Variability of Visual Recovery with Time in Epiretinal Membrane Surgery: A Predictive Analysis Based on Retinal Layer OCT Thickness Changes
Abstract
:1. Introduction
2. Materials and Methods
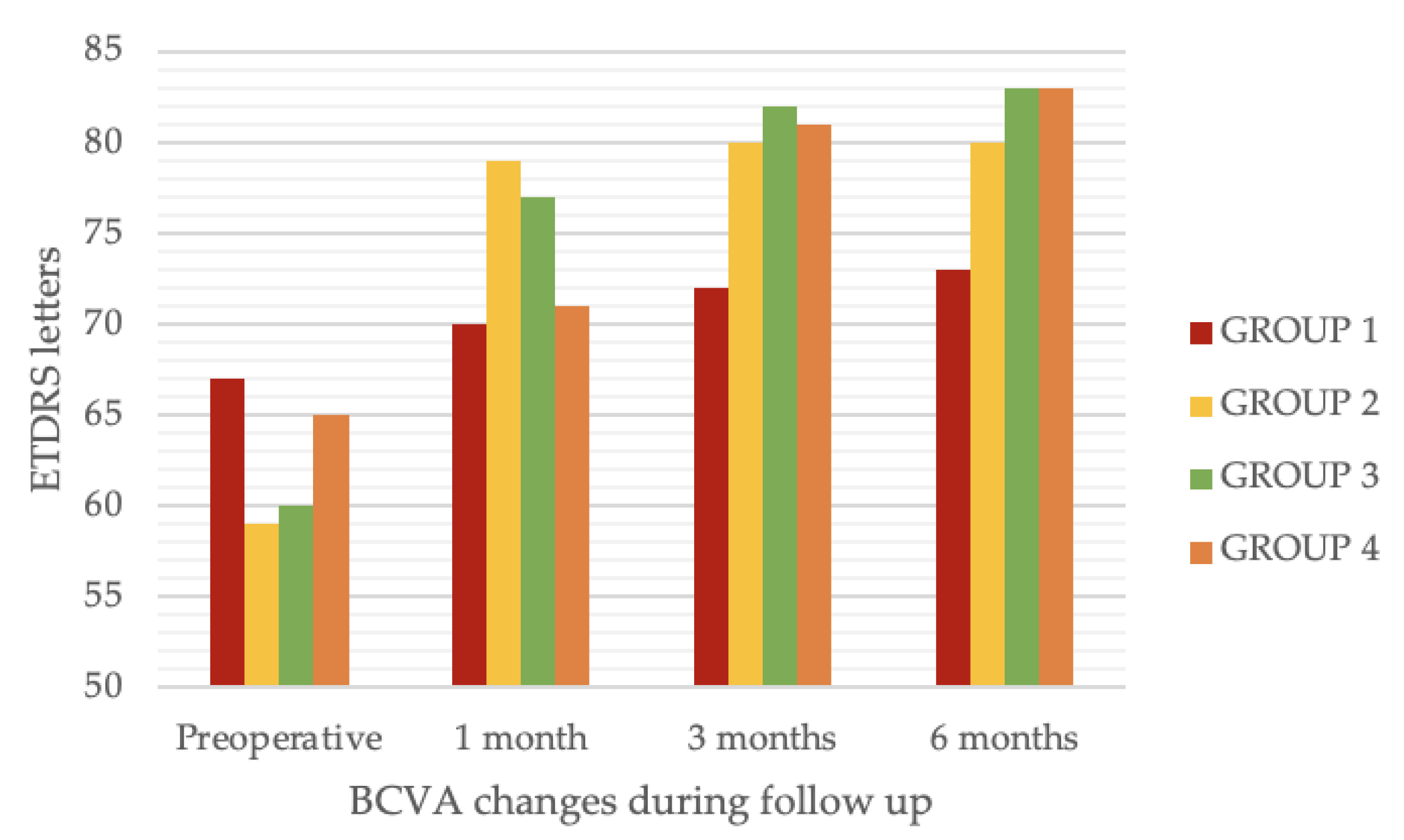
- Immediate (1 month after surgery) improvement of visual acuity without further improvements at later follow-ups (GROUP 2); or
- Immediate (1 month after surgery) improvement of visual acuity with further improvements at later follow-ups (GROUP 3); or
- Delayed improvement of visual acuity (no or minimal change at 1 month follow-up and >15 ETDRS letter change at 3 or 6 months follow up) (GROUP 4).
