Dyslipidemia Exacerbates Meibomian Gland Dysfunction: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Database Retrieval and Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Data Extraction
2.4. Risk-of-Bias Assessment
2.5. Assessment of Study Quality
2.6. Statistical Analysis
3. Results
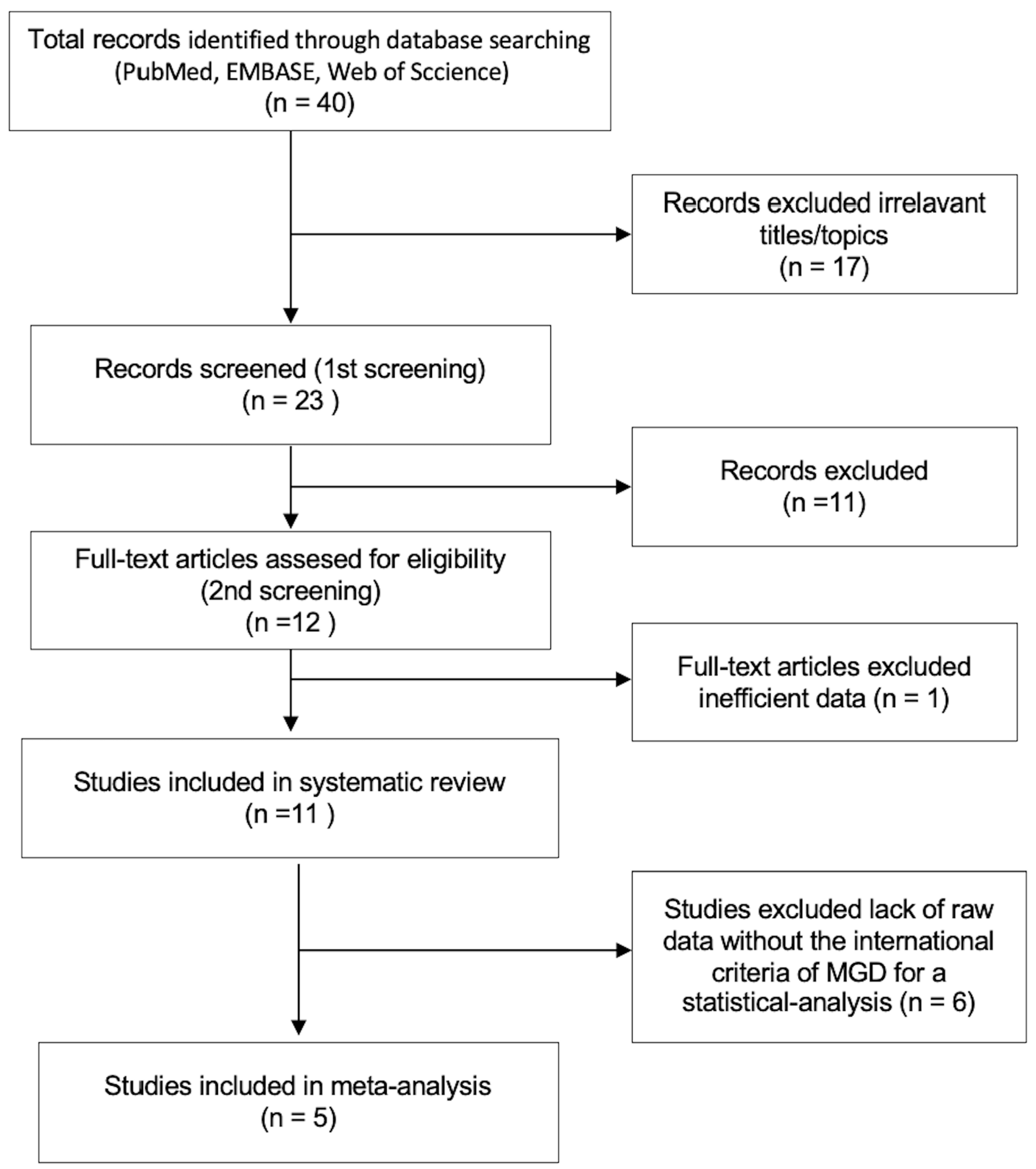
3.1. Study Selection
3.2. Study Characteristics
3.3. Quality of Evidence Assessment
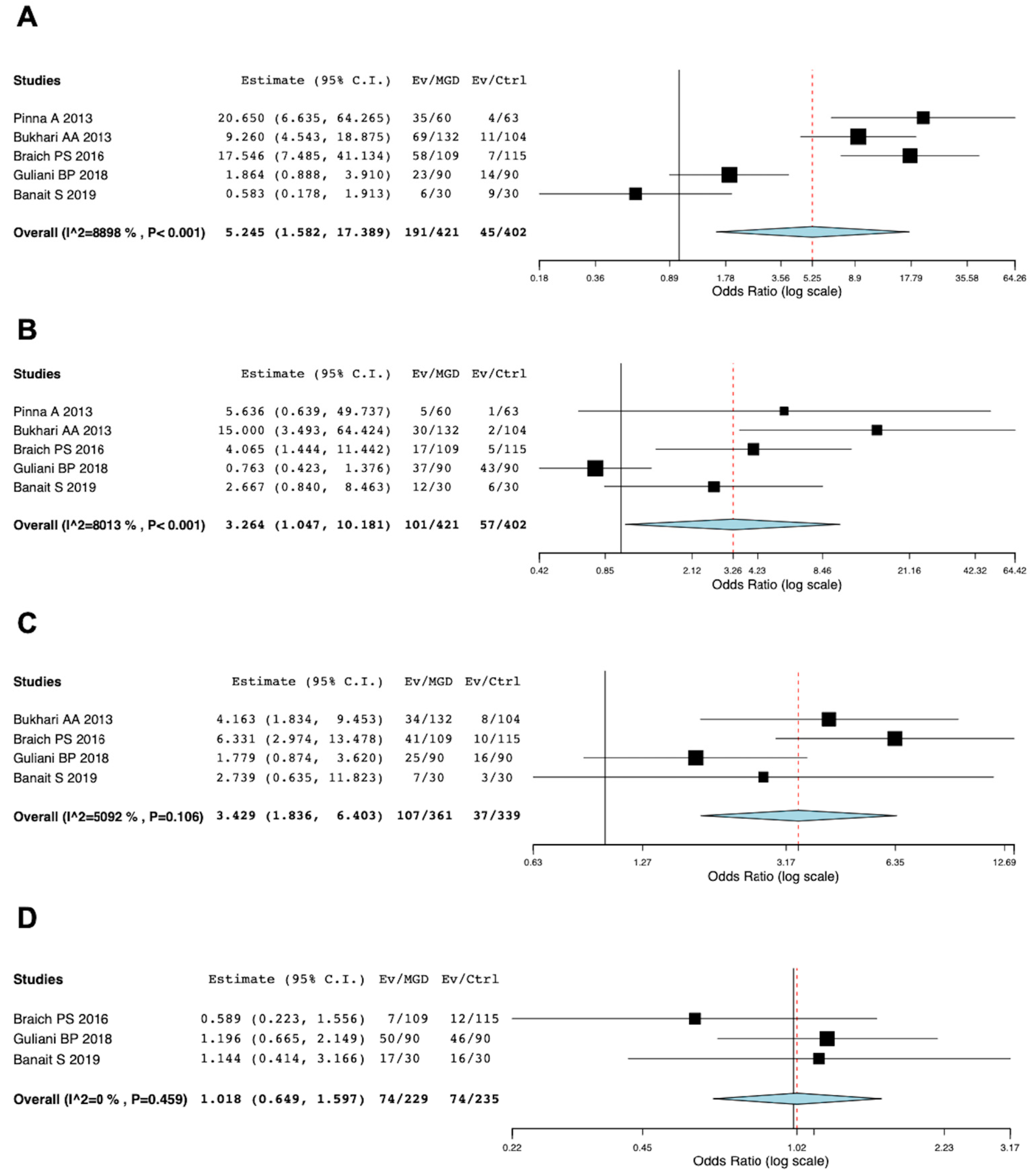
3.4. Risk of MGD in Dyslipidemia Patients
3.5. Meta-Analysis of Risk of Dyslipidemia in MGD Patients
3.6. MGD Severity and Dyslipidemia
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Clayton, J.A. Dry Eye. N. Engl. J. Med. 2018, 378, 2212–2223. [Google Scholar] [CrossRef] [PubMed]
- Craig, J.P.; Nelson, J.D.; Azar, D.T.; Belmonte, C.; Bron, A.J.; Chauhan, S.K.; de Paiva, C.S.; Gomes, J.A.P.; Hammitt, K.M.; Jones, L.; et al. TFOS DEWS II Report Executive Summary. Ocul. Surf. 2017, 15, 802–812. [Google Scholar] [CrossRef]
- McCann, P.; Abraham, A.G.; Mukhopadhyay, A.; Panagiotopoulou, K.; Chen, H.; Rittiphairoj, T.; Gregory, D.G.; Hauswirth, S.G.; Ifantides, C.; Qureshi, R.; et al. Prevalence and Incidence of Dry Eye and Meibomian Gland Dysfunction in the United States: A Systematic Review and Meta-analysis. JAMA Ophthalmol. 2022, 140, 1181–1192. [Google Scholar] [CrossRef] [PubMed]
- Tutt, R.; Bradley, A.; Begley, C.; Thibos, L.N. Optical and visual impact of tear break-up in human eyes. Investig. Ophthalmol. Vis. Sci. 2000, 41, 4117–4123. [Google Scholar]
- Stapleton, F.; Alves, M.; Bunya, V.Y.; Jalbert, I.; Lekhanont, K.; Malet, F.; Na, K.S.; Schaumberg, D.; Uchino, M.; Vehof, J.; et al. TFOS DEWS II Epidemiology Report. Ocul. Surf. 2017, 15, 334–365. [Google Scholar] [CrossRef] [PubMed]
- Amano, S.; Inoue, K. Estimation of Prevalence of Meibomian Gland Dysfunction in Japan. Cornea 2017, 36, 684–688. [Google Scholar] [CrossRef] [PubMed]
- Schein, O.D.; Munoz, B.; Tielsch, J.M.; Bandeen-Roche, K.; West, S. Prevalence of dry eye among the elderly. Am. J. Ophthalmol. 1997, 124, 723–728. [Google Scholar] [CrossRef] [PubMed]
- Viso, E.; Rodriguez-Ares, M.T.; Abelenda, D.; Oubina, B.; Gude, F. Prevalence of asymptomatic and symptomatic meibomian gland dysfunction in the general population of Spain. Investig. Ophthalmol. Vis. Sci. 2012, 53, 2601–2606. [Google Scholar] [CrossRef]
- Jie, Y.; Xu, L.; Wu, Y.Y.; Jonas, J.B. Prevalence of dry eye among adult Chinese in the Beijing Eye Study. Eye 2009, 23, 688–693. [Google Scholar] [CrossRef] [PubMed]
- Arita, R.; Mizoguchi, T.; Kawashima, M.; Fukuoka, S.; Koh, S.; Shirakawa, R.; Suzuki, T.; Morishige, N. Meibomian Gland Dysfunction and Dry Eye Are Similar but Different Based on a Population-Based Study: The Hirado-Takushima Study in Japan. Am. J. Ophthalmol. 2019, 207, 410–418. [Google Scholar] [CrossRef]
