-axis (sensitivity) for varying test thresholds, thereby plotting the performance of a diagnostic test. The predictive accuracy was measured by the area under the ROC curve (AUC) whereby higher AUC values represent greater accuracy. An AUC of 1.0 represents perfect discrimination (perfect sensitivity and specificity), whereas an AUC of 0.5 represents an essentially worthless test. The cut-off values for continuous variables were chosen by receiver operator characteristic (ROC) curves analysis and calculation of the Youden index according to each point (Youden’s J statistic: J = sensitivity + specificity − 1) [31]. These values were used for the subsequent uni- and multivariate analyses. Uni- and multivariate logistic regression analyses were used to evaluate the association between independent variables and binary postoperative outcomes (CD ≥ IIIb and mortality). For the multivariate analyses, we used a forward stepwise regression model including only clinically relevant variables and those presenting p < 0.05 in univariate analysis.
SPSS software, version 21.0 (SPSS Inc., Chicago, IL, USA) and GraphPad Prism software, version 9.4.1 (GraphPad Software, San Diego, CA, USA) were used for statistical analysis and graphs. A p value < 0.05 was considered statistically significant. Unless otherwise indicated, the baseline data are presented as median values with the standard deviation (SD).
3. Results
3.1. Baseline Characteristics
The overall characteristics of the patients and their surgeries are summarized in Table 1. The indications for surgery were 116 malignant (76.3%) and 36 benign (23.7%) tumors. Liver resection (LR) was performed by 107 open liver resections (OLR) (70.4%), 36 laparoscopic liver resections (LLR) (23.7%), and nine hand-assisted laparoscopic liver resections (HALLR) (5.9%). Ten patients received portal vein embolization prior to extended hemihepatectomy. In one case an in situ split with liver segment resection was performed followed by a left hemihepatectomy eight days later. Sixty-eight patients (56.6%) received surgery on the upper abdomen prior to LR, and in seven cases (4.6%) a conversion from LLR to OLR was necessary. The operative procedures included 102 minor (67.1%) and 50 major (32.9%) LRs. Due to tumor invasion, the following extrahepatic resections were necessary: hemicolectomy (N = 2), partial diaphragm (N = 6), colon section (N = 2), extrahepatic bile duct (N = 14), adrenal gland (N = 1), partial kidney (N = 1), portal vein bifurcation (N = 3), and atypical partial lung (N = 3) resection.

Table 1.
Baseline characteristics of the overall study population.
The median hospital stay was 9.0 ± 12.2 days (range: 3–72 days). The overall postoperative complications rate was 38.8% (N = 59) and the overall mortality rate was 4.6% (N = 7). The summary of the postoperative outcomes is shown in Table 2. All deaths occurred after extended hemihepatectomies within the first months after LR (range: 3–20 days). Five of these patients received a biliodigestive anastomosis. The causes of death included septic shock and multiple organ failure (N = 4), liver insufficiency (N = 1), bowel ischemia (N = 1), and pulmonary embolism and cardiac arrest (N = 1), respectively.

Table 2.
Summary of the postoperative outcome in 152 patients after liver resection.
3.2. Correlation of Lactate Levels with Postoperative Outcomes
The distribution of lactate levels in the study populations before and after resection as well as on the first postoperative day is shown in Figure 1. On day 0 after LR (LAC-POST, 1.8 ± 1.6 mmol/L) and one day after the surgery (LAC-POD1,1.8 ± 2.5 mmol/L), the average lactate levels showed significant differences compared to the lactate concentrations before liver resection (LAC-PRE, 0.8 ± 0.5, mmol/L) (p < 0.001 each), whereas LAC-POST and LAC-POD1 were comparable (p = 0.310). One day after surgery, the lactate concentration was still elevated (≥2 mmol/L) in 41% of the patients.
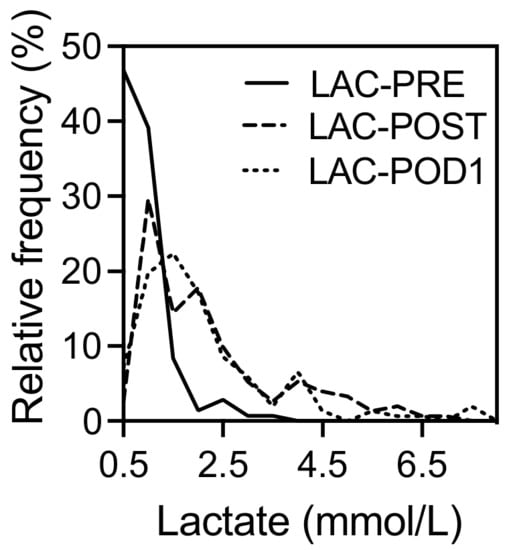
Figure 1.
