Preoperative Decreased Hounsfield Unit Values of Cervical Vertebrae and the Relative Cross-Sectional Area of Flexion/Extension Paraspinal Muscles Are Novel Risk Factors for the Loss of Cervical Lordosis after Open-Door Laminoplasty
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
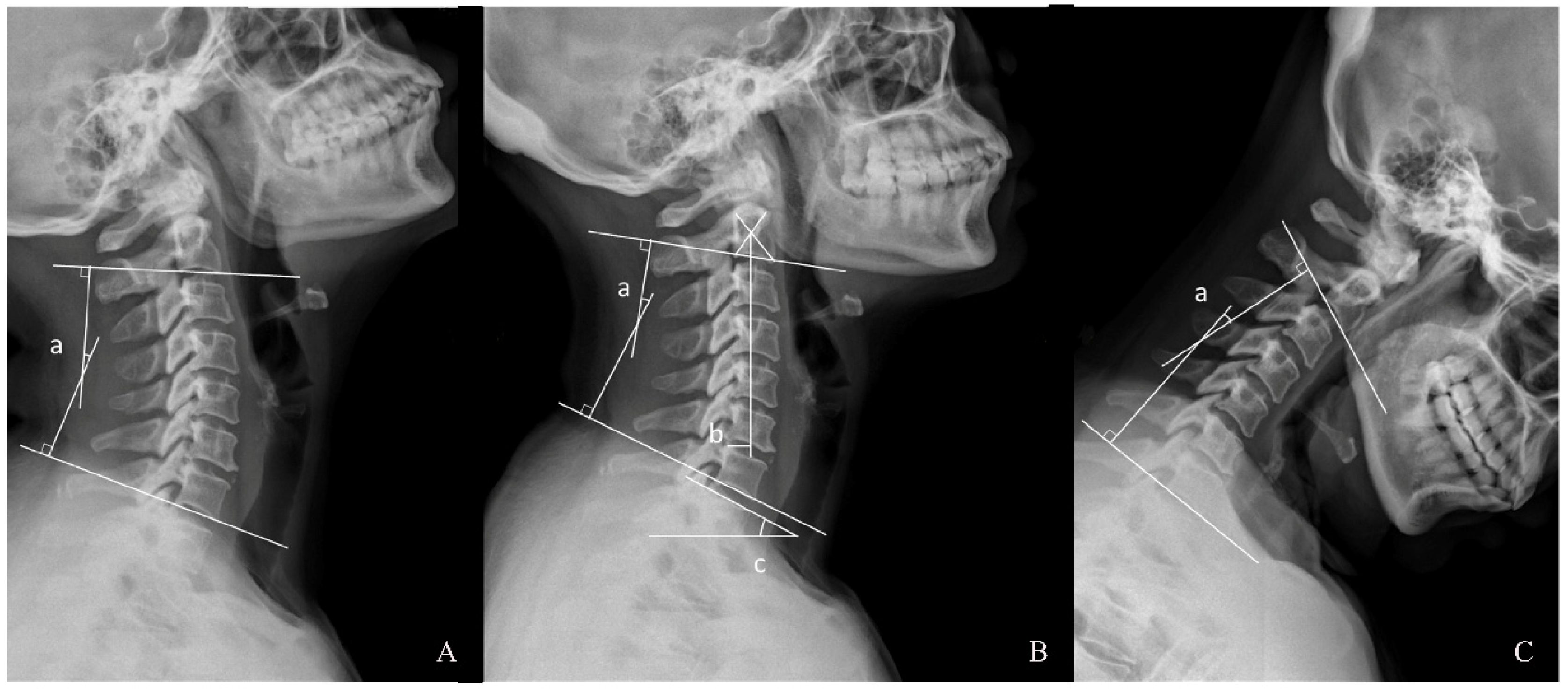
2.2. Radiographic Assessment
2.3. Clinical Function Assessment
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hirabayashi, K.; Watanabe, K.; Wakano, K.; Suzuki, N.; Satomi, K.; Ishii, Y. Expansive open-door laminoplasty for cervical spinal stenotic myelopathy. Spine 1983, 8, 693–699. [Google Scholar] [CrossRef]
- Hirabayashi, K.; Watanabe, K. A Review of My Invention of Expansive Laminoplasty. Neurospine 2019, 16, 379–382. [Google Scholar] [CrossRef] [PubMed]
- Aita, I.; Wadano, Y.; Yabuki, T. Curvature and range of motion of the cervical spine after laminaplasty. J. Bone Jt. Surg. Am. 2000, 82, 1743–1748. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, Y.; Toyama, Y.; Chiba, K.; Matsumoto, M.; Nakamura, M.; Takaishi, H.; Hirabayashi, H.; Hirabayashi, K. Long-term results of expansive open-door laminoplasty for ossification of the posterior longitudinal ligament of the cervical spine. J. Neurosurg. Spine 2004, 1, 168–174. [Google Scholar] [CrossRef]
- Suk, K.S.; Kim, K.T.; Lee, J.H.; Lee, S.H.; Lim, Y.J.; Kim, J.S. Sagittal alignment of the cervical spine after the laminoplasty. Spine 2007, 32, E656–E660. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Son, D.W.; Lee, S.H.; Kim, D.H.; Lee, S.W.; Song, G.S. The Predictable Factors of the Postoperative Kyphotic Change of Sagittal Alignment of the Cervical Spine after the Laminoplasty. J. Korean Neurosurg. Soc. 2017, 60, 577–583. [Google Scholar] [CrossRef] [Green Version]
- Xu, C.; Zhang, Y.; Dong, M.; Wu, H.; Yu, W.; Tian, Y.; Cao, P.; Chen, H.; Wang, X.; Shen, X.; et al. The relationship between preoperative cervical sagittal balance and clinical outcome of laminoplasty treated cervical ossification of the posterior longitudinal ligament patients. Spine J. 2020, 20, 1422–1429. [Google Scholar] [CrossRef]