Statistical Analysis
3. Results
Distinctive OCT Thickness Variation Trends for Each Group
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Steel, D.H.W.; Lotery, A.J. Idiopathic vitreomacular traction and macular hole: A comprehensive review of pathophysiology, diagnosis, and treatment. Eye 2013, 27 (Suppl. S1), S1–S21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romano, M.R.; Ilardi, G.; Ferrara, M.; Cennamo, G.; Parolini, B.; Mariotti, C.; Staibano, S.; Cennamo, G. Macular peeling-induced retinal damage: Clinical and histopathological evaluation after using different dyes. Graefes Arch. Clin. Exp. Ophthalmol. 2018, 256, 1573–1580. [Google Scholar] [CrossRef] [PubMed]
- Joshi, M.; Agrawal, S.; Christoforidis, J.B. Inflammatory mechanisms of idiopathic epiretinal membrane formation. Mediat. Inflamm. 2013, 2013, 192582. [Google Scholar] [CrossRef] [PubMed]
- Jackson, T.L.; Donachie, P.H.J.; Sparrow, J.M.; Johnston, R.L. United Kingdom National Ophthalmology Database Study of Vitreoretinal Surgery: Report 1; case mix, complications, and cataract. Eye 2013, 27, 644–651. [Google Scholar] [CrossRef] [Green Version]
- Meuer, S.M.; Myers, C.E.; Klein, B.E.K.; Swift, M.K.; Huang, Y.; Gangaputra, S.; Pak, J.W.; Danis, R.P.; Klein, R. The epidemiology of vitreoretinal interface abnormalities as detected by spectral-domain optical coherence tomography: The beaver dam eye study. Ophthalmology 2015, 122, 787–795. [Google Scholar] [CrossRef] [Green Version]
- Chang, W.-C.; Lin, C.; Lee, C.-H.; Sung, T.-L.; Tung, T.-H.; Liu, J.-H. Vitrectomy with or without internal limiting membrane peeling for idiopathic epiretinal membrane: A meta-analysis. PLoS ONE 2017, 12, e0179105. [Google Scholar] [CrossRef]
- Snead, D.R.J.; James, S.; Snead, M.P. Pathological changes in the vitreoretinal junction 1: Epiretinal membrane formation. Eye 2008, 22, 1310–1317. [Google Scholar] [CrossRef]
- Park, D.W.; Dugel, P.U.; Garda, J.; Sipperley, J.O.; Thach, A.; Sneed, S.R.; Blaisdell, J. Macular pucker removal with and without internal limiting membrane peeling: Pilot study. Ophthalmology 2003, 110, 62–64. [Google Scholar] [CrossRef]
- Azuma, K.; Ueta, T.; Eguchi, S.; Aihara, M. Effects of internal limiting membrane peeling combined with removal of idiopathic epiretinal membrane: A Systematic Review of Literature and Meta-Analysis. Retina 2017, 37, 1813–1819. [Google Scholar] [CrossRef]