Perioperative distribution of lactate concentration in 152 patients who underwent liver resection. LAC-PRE, lactate level before liver resection; LAC-POST, lactate after liver resection on day 0; LAC-POD1, lactate on day one after liver resection.
The average postoperative lactate concentrations (LAC-POST, LAC-POD1) were significantly increased in patients suffering from postoperative complications (Table 3).

Table 3.
Comparison of lactate levels and postoperative morbidity and mortality in 152 patients who underwent liver resection. of the postoperative outcome in 152 patients after liver resection.
Apart from the postoperative outcome, the lactate levels were also associated with intraoperative characteristics. There was a significant positive correlation between intraoperative blood loss, operation time, and both postoperative lactate levels (p < 0.001 each). A prolonged Pringle maneuver was also significantly associated with elevated LAC-POST (p = 0.008), but not with LAC-POD1 (p = 0.092). Postoperative lactate levels correlate with the extent of LR. Postoperative lactate levels were significantly increased in patients after extended hemihepatectomies (trisectorectomies) when compared with patients after minor LR (p < 0.001 each). The relationship between the intraoperative blood loss, duration of the Pringle maneuver, operation time, extent of liver resection, and lactate concentration after LRs is shown in Figure 2.
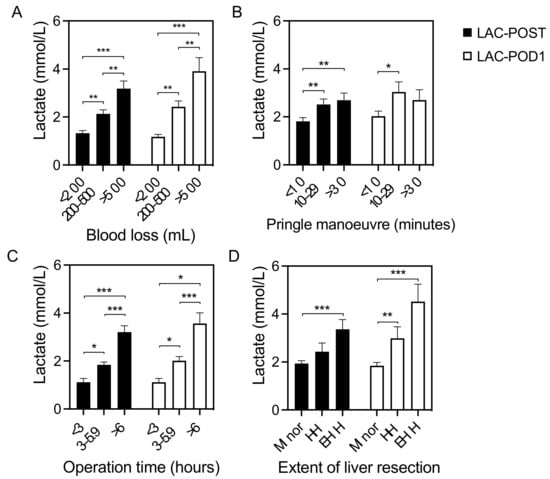
Figure 2.
Variables associated with postoperative lactate concentration. Relationship between the (A) intraoperative blood loss, (B) duration of the Pringle maneuver, (C) operation time, and (D) extent of liver resection and lactate concentration after liver resection. HH, hemihepatectomy; EHH, extended hemihepatectomy; LAC-POST, lactate after liver resection on day 0; LAC-POD1, lactate on day one after liver resection; minor, minor liver resection. Data are shown as mean values ± Standard error of the mean. * p < 0.05, ** p < 0.01, *** p < 0.001.
The prognostic values of the study parameters in predicting severe postoperative complications (CD ≥ IIIb) and mortality were assessed using ROC curve analysis and probability determination (Figure 3A,B). In our cohort, the LAC-PRE levels had no predictive power regarding morbidity or mortality after LR. However, the AUC values of LAC-POST and LAC-POD1 were moderately strong in predicting postoperative morbidity (0.681, and 0.768, respectively) whereby the corresponding cut-off values were 2.8 and 2.4 mmol/L. The AUC values of LAC-POST and LAC-POD1 regarding postoperative mortality indicated strong predictive accuracies (0.800, and 0.838, respectively) and the corresponding cut-off values were 3.1 and 5.4 mmol/L, respectively.
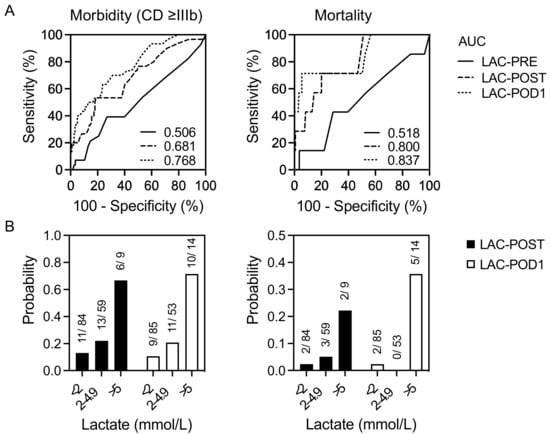
Figure 3.