- Lekhanont, K.; Rojanaporn, D.; Chuck, R.S.; Vongthongsri, A. Prevalence of dry eye in Bangkok, Thailand. Cornea 2006, 25, 1162–1167. [Google Scholar] [CrossRef]
- Uchino, M.; Dogru, M.; Yagi, Y.; Goto, E.; Tomita, M.; Kon, T.; Saiki, M.; Matsumoto, Y.; Uchino, Y.; Yokoi, N.; et al. The features of dry eye disease in a Japanese elderly population. Optom. Vis. Sci. Off. Publ. Am. Acad. Optom. 2006, 83, 797–802. [Google Scholar] [CrossRef]
- Lin, P.Y.; Tsai, S.Y.; Cheng, C.Y.; Liu, J.H.; Chou, P.; Hsu, W.M. Prevalence of dry eye among an elderly Chinese population in Taiwan: The Shihpai Eye Study. Ophthalmology 2003, 110, 1096–1101. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.A.; Stolzing, A. The role of lipid metabolism in aging, lifespan regulation, and age-related disease. Aging Cell 2019, 18, e13048. [Google Scholar] [CrossRef]
- Ericsson, S.; Berglund, L.; Frostegard, J.; Einarsson, K.; Angelin, B. The influence of age on low density lipoprotein metabolism: Effects of cholestyramine treatment in young and old healthy male subjects. J. Intern. Med. 1997, 242, 329–337. [Google Scholar] [CrossRef]
- Liu, H.H.; Li, J.J. Aging and dyslipidemia: A review of potential mechanisms. Ageing Res. Rev. 2015, 19, 43–52. [Google Scholar] [CrossRef]
- Kreisberg, R.A.; Kasim, S. Cholesterol metabolism and aging. Am. J. Med. 1987, 82, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Barson, J.R.; Karatayev, O.; Gaysinskaya, V.; Chang, G.Q.; Leibowitz, S.F. Effect of dietary fatty acid composition on food intake, triglycerides, and hypothalamic peptides. Regul. Pept. 2012, 173, 13–20. [Google Scholar] [CrossRef]
- Kuriakose, R.K.; Braich, P.S. Dyslipidemia and its Association with Meibomian Gland Dysfunction: A Systematic Review. Int. Ophthalmol. 2018, 38, 1809–1816. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Stroup, D.F.; Berlin, J.A.; Morton, S.C.; Olkin, I.; Williamson, G.D.; Rennie, D.; Moher, D.; Becker, B.J.; Sipe, T.A.; Thacker, S.B. Meta-analysis of observational studies in epidemiology: A proposal for reporting. Meta-analysis of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000, 283, 2008–2012. [Google Scholar] [CrossRef] [PubMed]
- Hoy, D.; Brooks, P.; Woolf, A.; Blyth, F.; March, L.; Bain, C.; Baker, P.; Smith, E.; Buchbinder, R. Assessing risk of bias in prevalence studies: Modification of an existing tool and evidence of interrater agreement. J. Clin. Epidemiol. 2012, 65, 934–939. [Google Scholar] [CrossRef]
- Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010, 25, 603–605. [Google Scholar] [CrossRef]
