Abstract
Endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) is an essential endoscopic tissue sampling method for diagnosing pancreatobiliary diseases; however, determining the presence of target specimens mixed in the blood by conventional observation is challenging due to the small size of the obtained sample. This study investigated the usefulness of a target sample check illuminator (TSCI) that emits a specific wavelength of light to determine the presence of target specimens. Twenty-seven patients who underwent EUS-FNA at our hospital were included. Conventional white light observation was performed for the collected samples, followed by TSCI; six people evaluated the presence of the target specimen on a 5-point scale. The target specimen discrimination score using TSCI (median: 5) was significantly higher than that using conventional white light observation (median: 1) (p < 0.001). No significant difference was observed in the discrimination score between the evaluator (novice vs. expert, p = 0.162) and puncture needle (22G vs. 25G, p = 0.196). The discriminability of TSCI in the samples obtained using EUS-FNA was significantly higher than that of conventional observation. TSCI does not depend on the evaluator or puncture needle for the identification of the target specimen; hence, it can provide a good pathological specimen and may contribute to the improvement of the diagnostic ability.
1. Introduction
The tissue samples of target lesions for pancreatobiliary diseases were difficult to collect, especially for those patients with pancreatic diseases; hence, treatment decisions were often made based on imaging diagnoses without histological examinations. As a result, a certain number of patients could not be surgically treated or have chemotherapy, radiation therapy, or follow-up care in accordance with the true diagnosis, and, thus, could not be provided with appropriate medical care [1]. Therefore, in 1980, Strohm et al. reported the usefulness of endoscopic ultrasonography (EUS) for visualizing adjacent organs through the gastrointestinal tract, which was an epoch-making event in the clinical practice of pancreatobiliary diseases [2]. Furthermore, in 1992, Vilmann et al. reported endoscopic ultrasound-guided fine needle aspiration (EUS-FNA), which is a technique that allows for tissue sampling under EUS and pathological evaluation for pancreatic lesions that were difficult in the past [3].
Recently, EUS, with its high image resolution, plays a central role in the diagnosis of pancreatobiliary diseases, subepithelial lesions (SELs), and enlarged lymph nodes. Since the differentiation between benign and malignant diseases significantly impacts the treatment plan, it is especially important to perform a definitive pathological diagnosis; EUS-FNA is widely used as a tissue sampling method [4,5,6]. However, the sampled tissue is small and mixed with blood, so it is often difficult to determine whether the appropriate target specimen has been acquired with conventional observation. Rapid on-site evaluation (ROSE) [7,8], which involves immediate cytological diagnosis, has been reported as a solution to this problem. However, the shortage of cytologists in Japan is a chronic problem, and this causes the introduction of ROSE to be difficult. Therefore, to improve the diagnostic ability, it is necessary to increase the number of punctures to ensure a sufficient sample volume; however, this may increase the risk of adverse events and peritoneal dissemination [9,10]. To address these problems, it is important to ensure it is easy for novices to evaluate the samples. We have developed a target sample check illuminator (TSCI) to determine the presence of target specimens in the samples acquired using EUS-FNA without the loss of quantity and quality and have reported its usefulness for pancreatic tumors, swollen lymph nodes, and SELs [11,12]. However, the versatility of TSCI has not been verified since evaluations using TSCI are performed with special wavelengths of light not usually experienced in medical practice.
This study aimed to examine the usefulness of TSCI for EUS-FNA samples, the experience of using TSCI, and differences in the ability to identify target specimens with the different puncture needle diameters used for EUS-FNA.
2. Materials and Methods
2.1. Study Design and Population
This was a single-center, retrospective, cohort study conducted at Tottori University Hospital in accordance with the ethical standards set forth in the Declaration of Helsinki in 1964; it was approved by the Institutional Review Board of our University Hospital (approval number: 2335). Twenty-seven patients who underwent EUS-FNA and presented with lesions detected using abdominal ultrasonography, computed tomography, magnetic resonance imaging, and esophagogastroduodenoscopy that required pathological diagnosis at our hospital from June to September 2014 were enrolled (Table 1).

Table 1.
Patient characteristics.
2.2. EUS-FNA Procedure
We used a GF-UCT260 endoscope (Olympus Optical, Tokyo, Japan) and an EU-ME2 processor (Olympus Optical, Tokyo, Japan) in all cases. The puncture needle was selected from 19G, 22G, and 25G (Expect™, Boston Scientific. Marlboro, MA, USA) by an endoscopist, based on the patient background and lesion characteristics. All EUS-FNA procedures were performed through the esophagus, stomach, and duodenum, under midazolam conscious sedation. After visualizing the lesion and confirming the lack of vessels in the puncture pathway using EUS, the FNA needle was punctured to collect tissue samples with approximately 20 back-and-forth movements using 20 mL of suction. We performed both the conventional and TSCI methods to determine whether the target specimen was obtained. The procedure was terminated if a sufficient amount of sample was successfully collected; otherwise, additional punctures were performed when the sample was insufficient.