ROC curves and probability of perioperative lactate levels in predicting postoperative complications and mortality in 152 patients undergoing liver resection. Receiver operator characteristic (ROC) curves (A) for lactate as discriminators of the increased rate of postoperative morbidity and mortality. Probability (B) of postoperative morbidity and mortality according to lactate concentration. CD ≥ IIIb, Clavien–Dindo ≥ IIIb; LAC-POST, lactate after liver resection on day 0; LAC-POD1, lactate on day one after liver resection.
The predictors of postoperative morbidity and mortality after LR are shown in Table 4 and Table 5, respectively. The cut-offs obtained from the ROC analysis were used to divide patients into two groups for uni- and multivariate logistic regression analysis (MVA). In the univariate analysis, LAC-POST and LAC-POD1 were significantly associated with postoperative morbidity (CD ≥ IIIb) and mortality (p < 0.001/0.001 and p = 0.009/0.021). After adjusting for co-variables in MVA, the patient’s age, complex resections (e.g., biliodigestive anastomosis or vascular reconstruction), duration of the Pringle maneuver, and LAC-POST (OR 9.28; 95% CI: 2.88–29.9; p < 0.001) were significant predictors for severe postoperative complications (CD ≥ IIIb). Regarding postoperative mortality, MVA revealed complex resection and LAC-POST (OR 11.69; 95% CI: 1.76–77.7; p = 0.011) as independent risk factors whereby LAC-POD1 failed to be identified as an independent predictor of morbidity and mortality in MVA. However, calculation and analysis of lactate differences one day after LR showed advantages regarding the prediction of postoperative complications, especially mortality (OR 20.72; 95% CI: 2.72–157.81; p = 0.003).

Table 4.
Predictors of postoperative morbidity in 152 patients who underwent liver resection.

Table 5.
Predictors of postoperative mortality in 152 patients undergoing liver resection.
4. Discussion
Based on the results, the predictive value of perioperative lactate levels on the short-term outcomes after liver resection is discussed here.
4.1. Preoperative Lactate Level
In the current study, preoperative lactate levels failed to predict the outcome after LR. In reviewing the literature, only two publications were found on this specific topic. In a recent study by Popescu et al., which investigated the rates of postoperative liver failure in a group of 55 patients after major LR, the lactate levels prior to the operation failed to predict its outcome [24]. In contrast, a retrospective study by Riediger et al. including 337 patients undergoing OLR demonstrated preoperative lactate levels as independent risk factors for in-hospital death (OR: 1.474; p = 0.004) [25]. The divergent results of these two studies may be explained by their different study populations, as hepatic lactate metabolism is determined by the quality of liver parenchyma and patient comorbidities, among other factors. Whereas Riediger et al. included a heterogeneous group of patients, who could have various causes of increased preoperative lactate levels, such as liver trauma, hepatitis, or liver cirrhosis, Popescu et al. excluded those patients with a potentially worse postoperative outcome [24,25]. In our cohort, all operations were elective. The patients were preselected due to routine clinical evaluation (including LiMAx® [maximum liver function capacity] test), and their lactate levels were mainly determined by intra- and postoperative procedures.
4.2. Early Postoperative Lactate Level (Day 0)
Lactate levels measured directly after surgery correlate with morbidity and mortality after LR [17,19,20,22,23,24,26]. Our results are in line with previous studies, which revealed lactate levels at the end of surgery as being an independent risk factor for postoperative complications and death [17,19,26]. In the current study, the lactate cut-off values are almost identical to the results of the multi-center analysis by Vibert et al., who defined cut-off values of 2.8 mmol/L and 3.0 mmol/L for the prediction of severe morbidity and 90-day mortality after LR, respectively [19]. These results may be relevant for surgeons and intensive care physicians in their daily clinical practice. They support the proposal by Wiggans et al., who postulated that lactate levels could be used to guide clinicians regarding the requirement of intensive care after LR, as patients with a normal postoperative lactate level are unlikely to suffer from hepatic dysfunction [20]. Furthermore, in the intensive care unit, the early postoperative lactate could be used to optimize hemodynamics and fluid therapy [23].