- Koda, M.; Mochizuki, M.; Konishi, H.; Aiba, A.; Kadota, R.; Inada, T.; Kamiya, K.; Ota, M.; Maki, S.; Takahashi, K.; et al. Comparison of clinical outcomes between laminoplasty, posterior decompression with instrumented fusion, and anterior decompression with fusion for K-line (-) cervical ossification of the posterior longitudinal ligament. Eur. Spine J. 2016, 25, 2294–2301. [Google Scholar] [CrossRef]
- Zhang, J.T.; Li, J.Q.; Niu, R.J.; Liu, Z.; Tong, T.; Shen, Y. Predictors of cervical lordosis loss after laminoplasty in patients with cervical spondylotic myelopathy. Eur. Spine J. 2017, 26, 1205–1210. [Google Scholar] [CrossRef]
- Kim, K.R.; Lee, C.K.; Park, J.Y.; Kim, I.S. Preoperative Parameters for Predicting the Loss of Lordosis After Cervical Laminoplasty. Spine 2020, 45, 1476–1484. [Google Scholar] [CrossRef]
- Kim, B.; Yoon, D.H.; Ha, Y.; Yi, S.; Shin, D.A.; Lee, C.K.; Lee, N.; Kim, K.N. Relationship between T1 slope and loss of lordosis after laminoplasty in patients with cervical ossification of the posterior longitudinal ligament. Spine J. 2016, 16, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Son, D.W.; Lee, J.S.; Sung, S.K.; Lee, S.W.; Song, G.S. Does Extension Dysfunction Affect Postoperative Loss of Cervical Lordosis in Patients Who Undergo Laminoplasty? Spine 2019, 44, E456–E464. [Google Scholar] [CrossRef] [PubMed]
- Fujishiro, T.; Nakano, A.; Yano, T.; Nakaya, Y.; Hayama, S.; Usami, Y.; Nozawa, S.; Baba, I.; Neo, M. Significance of flexion range of motion as a risk factor for kyphotic change after cervical laminoplasty. J. Clin. Neurosci. Off. J. Neurosurg. Soc. Australas. 2020, 76, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.J.; Park, J.H.; Jeon, S.R.; Rhim, S.C.; Roh, S.W. Importance of the preoperative cross-sectional area of the semispinalis cervicis as a risk factor for loss of lordosis after laminoplasty in patients with cervical spondylotic myelopathy. Eur. Spine J. 2018, 27, 2720–2728. [Google Scholar] [CrossRef]