- Takagi, H.; Hari, Y.; Kawai, N.; Ando, T.; Group, A. Meta-Analysis and Meta-Regression of Transcatheter Aortic Valve Implantation for Pure Native Aortic Regurgitation. Heart Lung Circ. 2020, 29, 729–741. [Google Scholar] [CrossRef]
- Inomata, T.; Kitazawa, K.; Kuno, T.; Sung, J.; Nakamura, M.; Iwagami, M.; Takagi, H.; Midorikawa-Inomata, A.; Zhu, J.; Fujimoto, K.; et al. Clinical and Prodromal Ocular Symptoms in Coronavirus Disease: A Systematic Review and Meta-Analysis. Investig. Ophthalmol. Vis. Sci. 2020, 61, 29. [Google Scholar] [CrossRef] [PubMed]
- Bukhari, A.A. Associations between the grade of meibomian gland dysfunction and dyslipidemia. Ophthalmic Plast. Reconstr. Surg. 2013, 29, 101–103. [Google Scholar] [CrossRef]
- Braich, P.S.; Howard, M.K.; Singh, J.S. Dyslipidemia and its association with meibomian gland dysfunction. Int. Ophthalmol. 2016, 36, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Guliani, B.P.; Bhalla, A.; Naik, M.P. Association of the severity of meibomian gland dysfunction with dyslipidemia in Indian population. Indian J. Ophthalmol. 2018, 66, 1411–1416. [Google Scholar] [PubMed]
- Pinna, A.; Blasetti, F.; Zinellu, A.; Carru, C.; Solinas, G. Meibomian gland dysfunction and hypercholesterolemia. Ophthalmology 2013, 120, 2385–2389. [Google Scholar] [CrossRef] [PubMed]
- Banait, S.H.Y. Association of meibomian gland dysfunction with dyslipidemia contributors. J. Datta Meghe Inst. Med. Sci. Univ. 2019, 14, 346–351. [Google Scholar]
- Dao, A.H.; Spindle, J.D.; Harp, B.A.; Jacob, A.; Chuang, A.Z.; Yee, R.W. Association of dyslipidemia in moderate to severe meibomian gland dysfunction. Am. J. Ophthalmol. 2010, 150, 371–375.e371. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; Chen, H.T.; Chen, H.C.; Chen, Y.T.; Hwang, Y.H.; Sun, C.C.; Hsiao, C.H.; Ma, D.H.; Wu, W.C.; Lai, C.C. Asymptomatic Meibomian Gland Dysfunction and Cardiovascular Disease Risk Factors in a Middle-Aged Population in Taiwan—A Cross-sectional Analysis. Sci Rep. 2017, 7, 4935. [Google Scholar] [CrossRef]
- Ha, M.; Song, J.; Park, S.; Han, K.; Hwang, H.S.; Kim, H.S.; Arita, R.; Na, K.S. Relationship between serum lipid level and meibomian gland dysfunction subtype in Korea using propensity score matching. Sci. Rep. 2021, 11, 16102. [Google Scholar] [CrossRef] [PubMed]
- Irfan, K.S.A.; Agrawal, A.; Singh, A.; Mittal, S.K.; Samanta, R.; Shrinkhal. Association of Lipid Profile with Severity of Meibomian Gland Dysfunction. Nepal. J. Ophthalmol. 2020, 12, 216–235. [Google Scholar] [CrossRef] [PubMed]
- Tulsyan, N.; Gupta, N.; Agrawal, N. Risk Factors Associated with Meibomian Gland Dysfunction: A Hospital Based Study. Nepal. J. Ophthalmol. 2021, 13, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, H.; Rastad, H.; Emamian, M.H.; Fotouhi, A. Meibomian gland dysfunction and its determinants in Iranian adults: A population-based study. Contact Lens Anterior Eye 2017, 40, 213–216. [Google Scholar] [CrossRef] [PubMed]