In our study, surgery time, duration of the Pringle maneuver, intraoperative blood loss, and extent of LR correlate with elevated postoperative lactate levels although none of the parameters were independently associated with postoperative morbidity or mortality. Previous studies showed several pre- and intraoperative factors to be associated with postoperative hyperlactatemia [17,19,20], and especially patients with diabetes display elevated lactate levels compared to healthy subjects, which most likely is due to the impairment of gluconeogenesis [19,20,32]. Unfortunately, in our cohort, data regarding patients’ pretreatment and comorbidities, such as the number and type of chemotherapies, diabetic medication, and other specific drugs associated with increased lactate levels, are incomplete, and conclusions about their influence on postoperative outcome cannot be drawn.
4.3. Lactate Clearance (Day 1)
In general, reduced lactate clearance indicates restricted microcirculation [33] and decreased liver function since up to 70% of the lactate in the human body is eliminated by the liver [15]. Especially in critically ill patients such as those suffering from sepsis, lactate clearance is a strong predictor of mortality [13,34]. In reviewing the literature, only one previous study was found, which analyzed the association between lactate clearance and the early postoperative outcome after LR. In their retrospective study published in 2015, Pagano et al. analyzed lactate clearance on day five after LR (defined as lactate at postoperative day five minus lactate at ICU presentation), without showing any significance in multivariate analysis [23]. However, the study population consisted of only 45 patients undergoing exclusively extended hepatectomies. In our study cohort, the lactate level one day after LR also failed to be an independent predictor of morbidity and mortality although the lactate clearance within the first postoperative day was a strong prognostic factor of patient survival. All deaths in our study population occurred after extended resections, which can lead to transient or permanent hepatic insufficiency and impairment of the lactate metabolism. It can be assumed that hyperlactatemia is not a primary manifestation of increased lactate production, as is the case in septic patients, but rather a result of decreased liver function and lactate clearance. Therefore, the determination of early lactate clearance as an indicator of reduced liver function seems to have a potential benefit in patients after major liver resection.
4.4. Postoperative Mortality
Our overall morbidity and mortality rates are comparable to those of other high-volume centers. In our study, the mortality rate was 22.6% for patients after extended hemihepatectomies (7 out of 31 patients), which is in line with previous reports. In a retrospective analysis by Filmann et al. including about 110,000 liver procedures performed in Germany between 2010 and 2015, the average hospital mortality rate after major liver resection was 10.4%. In addition, extended hemihepatectomies with biliodigestive anastomosis even led to a hospital mortality rate of 25.5% [5]. Besides postoperative hepatic insufficiency, bile leakage after biliary reconstruction contributes to the high postoperative complication rate after major liver resections [35].
4.5. Limitations
There are some notable limitations of this study that should be mentioned, the first of which is the relatively small number of patients that were included and its retrospective design. Second, the data concerning the lactate-contributing factors during anesthesia in the operating room and the intensive care unit are incomplete. Further studies with the measurement of factors such as the administration of catecholamines and vasoactive drugs, drugs associated with increased lactate levels, and circulatory parameters would be of interest.
5. Conclusions
Our study presents early postoperative lactate levels and clearance as a predictor of the postoperative outcome after liver resections. Furthermore, the correlation was independent of the diagnosis, the complexity of the resection, and the resection technique. Patients with elevated lactate should be monitored carefully due to the higher risk of morbidity and mortality. Further prospective studies are required to confirm our results and to determine the optimal postoperative care and therapy for these patients.
Author Contributions
Conceptualization, S.R. (Sebastian Recknagel) and U.S.; data acquisition, S.R. (Sebastian Recknagel) and U.S.; formal analysis, S.R. (Sebastian Recknagel), S.R. (Sebastian Rademacher), U.S. and R.S.; writing—review and editing, U.S., S.R. (Sebastian Recknagel), S.R. (Sebastian Rademacher), A.A.L., U.G.L., T.H., C.H., D.S. and R.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Open Access Publication Fund of the University of Leipzig. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. There are no potential conflicts of interest arising from associations with commercial or corporate interests in connection with the work submitted.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the local ethical commission board from the University of Leipzig (142/18-EK).