- Schreiber, J.J.; Anderson, P.A.; Rosas, H.G.; Buchholz, A.L.; Au, A.G. Hounsfield units for assessing bone mineral density and strength: A tool for osteoporosis management. J. Bone Jt. Surg. Am. 2011, 93, 1057–1063. [Google Scholar] [CrossRef]
- Fortin, M.; Battié, M.C. Quantitative paraspinal muscle measurements: Inter-software reliability and agreement using OsiriX and ImageJ. Phys. Ther. 2012, 92, 853–864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Z.; Bai, Z.; Yan, J.; Zhang, Y.; Li, S.; Yuan, L.; Huang, D.; Ye, W. Association Between Muscle Morphology Changes, Cervical Spine Degeneration, and Clinical Features in Patients with Chronic Nonspecific Neck Pain: A Magnetic Resonance Imaging Analysis. World Neurosurg. 2022, 159, e273–e284. [Google Scholar] [CrossRef]
- Furlan, J.C.; Catharine Craven, B. Psychometric analysis and critical appraisal of the original, revised, and modified versions of the Japanese Orthopaedic Association score in the assessment of patients with cervical spondylotic myelopathy. Neurosurg. Focus 2016, 40, E6. [Google Scholar] [CrossRef] [Green Version]
- Lee, B.S.; Walsh, K.M.; Lubelski, D.; Knusel, K.D.; Steinmetz, M.P.; Mroz, T.E.; Schlenk, R.P.; Kalfas, I.H.; Benzel, E.C. The effect of C2-3 disc angle on postoperative adverse events in cervical spondylotic myelopathy. J. Neurosurg. Spine 2018, 30, 38–45. [Google Scholar] [CrossRef] [Green Version]
- Sharma, R.; Borkar, S.; Katiyar, V.; Goda, R.; Phalak, M.; Joseph, L.; Suri, A.; Chandra, P.S.; Kale, S.S. Interplay of Dynamic Extension Reserve and T1 Slope in Determining the Loss of Cervical Lordosis Following Laminoplasty: A Novel Classification System. World Neurosurg. 2020, 136, e33–e40. [Google Scholar] [CrossRef]
- Fujishiro, T.; Hayama, S.; Obo, T.; Nakaya, Y.; Nakano, A.; Usami, Y.; Nozawa, S.; Baba, I.; Neo, M. Gap between flexion and extension ranges of motion: A novel indicator to predict the loss of cervical lordosis after laminoplasty in patients with cervical spondylotic myelopathy. J. Neurosurg. Spine 2021, 35, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Iizuka, H.; Nakajima, T.; Iizuka, Y.; Sorimachi, Y.; Ara, T.; Nishinome, M.; Takagishi, K. Cervical malalignment after laminoplasty: Relationship to deep extensor musculature of the cervical spine and neurological outcome. J. Neurosurg. Spine 2007, 7, 610–614. [Google Scholar] [CrossRef] [PubMed]