- Nelson, J.D.; Shimazaki, J.; Benitez-del-Castillo, J.M.; Craig, J.P.; McCulley, J.P.; Den, S.; Foulks, G.N. The international workshop on meibomian gland dysfunction: Report of the definition and classification subcommittee. Investig. Ophthalmol. Vis. Sci. 2011, 52, 1930–1937. [Google Scholar] [CrossRef]
- Tomlinson, A.; Bron, A.J.; Korb, D.R.; Amano, S.; Paugh, J.R.; Pearce, E.I.; Yee, R.; Yokoi, N.; Arita, R.; Dogru, M. The international workshop on meibomian gland dysfunction: Report of the diagnosis subcommittee. Investig. Ophthalmol. Vis. Sci. 2011, 52, 2006–2049. [Google Scholar] [CrossRef]
- Nichols, K.K.; Foulks, G.N.; Bron, A.J.; Glasgow, B.J.; Dogru, M.; Tsubota, K.; Lemp, M.A.; Sullivan, D.A. The international workshop on meibomian gland dysfunction: Executive summary. Investig. Ophthalmol. Vis. Sci. 2011, 52, 1922–1929. [Google Scholar] [CrossRef] [PubMed]
- Foulks, G.N.; Bron, A.J. Meibomian Gland Dysfunction: A Clinical Scheme for Description, Diagnosis, Classification, and Grading. Ocul. Surf. 2003, 1, 107–126. [Google Scholar] [CrossRef]
- Suzuki, T.; Kitazawa, K.; Cho, Y.; Yoshida, M.; Okumura, T.; Sato, A.; Kinoshita, S. Alteration in meibum lipid composition and subjective symptoms due to aging and meibomian gland dysfunction. Ocul. Surf. 2022, 26, 310–317. [Google Scholar] [CrossRef]
- Borchman, D.; Ramasubramanian, A.; Foulks, G.N. Human Meibum Cholesteryl and Wax Ester Variability With Age, Sex, and Meibomian Gland Dysfunction. Investig. Ophthalmol. Vis. Sci. 2019, 60, 2286–2293. [Google Scholar] [CrossRef]
- Siak, J.J.; Tong, L.; Wong, W.L.; Cajucom-Uy, H.; Rosman, M.; Saw, S.M.; Wong, T.Y. Prevalence and risk factors of meibomian gland dysfunction: The Singapore Malay eye study. Cornea 2012, 31, 1223–1228. [Google Scholar] [CrossRef]
- Jacob, J.M.; Pillai, P.S.; Goudinho, S.J. The association of meibomian gland dysfunction with dyslipidemia-A case-control study. World J. Pharm. Res. 2016, 5, 1390–1396. [Google Scholar]
- Şen, F.; Zengin, N. Meibomian Gland Dysfunction and Dyslipidemia. Harran Üniversitesi Tıp Fakültesi Derg. 2020, 17, 56–60. [Google Scholar]
- Bu, J.; Wu, Y.; Cai, X.; Jiang, N.; Jeyalatha, M.V.; Yu, J.; He, X.; He, H.; Guo, Y.; Zhang, M.; et al. Hyperlipidemia induces meibomian gland dysfunction. Ocul. Surf. 2019, 17, 777–786. [Google Scholar] [CrossRef]
- Osae, E.A.; Bullock, T.; Chintapalati, M.; Brodesser, S.; Hanlon, S.; Redfern, R.; Steven, P.; Smith, C.W.; Rumbaut, R.E.; Burns, A.R. Obese Mice with Dyslipidemia Exhibit Meibomian Gland Hypertrophy and Alterations in Meibum Composition and Aqueous Tear Production. Int. J. Mol. Sci. 2020, 21, 8772. [Google Scholar] [CrossRef] [PubMed]