- Chang, S. Controversies regarding internal limiting membrane peeling in idiopathic epiretinal membrane and macular hole. Retina 2012, 32 (Suppl. S2), S200–S203; discussion S203–S204. [Google Scholar] [CrossRef]
- Chang, S.; Gregory-Roberts, E.M.; Park, S.; Laud, K.; Smith, S.D.; Hoang, Q.V. Double peeling during vitrectomy for macular pucker: The Charles L. Schepens Lecture. JAMA Ophthalmol. 2013, 131, 525–530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Govetto, A.; Lalane, R.A.; Sarraf, D.; Figueroa, M.S.; Hubschman, J.P. Insights Into Epiretinal Membranes: Presence of Ectopic Inner Foveal Layers and a New Optical Coherence Tomography Staging Scheme. Am. J. Ophthalmol. 2017, 175, 99–113. [Google Scholar] [CrossRef] [PubMed]
- Oberwahrenbrock, T.; Weinhold, M.; Mikolajczak, J.; Zimmermann, H.; Paul, F.; Beckers, I.; Brandt, A.U. Reliability of Intra-Retinal Layer Thickness Estimates. PLoS ONE 2015, 10, e0137316. [Google Scholar] [CrossRef] [PubMed]
- Crincoli, E.; Savastano, M.C.; Savastano, A.; Caporossi, T.; Bacherini, D.; Miere, A.; Gambini, G.; De Vico, U.; Baldascino, A.; Minnella, A.M.; et al. New artificial intelligence analysis for prediction of long-term visual improvement after epiretinal membrane surgery. Retina 2023, 43, 173–181. [Google Scholar] [CrossRef]
- Catania, F.; Allegrini, D.; Nembri, A.; Confalonieri, F.; Zollet, P.; Crincoli, E.; Romano, M.R. Macular Microvascular Modifications in Progressive Lamellar Macular Holes. Diagnostics 2021, 11, 1717. [Google Scholar] [CrossRef]
- Mitchell, P.; Smith, W.; Chey, T.; Wang, J.J.; Chang, A. Prevalence and associations of epiretinal membranes. The Blue Mountains Eye Study, Australia. Ophthalmology 1997, 104, 1033–1040. [Google Scholar] [CrossRef]
- Aung, K.Z.; Makeyeva, G.; Adams, M.K.; Chong, E.W.-T.; Busija, L.; Giles, G.G.; English, D.R.; Hopper, J.; Baird, P.N.; Guymer, R.H.; et al. The prevalence and risk factors of epiretinal membranes: The Melbourne Collaborative Cohort Study. Retina 2013, 33, 1026–1034. [Google Scholar] [CrossRef]
- Song, S.J.; Lee, M.Y.; Smiddy, W.E. Ganglion cell layer thickness and visual improvement after epiretinal membrane surgery. Investig. Ophthalmol. Vis. Sci. 2015, 56, 5054. [Google Scholar] [CrossRef]
- Inoue, M.; Morita, S.; Watanabe, Y.; Kaneko, T.; Yamane, S.; Kobayashi, S.; Arakawa, A.; Kadonosono, K. Preoperative inner segment/outer segment junction in spectral-domain optical coherence tomography as a prognostic factor in epiretinal membrane surgery. Retina 2011, 31, 1366–1372. [Google Scholar] [CrossRef]