- Iizuka, H.; Shimizu, T.; Tateno, K.; Toda, N.; Edakuni, H.; Shimada, H.; Takagishi, K. Extensor musculature of the cervical spine after laminoplasty: Morphologic evaluation by coronal view of the magnetic resonance image. Spine 2001, 26, 2220–2226. [Google Scholar] [CrossRef] [PubMed]
- Vasavada, A.N.; Li, S.; Delp, S.L. Influence of muscle morphometry and moment arms on the moment-generating capacity of human neck muscles. Spine 1998, 23, 412–422. [Google Scholar] [CrossRef]
- Lin, S.; Lin, T.; Wu, Z.; Chen, G.; Shangguan, Z.; Wang, Z.; Liu, W. Does the asymmetry and extension function of the preoperative cervical paraspinal extensor predict postoperative cervical sagittal deformity in patients who undergo modified laminoplasty? Spine J. 2022, 22, 1953–1963. [Google Scholar] [CrossRef]
- Fujibayashi, S.; Neo, M.; Yoshida, M.; Miyata, M.; Takemoto, M.; Nakamura, T. Neck muscle strength before and after cervical laminoplasty: Relation to axial symptoms. J. Spinal Disord. Tech. 2010, 23, 197–202. [Google Scholar] [CrossRef]
- Colantonio, D.F.; Saxena, S.K.; Vanier, A.; Rodkey, D.; Tintle, S.; Wagner, S.C. Cervical Spine Computed Tomography Hounsfield Units Accurately Predict Low Bone Mineral Density of the Femoral Neck. Clin. Spine Surg. 2020, 33, E58–E62. [Google Scholar] [CrossRef]
- Lovecchio, F.; Ang, B.; Louie, P.K.; Chaudary, C.; Shah, S.P.; Punyala, A.; Yao, Y.C.; Steinhaus, M.; McCarthy, M.H.; Huang, R.; et al. Bone Density Distribution in the Cervical Spine. Glob. Spine J. 2022, 21925682221098965. [Google Scholar] [CrossRef]
- Yang, Z.; Griffith, J.F.; Leung, P.C.; Lee, R. Effect of osteoporosis on morphology and mobility of the lumbar spine. Spine 2009, 34, E115–E121. [Google Scholar] [CrossRef]
- Landham, P.R.; Gilbert, S.J.; Baker-Rand, H.L.; Pollintine, P.; Robson Brown, K.A.; Adams, M.A.; Dolan, P. Pathogenesis of Vertebral Anterior Wedge Deformity: A 2-Stage Process? Spine 2015, 40, 902–908. [Google Scholar] [CrossRef]