- Lo Sasso, G.; Schlage, W.K.; Boue, S.; Veljkovic, E.; Peitsch, M.C.; Hoeng, J. The Apoe(-/-) mouse model: A suitable model to study cardiovascular and respiratory diseases in the context of cigarette smoke exposure and harm reduction. J. Transl. Med. 2016, 14, 146. [Google Scholar] [CrossRef]
- Butovich, I.A. Meibomian glands, meibum, and meibogenesis. Exp. Eye Res. 2017, 163, 2–16. [Google Scholar] [CrossRef] [PubMed]
- Butovich, I.A.; Wilkerson, A.; Yuksel, S. Differential effects of dietary cholesterol and triglycerides on the lipid homeostasis in Meibomian glands. J. Steroid Biochem. Mol. Biol. 2021, 211, 105894. [Google Scholar] [CrossRef] [PubMed]
- Butovich, I.A.; Suzuki, T. Delineating a novel metabolic high triglycerides-low waxes syndrome that affects lipid homeostasis in meibomian and sebaceous glands. Exp. Eye Res. 2020, 199, 108189. [Google Scholar] [CrossRef] [PubMed]
- Borchman, D.; Foulks, G.N.; Yappert, M.C.; Milliner, S.E. Differences in human meibum lipid composition with meibomian gland dysfunction using NMR and principal component analysis. Investig. Ophthalmol. Vis. Sci. 2012, 53, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Kitazawa, K.; Inomata, T.; Shih, K.; Hughes, J.B.; Bozza, N.; Tomioka, Y.; Numa, K.; Yokoi, N.; Campisi, J.; Dana, R.; et al. Impact of aging on the pathophysiology of dry eye disease: A systematic review and meta-analysis. Ocul. Surf. 2022, 25, 108–118. [Google Scholar] [CrossRef]
- Villani, E.; Canton, V.; Magnani, F.; Viola, F.; Nucci, P.; Ratiglia, R. The aging Meibomian gland: An in vivo confocal study. Investig. Ophthalmol. Vis. Sci. 2013, 54, 4735–4740. [Google Scholar] [CrossRef]
- Alsuhaibani, A.H.; Carter, K.D.; Abramoff, M.D.; Nerad, J.A. Utility of meibography in the evaluation of meibomian glands morphology in normal and diseased eyelids. Saudi J. Ophthalmol. Off. J. Saudi Ophthalmol. Soc. 2011, 25, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Yoon, C.H.; Ryu, J.S.; Hwang, H.S.; Kim, M.K. Comparative Analysis of Age-Related Changes in Lacrimal Glands and Meibomian Glands of a C57BL/6 Male Mouse Model. Int. J. Mol. Sci. 2020, 21, 4169. [Google Scholar] [CrossRef] [PubMed]
- Parfitt, G.J.; Xie, Y.; Geyfman, M.; Brown, D.J.; Jester, J.V. Absence of ductal hyper-keratinization in mouse age-related meibomian gland dysfunction (ARMGD). Aging 2013, 5, 825–834. [Google Scholar] [CrossRef]
- Sasaki, L.; Hamada, Y.; Yarimizu, D.; Suzuki, T.; Nakamura, H.; Shimada, A.; Pham, K.T.N.; Shao, X.; Yamamura, K.; Inatomi, T.; et al. Intracrine activity involving NAD-dependent circadian steroidogenic activity governs age-associated meibomian gland dysfunction. Nat. Aging 2022, 2, 105–114. [Google Scholar] [CrossRef]