- Shimozono, M.; Oishi, A.; Hata, M.; Matsuki, T.; Ito, S.; Ishida, K.; Kurimoto, Y. The significance of cone outer segment tips as a prognostic factor in epiretinal membrane surgery. Am. J. Ophthalmol. 2012, 153, 698–704, 704.e1. [Google Scholar] [CrossRef]
- Michalewski, J.; Michalewska, Z.; Cisiecki, S.; Nawrocki, J. Morphologically functional correlations of macular pathology connected with epiretinal membrane formation in spectral optical coherence tomography (SOCT). Graefes Arch. Clin. Exp. Ophthalmol. 2007, 245, 1623–1631. [Google Scholar] [CrossRef] [PubMed]
- Scupola, A.; Grimaldi, G.; Abed, E.; Sammarco, M.G.; Giudiceandrea, A.; Greco, A.; Sasso, P.; Blasi, M.A. Arcuate nerve fiber layer changes after internal limiting membrane peeling in idiopathic epiretinal membrane. Retina 2018, 38, 1777–1785. [Google Scholar] [CrossRef] [PubMed]
- Iuliano, L.; Fogliato, G.; Gorgoni, F.; Corbelli, E.; Bandello, F.; Codenotti, M. Idiopathic epiretinal membrane surgery: Safety, efficacy and patient related outcomes. OPTH 2019, 13, 1253–1265. [Google Scholar] [CrossRef] [Green Version]
- Gartner, S.; Henkind, P. Aging and degeneration of the human macula. 1. Outer nuclear layer and photoreceptors. Br. J. Ophthalmol. 1981, 65, 23–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.S.; Chhablani, J.; Chan, C.K.; Cheng, L.; Kozak, I.; Hartmann, K.; Freeman, W.R. Retinal adherence and fibrillary surface changes correlate with surgical difficulty of epiretinal membrane removal. Am. J. Ophthalmol. 2012, 153, 692–697.e2. [Google Scholar] [CrossRef] [Green Version]
- Paovic, J.; Paovic, P.; Paovic, A. Correlation between epiretinal membrane bridging, visual acuity and central macular thickness. J. Cytol. Histol. 2017, 8, 17–20. [Google Scholar] [CrossRef]
- Yamashita, T.; Uemura, A.; Kita, H.; Sakamoto, T. Analysis of the retinal nerve fiber layer after indocyanine green-assisted vitrectomy for idiopathic macular holes. Ophthalmology 2006, 113, 280–284. [Google Scholar] [CrossRef]
- Kim, K.Y.; Yu, S.-Y.; Kim, M.S.; Kim, E.S.; Kwak, H.W. Changes of parafoveal retinal nerve fiber layer thickness analyzed by spectral-domain optical coherence tomography after pars plana vitrectomy. Retina 2013, 33, 776–784. [Google Scholar] [CrossRef]