- Salzmann, S.N.; Okano, I.; Miller, C.O.; Chiapparelli, E.; Reisener, M.J.; Amini, D.A.; Winter, F.; Shue, J.; Carrino, J.A.; Sama, A.A.; et al. The cervical spine demonstrates less postoperative bone loss than the lumbar spine. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2022, 40, 654–660. [Google Scholar] [CrossRef] [PubMed]
- Demir, Ö.; Öksüz, E.; Deniz, F.E.; Demir, O. Assessing the effects of lumbar posterior stabilization and fusion to vertebral bone density in stabilized and adjacent segments by using Hounsfield unit. J. Spine Surg. 2017, 3, 548–553. [Google Scholar] [CrossRef] [PubMed]


| Variable | Descriptive Statistics | p |
|---|---|---|
| Age (year) | 56.8 ± 8.4 | |
| Sex | ||
| Male | 28 (66.7%) | |
| Female | 14 (33.3%) | |
| BMI (kg/m2) | 25.7 ± 2.29 | |
| Follow-up period (month) | 24.9 ± 21.7 | |
| Operation level | ||
| C3–6 | 26 (61.9%) | |
| Involve C2/C7 | 16 (38.1%) | |
| JOA score | ||
| Preoperative | 12.2 ± 1.2 | |
| Follow-up | 14.1 ± 0.72 | <0.01 ** |
| JOA mean recovery rate | 37.8 ± 14.7% | |
| C2–7 Cobb (°) | ||
| Preoperative | 18.0 ± 9.7 | |
| Follow-up | 12.9 ± 10.8 | <0.01 ** |
| C2–7 SVA (mm) | ||
| Preoperative | 12.9 ± 6.5 | |
| Follow-up | 17.2 ± 11.9 | 0.027 * |
| T1S (°) | ||
| Preoperative | 24.9 ± 6.4 | |
| Follow-up | 22.7 ± 7.8 | 0.024 * |
| fROM (°) | 33.5 ± 9.8 | |
| eROM (°) | 8.5 ± 5.0 | |
| ROM (°) | 42.1 ± 9.7 | |
| fROM/eROM | 9.8 ± 23.9 | |
| eROM/fROM | 0.21 ± 0.13 |
| Variable | LCL | NLCL | p | |
|---|---|---|---|---|
| Age | 58.1 ± 8.0 | 55.2 ± 8.9 | 0.264 | |
| Sex (M/F) | 18/6 | 10/8 | 0.186 | |
| BMI (kg/m2) | 25.74 | 25.90 | 0.268 | |
| C2/C7 Involved (Yes/No) | 8/16 | 10/8 | 0.211 | |
| Follow-up period (month) | 23.7 ± 20.3 | 26.7 ± 23.9 | 0.664 | |
| JOA score | Preoperative | 12.2 ± 1.0 | 12.2 ± 1.5 | 0.887 |
| Follow-up | 13.9 ± 0.6 | 14.4 ± 0.8 | 0.021 * | |
| Mean JOA recovery rate | 34.1 ± 11.6 | 42.9 ± 17.1 | 0.053 |
| Variable | LCL | NLCL | p | |
|---|---|---|---|---|
| C2–7 Cobb (°) | Preoperative | 19.49 ± 8.20 | 15.98 ± 11.41 | 0.253 |
| Follow-up | 9.20 ± 9.33 | 17.88 ± 10.88 | <0.01 ** | |