Informed Consent Statement
Written informed consent from any patient for data collection in a prospectively collected database is available. However, written informed consent to the study was waived by the local Ethics Committee (Ethics Committee of the first affiliated University Hospital of Leipzig University) in view of the retrospective design of the study, according to the national and local guidelines, such as the fact that all clinical/laboratory measurements and procedures were part of the routine care.
Data Availability Statement
Our database contains highly sensitive data that may provide insight into clinical and personnel information about our patients and lead to the identification of these patients. Therefore, according to organizational restrictions and regulations, these data cannot be made publicly available. However, the datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors declare that they have no conflict of interest.
Abbreviations
| ASA | American Society of Anesthesiology |
| AUC | Area under the curve |
| BMI | Body mass index |
| CCA | Cholangiocarcinoma |
| CRLM | Colorectal liver metastases |
| FFP | Fresh frozen plasma |
| HALLR | Hand-assisted laparoscopic liver resection |
| HCC | Hepatocellular carcinoma |
| ICU | Intensive care unit |
| LAC-POD1 | Lactate on day one after liver resection |
| LAC-POST | Lactate after liver resection on day 0 |
| LAC-PRE | Lactate before liver resection |
| LLR | Laparoscopic liver resection |
| LR | Liver resection |
| MVA | Multivariate analysis |
| nCRLM | Non-colorectal liver metastases |
| OLR | Open liver resection |
| PM | Pringle maneuver |
| POD | Postoperative day |
| RBC | Red blood cells |
| SD | Standard deviation |
| UVA | Univariate analysis |
References
- Aloia, T.A.; Fahy, B.N.; Fischer, C.P.; Jones, S.L.; Duchini, A.; Galati, J.; Gaber, A.O.; Ghobrial, R.M.; Bass, B.L. Predicting poor outcome following hepatectomy: Analysis of 2313 hepatectomies in the NSQIP database. HPB 2009, 11, 510–515. [Google Scholar] [CrossRef] [PubMed]
- Virani, S.; Michaelson, J.S.; Hutter, M.M.; Lancaster, R.T.; Warshaw, A.L.; Henderson, W.G.; Khuri, S.F.; Tanabe, K.K. Morbidity and mortality after liver resection: Results of the patient safety in surgery study. J. Am. Coll. Surg. 2007, 204, 1284–1292. [Google Scholar] [CrossRef] [PubMed]
- Dokmak, S.; Ftériche, F.S.; Borscheid, R.; Cauchy, F.; Farges, O.; Belghiti, J. 2012 Liver resections in the 21st century: We are far from zero mortality. HPB 2013, 15, 908–915. [Google Scholar] [CrossRef] [PubMed]
- Farges, O.; Goutte, N.; Bendersky, N.; Falissard, B.; ACHBT-French Hepatectomy Study Group. Incidence and risks of liver resection: An all-inclusive French nationwide study. Ann. Surg. 2012, 256, 697–704, discussion 704–705. [Google Scholar] [CrossRef]
- Filmann, N.; Walter, D.; Schadde, E.; Bruns, C.; Keck, T.; Lang, H.; Oldhafer, K.; Schlitt, H.J.; Schön, M.R.; Herrmann, E.; et al. Mortality after liver surgery in Germany. Br. J. Surg. 2019, 106, 1523–1529. [Google Scholar] [CrossRef]
- Tzeng, C.W.; Cooper, A.B.; Vauthey, J.N.; Curley, S.A.; Aloia, T.A. Predictors of morbidity and mortality after hepatectomy in elderly patients: Analysis of 7621 NSQIP patients. HPB 2014, 16, 459–468. [Google Scholar] [CrossRef]
- Hoffmann, K.; Hinz, U.; Stravodimos, C.; Knoblich, T.; Schön, M.R.; Büchler, M.W.; Mehrabi, A. Risk assessment for liver resection. Surgery 2018, 164, 998–1005. [Google Scholar] [CrossRef]