- Nien, C.J.; Massei, S.; Lin, G.; Nabavi, C.; Tao, J.; Brown, D.J.; Paugh, J.R.; Jester, J.V. Effects of age and dysfunction on human meibomian glands. Arch. Ophthalmol. 2011, 129, 462–469. [Google Scholar] [CrossRef]
- Jester, J.V.; Potma, E.; Brown, D.J. PPARgamma Regulates Mouse Meibocyte Differentiation and Lipid Synthesis. Ocul. Surf. 2016, 14, 484–494. [Google Scholar] [CrossRef]
- Frishman, W.H.; Ooi, W.L.; Derman, M.P.; Eder, H.A.; Gidez, L.I.; Ben-Zeev, D.; Zimetbaum, P.; Heiman, M.; Aronson, M. Serum lipids and lipoproteins in advanced age. Intraindividual changes. Ann Epidemiol. 1992, 2, 43–50. [Google Scholar] [CrossRef]
- Gobal, F.A.; Mehta, J.L. Management of dyslipidemia in the elderly population. Ther. Adv. Cardiovasc. Dis. 2010, 4, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Yashin, A.I.; Akushevich, I.V.; Arbeev, K.G.; Akushevich, L.; Ukraintseva, S.V.; Kulminski, A. Insights on aging and exceptional longevity from longitudinal data: Novel findings from the Framingham Heart Study. Age 2006, 28, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, A.; Barrett-Connor, E.; Shan, J. Total, LDL, and HDL cholesterol decrease with age in older men and women. The Rancho Bernardo Study 1984–1994. Circulation 1997, 96, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Abbott, R.D.; Garrison, R.J.; Wilson, P.W.; Epstein, F.H.; Castelli, W.P.; Feinleib, M.; LaRue, C. Joint distribution of lipoprotein cholesterol classes. The Framingham study. Arteriosclerosis 1983, 3, 260–272. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.Q.; Zhang, Y.Z.; Wu, Y.; Zhang, J.J.; Li, T.B.; Jiang, T.; Xiong, X.M.; Luo, X.J.; Ma, Q.L.; Peng, J. Myeloperoxidase-derived hypochlorous acid promotes ox-LDL-induced senescence of endothelial cells through a mechanism involving beta-catenin signaling in hyperlipidemia. Biochem. Biophys. Res. Commun. 2015, 467, 859–865. [Google Scholar] [CrossRef]
- Coppe, J.P.; Patil, C.K.; Rodier, F.; Sun, Y.; Munoz, D.P.; Goldstein, J.; Nelson, P.S.; Desprez, P.Y.; Campisi, J. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 2008, 6, 2853–2868. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, M.; Okada, S.; Nakagomi, A.; Moriya, J.; Shimizu, I.; Nojima, A.; Yoshida, Y.; Ichimiya, H.; Kamimura, N.; Kobayashi, Y.; et al. Inhibition of endothelial p53 improves metabolic abnormalities related to dietary obesity. Cell Rep. 2014, 7, 1691–1703. [Google Scholar] [CrossRef] [PubMed]

| Inclusion Criteria |
| 1. Study objective: Potential involvement of dyslipidemia in the MGD were investigated. |
| 2. Study design: Retrospective (cross-sectional studies, case-control studies, case series, and case reports) and prospective studies |
| 3. Outcome: The level of TC, TG, LDL, and HDL in the patients with or without MGD or based on the severity of MGD |