- Kim, J.H.; Kang, S.W.; Kong, M.G.; Ha, H.S. Assessment of retinal layers and visual rehabilitation after epiretinal membrane removal. Graefes Arch. Clin. Exp. Ophthalmol. 2013, 251, 1055–1064. [Google Scholar] [CrossRef]
- Lee, E.K.; Yu, H.G. Ganglion cell-inner plexiform layer thickness after epiretinal membrane surgery: A spectral-domain optical coherence tomography study. Ophthalmology 2014, 121, 1579–1587. [Google Scholar] [CrossRef]
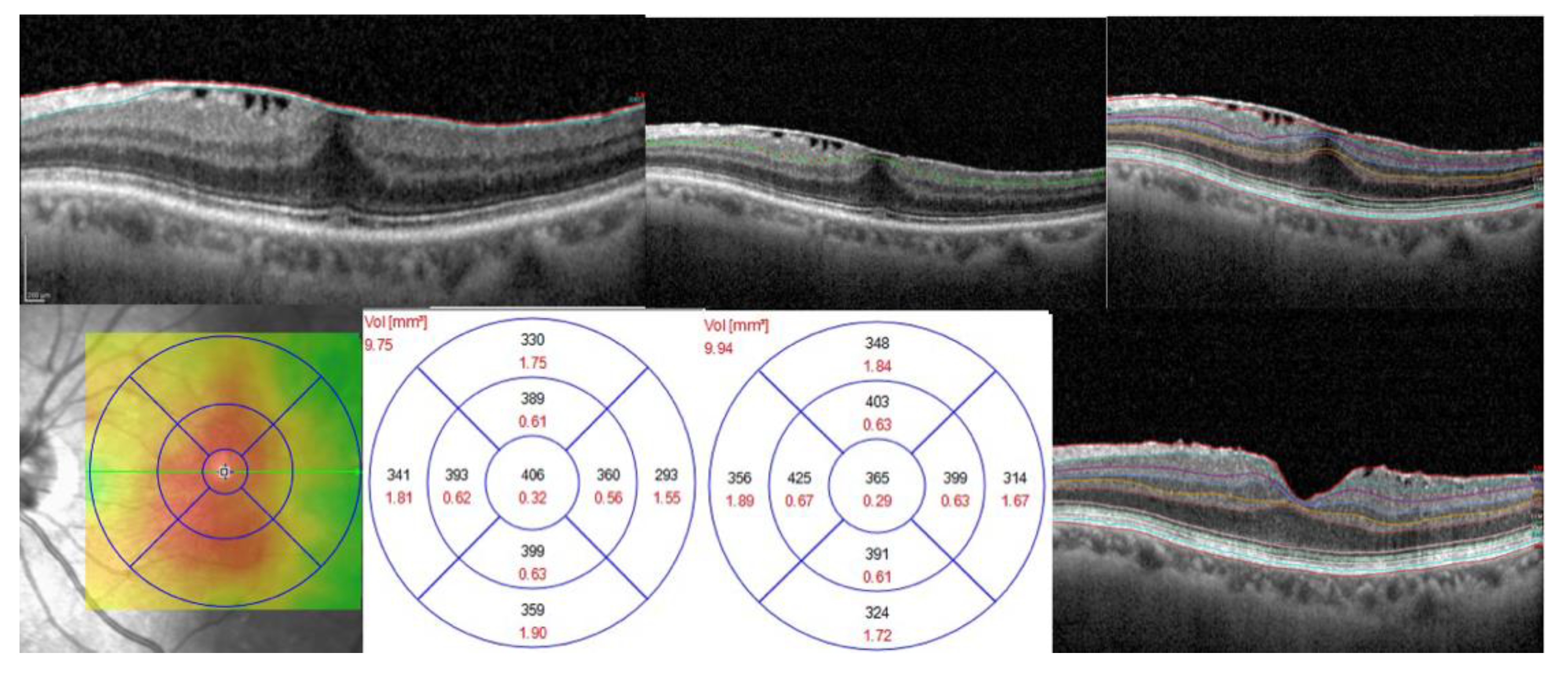

| Variable | Detail | GROUP 1 (20 pts) | GROUP 2 (35 pts) | GROUP 3 (15 pts) | GROUP 4 (15 pts) | p Regression |
|---|---|---|---|---|---|---|
| General data and comorbidities | Sex | 8/20 (40.0%) | 18/35 (51.4%) | 7/15 (46.7%) | 7/15 (46.7%) | 0.88 |
| Age | 72.14 ± 9.92 | 70.43 ± 6.88 | 71.57 ± 6.13 | 68.30 ± 9.49 | 0.73 | |
| CKD | 6/20 (30.0%) | 6/35 (17.2%) | 3/15 (20.0%) | 4/15 (26.7%) | 0.14 | |
| COPD | 5/20 (25.0%) | 7/35 (20.0%) | 4/15 (26.7%) | 4/15 (26.7%) | 0.26 | |
| SAH | 12/20 (60.0%) | 20/35 (57.1%) | 7/15 (46.7%) | 8/15 (53.3%) | 0.27 | |
| Visual acuity (ETDRS letters) | Preoperative BCVA | 67.2 ± 4.8 | 59.1 ± 5.1 | 60.9 ± 4.9 | 65.1 ± 5.0 | 0.35 |
| OCT preoperative macular layers (μm) | CMT | 448.20 ± 87.96 | 464.89 ± 116.35 | 526.00 ± 54.19 | 534.29 ± 75.43 | 0.131 |
| Inner layers | 355.33 ± 91.13 | 378.55 ± 123.60 | 436.88 ± 54.84 | 442.86 ± 84.81 | 0.17 | |
| RNFL | 69.93 ± 50.50 | 89.82 ± 106.01 | 108.88 ± 55.63 | 135.43 ± 111.31 | 0.44 | |
| GCL | 43.13 ± 14.31 | 44.31 ± 15.85 | 48.50 ± 15.65 | 44.43 ± 3.91 | 0.87 | |
| IPL | 42.13 ± 13.05 | 41.52 ± 13.07 | 51.00 ± 11.83 | 45.29 ± 5.06 | 0.26 | |
| INL | 55.40 ± 15.96 | 50.20 ± 15.69 | 57.38 ± 11.69 | 58.00 ± 13.71 | 0.40 | |
| OPL | 33.57 ± 9.52 | 36.40 ± 8.25 | 44.17 ± 6.71 | 34.43 ± 7.09 | 0.078 | |
| ONL | 111.21 ± 27.82 | 118.07 ± 30.18 | 134.00 ± 43.67 | 125.14 ± 28.73 | 0.47 |
| Variable | VA Trend | Preoperative | 1 Month after Surgery | 3 Months after Surgery | 6 Months after Surgery | p | F | Partial Eta Squared |
|---|---|---|---|---|---|---|---|---|
| CMT | GROUP 1 | 0.75 | ||||||
| Inner layers (μm) | GROUP 1 | 394.50 ± 58.40 | 451.00 ± 44.06 | 344.50 ± 55.15 | 305.50 ± 34.70 | <0.001 | 13.380 | 0.728 |
| GROUP 2 | 364.90 ± 24.90 | 325.90 ± 18.79 | 321.36 ± 23.51 | 301.00 ± 14.79 | ||||
| GROUP 3 | 468.66 ± 47.68 | 321.66 ± 35.97 | 288.33 ± 45.03 | 291.67 ± 28.33 | ||||
| GROUP 4 | 430.40 ± 36.94 | 324.40 ± 27.87 | 303.60 ± 34.88 | 330.20 ± 21.95 | ||||
| RNFL (μm) | GROUP 1 | 69.00 ± 51.56 | 111.50 ± 20.69 | 54.00 ± 16.19 | 31.00 ± 14.77 | 0.002 | 3.85 | 0.418 |