- Breitenstein, S.; DeOliveira, M.L.; Raptis, D.A.; Slankamenac, K.; Kambakamba, P.; Nerl, J.; Clavien, P.A. Novel and simple preoperative score predicting complications after liver resection in noncirrhotic patients. Ann. Surg. 2010, 252, 726–734. [Google Scholar] [CrossRef]
- McCormack, L.; Petrowsky, H.; Jochum, W.; Furrer, K.; Clavien, P.A. Hepatic Steatosis Is a Risk Factor for Postoperative Complications After Major Hepatectomy: A Matched Case-Control Study. Ann. Surg. 2007, 245, 923–930. [Google Scholar] [CrossRef]
- Sitzmann, J.V.; Greene, P.S. Perioperative predictors of morbidity following hepatic resection for neoplasm. A multivariate analysis of a single surgeon experience with 105 patients. Ann. Surg. 1994, 219, 13–17. [Google Scholar] [CrossRef]
- Strey, C.W.; Marquez-Pinilla, R.M.; Markiewski, M.M.; Siegmund, B.; Oppermann, E.; Lambris, J.D.; Bechstein, W.O. Early post-operative measurement of cytokine plasma levels combined with pre-operative bilirubin levels identify high-risk patients after liver resection. Int. J. Mol. Med. 2011, 27, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Casserly, B.; Phillips, G.S.; Schorr, C.; Dellinger, R.P.; Townsend, S.R.; Osborn, T.M.; Reinhart, K.; Selvakumar, N.; Levy, M.M. Lactate measurements in sepsis-induced tissue hypoperfusion: Results from the Surviving Sepsis Campaign database. Crit. Care Med. 2015, 43, 567–573. [Google Scholar] [CrossRef]
- Gyawali, B.; Ramakrishna, K.; Dhamoon, A.S. Sepsis: The evolution in definition, pathophysiology, and management. SAGE Open Med. 2019, 7, 2050312119835043. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.M.; An, W.S. New clinical criteria for septic shock: Serum lactate level as new emerging vital sign. J. Thorac. Dis. 2016, 8, 1388–1390. [Google Scholar] [CrossRef] [PubMed]
- Vitin, A.A.; Azamfirei, L.; Tomescu, D.; Lang, J.D. Perioperative Management of Lactic Acidosis in End-Stage Liver Disease Patient. J. Crit. Care Med. (Targu Mures) 2017, 3, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Giustiniano, E.; Procopio, F.; Costa, G.; Rocchi, L.; Ruggieri, N.; Cantoni, S.; Zito, P.C.; Gollo, Y.; Torzilli, G.; Raimondi, F. Serum lactate in liver resection with intermittent Pringle maneuver: The “square-root- shape. J. Hepatobiliary Pancreat. Sci. 2017, 24, 627–636. [Google Scholar] [CrossRef] [PubMed]
- Lemke, M.; Karanicolas, P.J.; Habashi, R.; Behman, R.; Coburn, N.G.; Hanna, S.S.; Law, C.H.L.; Hallet, J. Elevated Lactate is Independently Associated with Adverse Outcomes Following Hepatectomy. World J. Surg. 2017, 41, 3180–3188. [Google Scholar] [CrossRef]
- Meguro, M.; Mizuguchi, T.; Kawamoto, M.; Nishidate, T.; Ishii, M.; Tatsumi, H.; Kimura, Y.; Furuhata, T.; Hirata, K. Highest intraoperative lactate level could predict postoperative infectious complications after hepatectomy, reflecting the Pringle maneuver especially in chronic liver disease. J. Hepatobiliary Pancreat. Sci. 2014, 21, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Vibert, E.; Boleslawski, E.; Cosse, C.; Adam, R.; Castaing, D.; Cherqui, D.; Naili, S.; Régimbeau, J.M.; Cunha, A.S.; Truant, S.; et al. Arterial Lactate Concentration at the End of an Elective Hepatectomy Is an Early Predictor of the Postoperative Course and a Potential Surrogate of Intraoperative Events. Ann. Surg. 2015, 262, 787–792, discussion 792–793. [Google Scholar] [CrossRef]
- Wiggans, M.G.; Starkie, T.; Shahtahmassebi, G.; Woolley, T.; Birt, D.; Erasmus, P.; Anderson, I.; Bowles, M.J.; Aroori, S.; Stell, D.A. Serum arterial lactate concentration predicts mortality and organ dysfunction following liver resection. Perioper Med. 2013, 2, 21. [Google Scholar] [CrossRef]