| Exclusion Criteria |
| 1. Clinical guidelines, consensus documents, reviews, and conference proceedings |
| 2. Animal-based study |
| 3. Studies not involved in the study objective |
| 4. Lack of data to analyze |
| 5. Pre-printed articles |
| 6. Articles not published in English |
| Risk of Bias Domains | Bukhari, 2013 [26] | Pinna, 2013 [29] | Braich, 2016 [27] | Guliani, 2018 [28] | Banait, 2019 [30] |
|---|---|---|---|---|---|
| Representativeness of target population to national population | N | N | N | N | N |
| Representativeness of sampling frame to target population | Y | Y | Y | Y | Y |
| Sampling: random or census | N | N | N | N | N |
| Minimal non-response bias | Y | Y | Y | Y | Y |
| Data collected directly from participants | Y | Y | Y | Y | Y |
| Acceptable case definition | Y | Y | Y | Y | Y |
| Valid and reliable instrument | Y | Y | Y | Y | Y |
| Same mode of data collection for all participants | Y | Y | Y | Y | Y |
| Prevalence period appropriate | Y | Y | Y | Y | Y |
| Numerator(s) and denominator(s) appropriate | Y | Y | Y | Y | Y |
| Overall risk of bias | LHI | L | L | L | L |
| Risk of Bias Domains | Bukhari, 2013 [26] | Pinna, 2013 [29] | Braich, 2016 [27] | Guliani, 2018 [28] | Banait, 2019 [30] |
|---|---|---|---|---|---|
| Selection Overall risk of bias | **** | *** | *** | ** | *** |
| Comparability H—high quality; | ** | ** | ** | * | ** |
| Exposure | *** | ** | ** | *** | ** |
| Overall risk of bias | H | H | H | I | H |
| First Author | Year | Country | MGD | Participants (n) | Age (Mean) | TC ≥200 mg/dL | TG ≥ 150 mg/dL | LDL ≥ 130 mg/dL | HDL ≤ 40 mg/dL |
|---|---|---|---|---|---|---|---|---|---|
| Dao AH [31] | 2010 | United States | (+) | 46 | 27–82 * (52) | 67.4% | 15.2% | 39.1% | 6.5% |
| (−) | historical control | 20–65 over (47) | 45.1% | 33.1% | 32.8% | 15.7% | |||
| Pinna A [29] | 2013 | Italy | (+) | 60 | 18–54 (38) | 58.3% | 8.3% | - | - |
| (−) | 63 | 18–54 (36) | 6.3% | 1.1% | - | - | |||
| Bukhari AA [26] | 2013 | Saudi Arabia | (+) | 132 | 15–78 **(49) | 52.3% | 22.7%† | 25.8% | 3.8% |
| (−) | 104 | 10.6% | 1.9% | 7.3% | - | ||||
| Braich PS [27] | 2016 | United States | (+) | 109 | 20–72 (47) | 53.2% | 15.6% | 37.6% | 6.4% |
| (−) | 115 | 19–75 (46) | 6.1% | 4.3% | 8.7% | 10.4% | |||
| Chen A [32] | 2017 | Taiwan | (+) | 89 | 30–60 (49) | The number of patients with dyslipidemia was not described | |||
| (−) | 199 | 30–60 (49) | The number of patients with dyslipidemia was not described | ||||||
| Hashemi H [36] | 2017 | Iran | (+) | 4700 | 45–69 (55) | OR:1.000, 95%CI (1.000–1.002) | OR:1.0001, 95%CI (1.000–1.008) | - | OR:0.99, 95%CI (0.986–0.998) |
| Guliani BP [28] | 2018 | India | (+) | 90 | 18–54 | 25.6% | 41.1% | 27.8% | 55.6% |