2.3. TSCI (Target Sample Check Illuminator)
Based on the light absorption spectrum of human oxyhemoglobin (peak spectrum of deoxidized hemoglobin/oxidized hemoglobin: 550 nm/540 nm and 585 nm) reported by Zijlstra et al. in 1991 [13], we found that blood components and tissues could be clearly distinguished by transmitting a wavelength of 605 nm light through the collected samples [11]. Observing the specimens with transmitted light of this wavelength, blood absorbs light and appears dark brown, whereas tissue appears orange without light absorption, causing it to be easier to distinguish tissue components in specimens with mixed blood. TSCI is a device developed by Adachi Co., Ltd. (Osaka, Japan) that facilitates the identification of the presence of target specimens collected using EUS-FNA using transmitting 605 nm wavelength light from below. Currently, we use TSCI for evaluating the EUS-FNA samples in routine clinical practice at our hospital (Figure 1).

Figure 1.
Main unit of TSCI. On the front of the device, there is a tab to switch the 605 nm wavelength light (red arrow), a knob to adjust the light intensity (yellow arrow), and a lamp to check the operation status. In the center of the upper surface of the device, there is a white plate that emits transmitted light from below, and a Petri dish containing the specimen is placed on this plate (blue arrow). Abbreviation: TSCI, target sample check illuminator.
2.4. Evaluation of Sampling Tissue
The EUS-FNA samples were extruded from the puncture needle into a plastic Petri dish, and we evaluated whether the target specimen had been acquired by observation using the conventional method with white light. The presence of a target specimen is usually determined by the appearance of a white component in the thread-like red specimen (blood component). Subsequently, TSCI was performed. The Petri dish was set in the TSCI and irradiated with a 605 nm wavelength light to observe the transmitted light. Unlike conventional observation with white light, blood components appear dark brown and target specimens appear orange; therefore, the presence of the target specimen is determined based on these findings (Figure 2). The images of EUS-FNA specimens for discriminant scoring were taken at a fixed distance from directly above the specimen for both white light and TSCI observations. Therefore, there is no difference in the image quality from case to case. The solid component was fixed in formalin for histological diagnosis, and the liquid component was pathologically diagnosed using cytological diagnosis.

Figure 2.
Representative EUS-FNA sample images. The images of the EUS-FNA samples from the three cases with puncture needles of different sizes were observed using the conventional and TSCI methods. The solid components and blood and tissue components are clearly distinguished by the transmitted light in the TSCI method. (a) AIP, needle size: 19G, median score (conventional/TSCI): 3.5/5; (b) Pancreatic cancer, needle size: 22G, median score (conventional/TSCI): 1/5; and (c) Pancreatic cancer, needle size: 25G, median score (conventional/TSCI): 3.5/5. Abbreviations: AIP, autoimmune pancreatitis.
2.5. Study Protocol, Outcome Measures, and Statistical Analysis
Using the images of the EUS-FNA samples with conventional and TSCI observations, six evaluators rated whether the target specimens were visible in the samples mixed with blood on a five-point scale (1: bad, 2: poor, 3: moderate, 4: good, and 5: excellent). White light conventional observation images were rated first, followed by TSCI images, and the evaluators were blinded to discriminant scoring among themselves. The evaluators consisted of three experienced TSCI users and three TSCI novices. The TSCI novices were provided with a 15 min explanation of the TSCI observation methods with examples before sample evaluation. The statistical analyses were performed using SPSS ver. 25 (IBM, Armonk, NY, USA). The stratified categories for each clinical parameter were evaluated using the Wilcoxon signed-rank test, Paired Wilcoxon signed-rank test, and Chi-square tests. The Wilcoxon signed-rank test was used for the variables that were not normally distributed. The statistical significance was set at p < 0.05.
3. Results
The median age of the patients was 65.3 years (range: 23–80), with 17 men and 10 women. EUS-FNA was performed for 18 pancreatic tumors, three gastric SELs, three lymph nodes, and two others with three puncture needle sizes: 22G (13 cases, 48.1%), 25G (10 cases, 37%), and 19G (4 cases, 14.8%). As for the final diagnosis, with 10 cases, pancreatic cancer was the most commonly observed (37%), followed by 3 autoimmune pancreatitis cases (11.1%), 3 gastrointestinal stromal tumors (11.1%), 2 solid pseudopapillary neoplasms (7.4%), 2 intrapancreatic accessory spleen (7.4%), 2 benign reactive enlarged lymph nodes (7.4%), 1 lymphoepithelial cyst (3.7%), 1 liposarcoma (3.7%), 1 diffuse large B-cell lymphoma (3.7%), 1 papillitis of Vater (3.7%), and 1 esophageal achalasia (3.7%). No procedure-related adverse events were observed.