- Connolly, C.; Stättner, S.; Niederwieser, T.; Primavesi, F. Systematic review on peri-operative lactate measurements to predict outcomes in patients undergoing liver resection. J. Hepatobiliary Pancreat. Sci. 2020, 27, 359–370. [Google Scholar] [CrossRef] [PubMed]
- Niederwieser, T.; Braunwarth, E.; Dasari, B.V.M.; Pufal, K.; Szatmary, P.; Hackl, H.; Haselmann, C.; Connolly, C.E.; Cardini, B.; Öfner, D.; et al. Early postoperative arterial lactate concentrations to stratify risk of post-hepatectomy liver failure. Br. J. Surg. 2021, 108, 1360–1370. [Google Scholar] [CrossRef] [PubMed]
- Pagano, D.; Tropea, A.; Cintorino, D.; Biondi, A.; Spada, M.; Gruttadauria, S. The unreliability of continuous postoperative lactate monitoring after extended hepatectomies: Single center experience. Updates Surg. 2015, 67, 33–37. [Google Scholar] [CrossRef]
- Popescu, M.; Dima, S.; Brasoveanu, V.; Tudor, A.; Simionescu, M.; Tomescu, D. High perioperative lactate levels and decreased lactate clearance are associated with increased incidence of posthepatectomy liver failure. Hepatobiliary Pancreat. Dis. Int. 2021, 20, 592–594. [Google Scholar] [CrossRef] [PubMed]
- Riediger, C.; Mueller, M.W.; Hapfelmeier, A.; Bachmann, J.; Friess, H.; Kleeff, J. Preoperative Serum Bilirubin and Lactate Levels Predict Postoperative Morbidity and Mortality in Liver Surgery: A Single-Center Evaluation. Scand. J. Surg. 2015, 104, 176–184. [Google Scholar] [CrossRef]
- Watanabe, I.; Mayumi, T.; Arishima, T.; Takahashi, H.; Shikano, T.; Nakao, A.; Nagino, M.; Nimura, Y.; Takezawa, J. Hyperlactemia can predict the prognosis of liver resection. Shock 2007, 28, 35–38. [Google Scholar] [CrossRef]
- Stockmann, M.; Lock, J.F.; Malinowski, M.; Niehues, S.M.; Seehofer, D.; Neuhaus, P. The LiMAx test: A new liver function test for predicting postoperative outcome in liver surgery. HPB 2010, 12, 139–146. [Google Scholar] [CrossRef]
- Strasberg, S.M. Nomenclature of hepatic anatomy and resections: A review of the Brisbane 2000 system. J. Hepatobiliary Pancreat. Surg. 2005, 12, 351–355. [Google Scholar] [CrossRef]
- Rahbari, N.N.; Garden, O.J.; Padbury, R.; Brooke-Smith, M.; Crawford, M.; Adam, R.; Koch, M.; Makuuchi, M.; Dematteo, R.P.; Christophi, C.; et al. Posthepatectomy liver failure: A definition and grading by the International Study Group of Liver Surgery (ISGLS). Surgery 2011, 149, 713–724. [Google Scholar] [CrossRef]
- Dindo, D.; Demartines, N.; Clavien, P.A. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef]
- Habibzadeh, F.; Habibzadeh, P.; Yadollahie, M. On determining the most appropriate test cut-off value: The case of tests with continuous results. Biochem. Med. 2016, 26, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Adeva-Andany, M.; López-Ojén, M.; Funcasta-Calderón, R.; Ameneiros-Rodríguez, E.; Donapetry-García, C.; Vila-Altesor, M.; Rodríguez-Seijas, J. Comprehensive review on lactate metabolism in human health. Mitochondrion 2014, 17, 76–100. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, G.; Bellomo, R.; Bakker, J. The ten pitfalls of lactate clearance in sepsis. Intensive Care Med. 2019, 45, 82–85. [Google Scholar] [CrossRef]
- Zhang, Z.; Xu, X. Lactate clearance is a useful biomarker for the prediction of all-cause mortality in critically ill patients: A systematic review and meta-analysis. Crit. Care Med. 2014, 42, 2118–2125. [Google Scholar] [CrossRef] [PubMed]
- Seehofer, D.; Kamphues, C.; Neuhaus, P. Resektion von Klatskin-Tumoren [Resection of Klatskin tumors]. Chirurg 2012, 83, 221–228. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).