| T1S (°) | Preoperative | 27.46 ± 6.39 | 22.42 ± 6.48 | 0.016 * |
| Follow-up | 24.23 ± 8.42 | 21.25 ± 7.12 | 0.233 | |
| C2–7 SVA (mm) | Preoperative | 11.91 ± 5.70 | 14.28 ± 7.33 | 0.244 |
| Follow-up | 21.73 ± 11.88 | 11.10 ± 9.11 | <0.01 ** | |
| fROM (°) | Preoperative | 38.63 ± 7.54 | 26.71 ± 8.37 | <0.01 ** |
| eROM (°) | Preoperative | 7.11 ± 4.62 | 10.43 ± 4.95 | 0.031 * |
| ROM (°) | Preoperative | 45.7 ± 7.8 | 37.14 ± 9.97 | <0.01 ** |
| fROM/eROM | Preoperative | 14.67 ± 30.99 | 3.34 ± 2.18 | <0.01 ** |
| Muscles RCSA | ||||
| LCo + LCa | Preoperative | 0.47 ± 0.12 | 0.38 ± 0.10 | 0.019 * |
| SCM | Preoperative | 1.59 ± 0.40 | 1.39 ± 0.30 | 0.087 |
| Mult | Preoperative | 0.39 ± 0.15 | 0.46 ± 0.11 | 0.139 |
| SeCe | Preoperative | 0.49 ± 0.14 | 0.56 ± 0.17 | 0.144 |
| SeCa | Preoperative | 0.67 ± 0.24 | 0.77 ± 0.22 | 0.157 |
| SpCe + SpCa | Preoperative | 0.90 ± 0.21 | 0.84 ± 0.20 | 0.295 |
| LSc | Preoperative | 1.17 ± 0.22 | 1.19 ± 0.31 | 0.790 |
| Flexion muscles | Preoperative | 2.06 ± 0.48 | 1.77 ± 0.38 | 0.046 * |
| Extension muscles | Preoperative | 3.62 ± 0.72 | 3.81 ± 0.90 | 0.441 |
| Flexion/Extension muscles | Preoperative | 0.57 ± 0.11 | 0.47 ± 0.08 | <0.01 ** |
| HU values | ||||
| C2 | Preoperative | 354.75 ± 86.31 | 429.22 ± 81.02 | 0.007 ** |
| C3 | Preoperative | 310.29 ± 68.60 | 414.83 ± 83.33 | <0.01 ** |
| C4 | Preoperative | 328.13 ± 64.55 | 428.44 ± 102.03 | <0.01 ** |
| C5 | Preoperative | 317.04 ± 68.53 | 408.94 ± 97.88 | <0.01 ** |
| C6 | Preoperative | 260.67 ± 58.20 | 335.06 ± 87.22 | <0.01 ** |
| C7 | Preoperative | 244.58 ± 57.43 | 302.17 ± 65.23 | <0.01 ** |
| Mean HU values | Preoperative | 302.75 ± 64.70 | 386.61 ± 81.89 | <0.01 ** |
| Age | BMI | JOA Mean Recovery Rate | Pre- C2–7 Cobb | Pre-T1S | Pre- C2–7 SVA | fROM | eROM | ROM | fROM/eROM | LCo + LCa RCSA | SCMRCSA | Mult RCSA | SeCe RCSA | SeCa RCSA | SpCe + SpCa RCSA | LSc RCSA | Flexion Muscles RCSA | Extension Muscles RCSA | Flexion/Extension Muscles RCSA | HU Values | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LCL | r | −0.088 | 0.037 | −0.430 | −0.260 | 0.437 | 0.034 | 0.485 | −0.386 | 0.293 | 0.545 | 0.317 | 0.145 | −0.264 | −0.279 | −0.301 | 0.079 | −0.066 | 0.182 | −0.158 | 0.421 | −0.352 |