| GROUP 2 | 57.73 ± 21.99 | 25.82 ± 8.83 | 27.27 ± 6.90 | 18.45 ± 6.29 | ||||
| GROUP 3 | 135.67 ± 42.10 | 19.00 ± 16.90 | 19.33 ± 13.22 | 18.67 ± 12.06 | ||||
| GROUP 4 | 122.80 ± 32.62 | 44.60 ± 13.09 | 32.60 ± 10.24 | 37.20 ± 9.34 | ||||
| GCL (μm) | 0.88 | |||||||
| IPL (μm) | 0.37 | |||||||
| INL (μm) | 0.55 | |||||||
| OPL (μm) | 0.23 | |||||||
| ONL (μm) | GROUP 1 | 150.50 ± 26.41 | 161.50 ± 10.43 | 118.50 ± 14.83 | 120.50 ± 12.03 | 0.042 | 2.02 | 0.307 |
| GROUP 2 | 125.63 ± 11.28 | 121.73 ± 4.45 | 124.00 ± 6.32 | 120.00 ± 5.13 | ||||
| GROUP 3 | 139.33 ± 21.58 | 122.67 ± 8.52 | 108.67 ± 12.10 | 109.00 ± 9.82 | ||||
| GROUP 4 | 128.20 ± 16.72 | 120.00 ± 6.60 | 127.00 ± 9.38 | 131.20 ± 7.61 | ||||
| GCL/IPL | GROUP 1 | 1.02 ± 0.19 | 1.00 ± 0.37 | 0.95 ± 0.11 | 1.07 ± 0.49 | 0.034 | 2.14 | 0.389 |
| GROUP 2 | 1.07 ± 0.23 | 0.97 ± 0.15 | 1.02 ± 0.18 | 1.02 ± 0.16 | ||||
| GROUP 3 | 0.99 ± 0.33 | 1.04 ± 0.19 | 1.01 ± 0.20 | 1.06 ± 0.13 | ||||
| GROUP 4 | 0.99 ± 0.16 | 1.03 ± 0.13 | 0.95 ± 0.19 | 0.97 ± 0.14 | ||||
| GCL/CMT | GROUP 1 | 0.095 ± 0.012 | 0.090 ± 0.011 | 0.089 ± 0.013 | 0.088 ± 0.012 | 0.013 | 1.627 | 0.498 |
| GROUP 2 | 0.096 ± 0.010 | 0.10 ± 0.009 | 0.096 ± 0.011 | 0.097 ± 0.012 | ||||
| GROUP 3 | 0.093 ± 0.010 | 0.10 ± 0.012 | 0.10 ± 0.013 | 0.10 ± 0.010 | ||||
| GROUP 4 | 0.085 ± 0.012 | 0.10 ± 0.011 | 0.083 ± 0.012 | 0.082 ± 0.009 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romano, M.; Catania, F.; Vallejo-Garcia, J.L.; Sorrentino, T.; Crincoli, E.; Vinciguerra, P. Variability of Visual Recovery with Time in Epiretinal Membrane Surgery: A Predictive Analysis Based on Retinal Layer OCT Thickness Changes. J. Clin. Med. 2023, 12, 2107. https://doi.org/10.3390/jcm12062107
Romano M, Catania F, Vallejo-Garcia JL, Sorrentino T, Crincoli E, Vinciguerra P. Variability of Visual Recovery with Time in Epiretinal Membrane Surgery: A Predictive Analysis Based on Retinal Layer OCT Thickness Changes. Journal of Clinical Medicine. 2023; 12(6):2107. https://doi.org/10.3390/jcm12062107
Chicago/Turabian StyleRomano, Mary, Fiammetta Catania, Josè Luis Vallejo-Garcia, Tania Sorrentino, Emanuele Crincoli, and Paolo Vinciguerra. 2023. "Variability of Visual Recovery with Time in Epiretinal Membrane Surgery: A Predictive Analysis Based on Retinal Layer OCT Thickness Changes" Journal of Clinical Medicine 12, no. 6: 2107. https://doi.org/10.3390/jcm12062107