| (−) | 90 | 18–54 | 15.6% | 47.8% | 17.8% | 51.1% | |||
| Banait S [30] | 2019 | India | (+) | 30 | 31–80 (62) | 20.0% | 40.0% | 23.3% | 56.7% |
| (−) | 30 | 31–80 | 30.0% | 20.0% | 10.0% | 53.3% | |||
| Ha M [33] | 2021 | South Korea | (+) | 95 | 19–86 (58) | 41.1% | 25.2% | 20.0% | 5.3% |
| (−) | 475 | 19–80 (56) | The number of patients with dyslipidemia was not described | ||||||
| Irfan KSA [34] | 2021 | India | (+) | 58 | 18–65 (49) | 77.6% | 89.7% | 17.2% | 10.3% |
| (−) | 58 | 18–65 (49) | 19.0% | 43.1% | 3.4% | 1.7% | |||
| Tulsyan N [35] | 2021 | Nepal | (+) | 400 | 31- | 20.0% | 57.0% | 23.5% | 40.0% |
| First Author | Year | Country | MGD | Participants (n) | TC (mg/dL) | TG (mg/dL) | LDL (mg/dL) | HDL (mg/dL) |
|---|---|---|---|---|---|---|---|---|
| Pinna A [29] | 2013 | Italy | (+) | 60 | 210 (4) | 90 (47) | 128 (4) | 62 (2) |
| (−) | 63 | 163 (3) | 74 (27) | 94 (3) | 53 (1) | |||
| Braich PS [27] | 2016 | United states | (+) | 109 | 203 (13) | 99 (42) | 126 (10) | 53 (4) |
| (−) | 115 | 157 (15) | 82 (37) | 92 (12) | 46 (3) | |||
| Chen A [32] | 2017 | Taiwan | (+) | 89 | 213 (34) | 188 (109) | 135 (30) | 48 (12) |
| (−) | 199 | 188 (35) | 130 (74) | 108 (30) | 51 (13) | |||
| Guliani BP [28] | 2018 | India | (+) | 90 | 186 (59) | 150 (63) | 116 (50) | 43 (21) |
| (−) | 90 | N/A | N/A | N/A | N/A | |||
| Ha M [33] | 2021 | South Korea | (+) | 95 | 193 (34) | 128 (75) | 105 (31) | 62 (13) |
| (−) | 475 | 198 (38) | 135 (90) | 119 (34) | 51 (12) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomioka, Y.; Kitazawa, K.; Yamashita, Y.; Numa, K.; Inomata, T.; Hughes, J.-W.B.; Soda, R.; Nakamura, M.; Suzuki, T.; Yokoi, N.; et al. Dyslipidemia Exacerbates Meibomian Gland Dysfunction: A Systematic Review and Meta-Analysis. J. Clin. Med. 2023, 12, 2131. https://doi.org/10.3390/jcm12062131
Tomioka Y, Kitazawa K, Yamashita Y, Numa K, Inomata T, Hughes J-WB, Soda R, Nakamura M, Suzuki T, Yokoi N, et al. Dyslipidemia Exacerbates Meibomian Gland Dysfunction: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2023; 12(6):2131. https://doi.org/10.3390/jcm12062131
Chicago/Turabian StyleTomioka, Yasufumi, Koji Kitazawa, Yohei Yamashita, Kohsaku Numa, Takenori Inomata, Jun-Wei B. Hughes, Rina Soda, Masahiro Nakamura, Tomo Suzuki, Norihiko Yokoi, and et al. 2023. "Dyslipidemia Exacerbates Meibomian Gland Dysfunction: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 12, no. 6: 2131. https://doi.org/10.3390/jcm12062131
APA StyleTomioka, Y., Kitazawa, K., Yamashita, Y., Numa, K., Inomata, T., Hughes, J.-W. B., Soda, R., Nakamura, M., Suzuki, T., Yokoi, N., & Sotozono, C. (2023). Dyslipidemia Exacerbates Meibomian Gland Dysfunction: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 12(6), 2131. https://doi.org/10.3390/jcm12062131