| p | 0.869 | 0.814 | 0.005 ** | 0.096 | 0.004 ** | 0.833 | 0.001 ** | 0.011 ** | 0.060 | 0.001 ** | 0.041 * | 0.360 | 0.091 | 0.074 | 0.053 | 0.617 | 0.679 | 0.248 | 0.038 | 0.006 ** | 0.022 * |
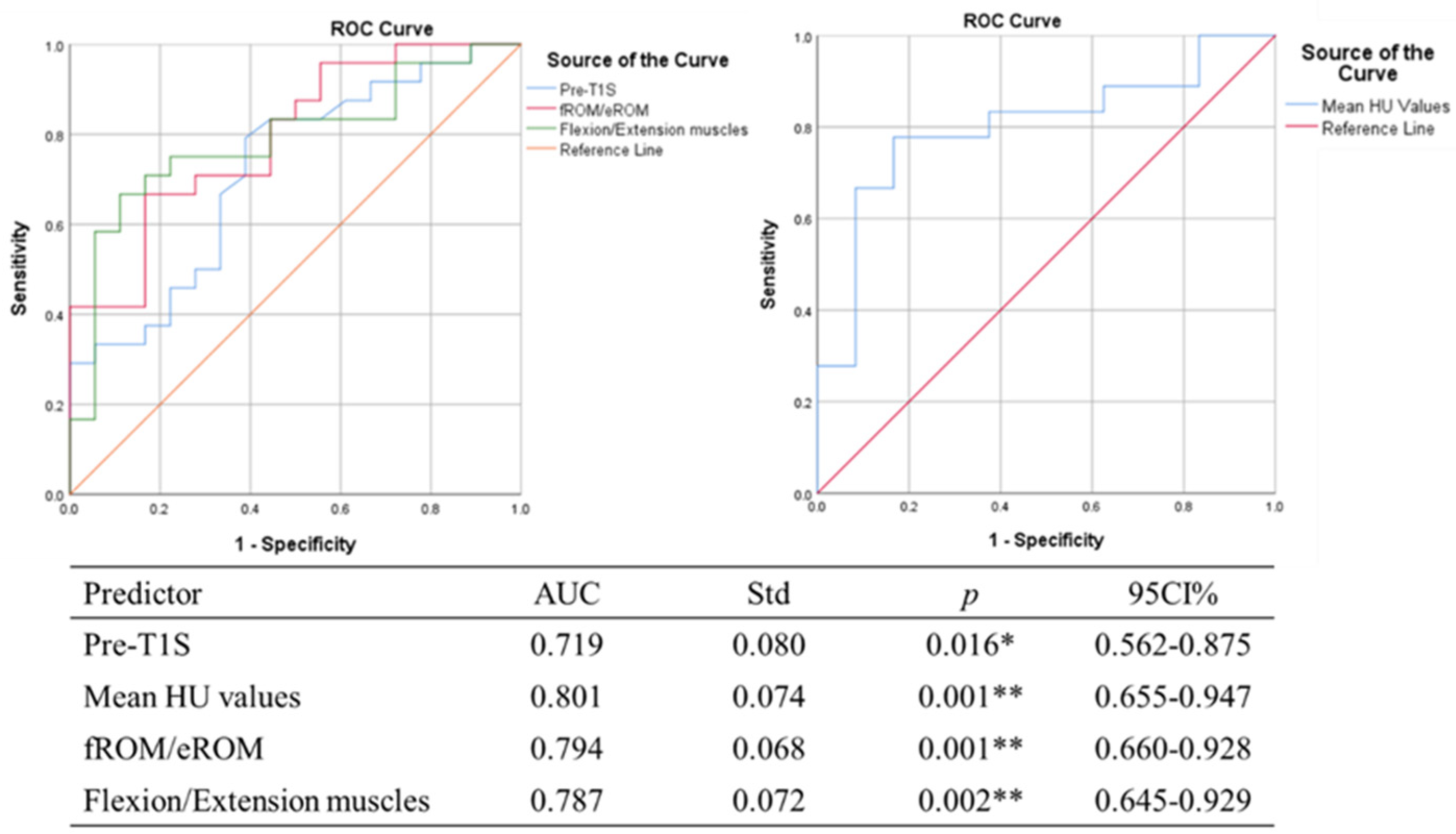
| Unstandardized Coefficients | Standardized Coefficients | p Value | |||
|---|---|---|---|---|---|
| Β | SE | β | t | ||
| Constant | −9.822 | 8.622 | −1.139 | 0.262 | |
| Pre-T1S | 0.422 | 0.145 | 0.367 | 2.910 | <0.01 ** |
| Mean HU values | −0.029 | 0.041 | −0.305 | 2.666 | 0.018 * |
| fROM/eROM | 0.111 | 0.012 | 0.336 | −2.479 | 0.011 * |
| Flexion/Extension muscles | 24.276 | 8.881 | 0.336 | 2.733 | 0.010 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, W.; Li, S.; Shi, H.; Li, Y.; Qiu, J.; Zhou, J.; Huang, D.; Peng, Y.; Gao, W.; Liang, A. Preoperative Decreased Hounsfield Unit Values of Cervical Vertebrae and the Relative Cross-Sectional Area of Flexion/Extension Paraspinal Muscles Are Novel Risk Factors for the Loss of Cervical Lordosis after Open-Door Laminoplasty. J. Clin. Med. 2023, 12, 2119. https://doi.org/10.3390/jcm12062119
Hu W, Li S, Shi H, Li Y, Qiu J, Zhou J, Huang D, Peng Y, Gao W, Liang A. Preoperative Decreased Hounsfield Unit Values of Cervical Vertebrae and the Relative Cross-Sectional Area of Flexion/Extension Paraspinal Muscles Are Novel Risk Factors for the Loss of Cervical Lordosis after Open-Door Laminoplasty. Journal of Clinical Medicine. 2023; 12(6):2119. https://doi.org/10.3390/jcm12062119
Chicago/Turabian StyleHu, Wenjun, Shaoguang Li, Huihong Shi, Yong Li, Jincheng Qiu, Jinlang Zhou, Dongsheng Huang, Yan Peng, Wenjie Gao, and Anjing Liang. 2023. "Preoperative Decreased Hounsfield Unit Values of Cervical Vertebrae and the Relative Cross-Sectional Area of Flexion/Extension Paraspinal Muscles Are Novel Risk Factors for the Loss of Cervical Lordosis after Open-Door Laminoplasty" Journal of Clinical Medicine 12, no. 6: 2119. https://doi.org/10.3390/jcm12062119