The diagnostic ability of EUS-FNA was as follows: sampling success rate, 96.3% (26/27); sensitivity, 88.2% (15/17); specificity, 90% (9/10); positive predictive value, 93.8% (15/16); negative predictive value, 81.8% (9/11); and accuracy, 88.9% (24/27) (Table 2). The discrimination scores for the target specimens using conventional observation were 1: 65.4%, 2: 13.6%, 3: 10.5%, 4: 7.4%, and 5: 3.1% (median 1, interquartile range [IQR] 1–2), while those using TSCI were 1: 7.5%, 2: 2.4%, 3: 9.3%, 4: 30.2%, and 5: 50.6% (median 5, IQR 4-5), resulting in significantly higher discrimination scores in the TSCI group (p < 0.001) (Figure 3). The TSCI group had a high discrimination ability, scoring 4 or higher, compared to the conventional observation group (88.2% vs. 5.2%, p < 0.001). In the TSCI observation group, there was no significant difference in the number of cases with a score of 4 or higher between novices and experienced raters (76.5% vs. 85.2%, p = 0.162), indicating that the experience with TSCI did not the affect discriminatory ability (Figure 4). Moreover, there was no significant difference in the discrimination ability between 22G and 25G puncture needles (median 5; IQR4–5 vs. 4; IQR4–5, p = 0.196) (Figure 2 and Figure 5).

Table 2.
Diagnostic ability of EUS-FNA.
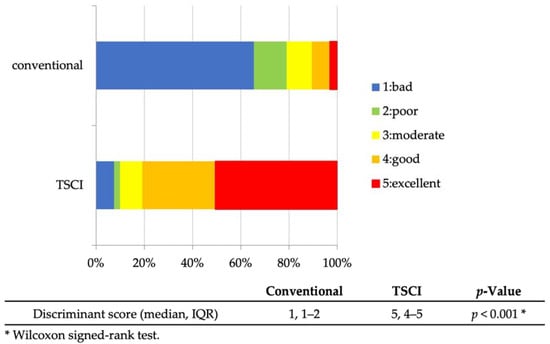
Figure 3.
Discriminant scores for EUS-FNA sample (conventional vs. TSCI). Discriminant scores for EUS-FNA samples by the six raters. The TSCI observations had significantly higher discriminant scores than the conventional method. Abbreviations: EUS-FNA, endoscopic ultrasound-guided fine-needle aspiration; TSCI, target sample check illuminator; and IQR, interquartile range.
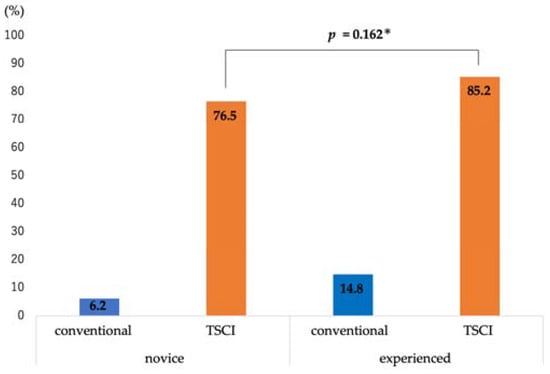
Figure 4.
Differences from experience in high discriminant scoring cases. In high-scoring cases (≥4), there was no significant difference between the novice and experienced raters. * Chi-square test. Abbreviations: TSCI, target sample check illuminator.
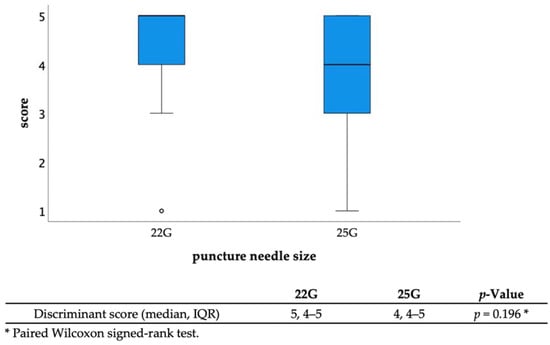
Figure 5.
Discriminant scores with different needle sizes (22G vs. 25G). There was no significant difference in the discriminant scores between different puncture needle sizes. Abbreviations: IQR, interquartile range.
4. Discussion
Pancreatic and biliary tract cancers have very poor prognoses; therefore, it is important to detect and treat them as early as possible [14]. EUS-FNA is a fundamental diagnostic technique for pancreatobiliary diseases and is useful for many other lesions, such as gastrointestinal SELs, lymph nodes, and intraperitoneal masses in a wide area from the mediastinum to the pelvis. Therefore, EUS-FNA is required to safely collect appropriate samples with a minimum number of punctures, and many studies have attempted to improve the diagnostic ability, increase the number of specimens, and reduce adverse events [15,16,17,18]. Specific items to improve diagnostic performance include a selection of the EUS scope, needle shape, needle diameter, number of punctures, puncture method, number of strokes, aspiration method, and specimen processing method. Regarding scope selection, it was reported that forward and anterior oblique viewing scopes had comparable results concerning both the diagnostic ability and safety, as well as the shape and puncture needle diameter. The use of a large-diameter (19G > 22G) or a Francine needle had a better diagnostic ability, and the adverse events rate was comparable [19,20]. It has also been reported that the diagnostic ability can be increased with lesser punctures by using the door-knocking method for a quick puncture and the fanning method, wherein the needle is repeatedly punctured while shifting its position within the target lesion [16,21]. Furthermore, when the inner wire is slowly pulled out while the puncture is repeated, it applies only a small amount of negative pressure to the needle lumen, resulting in less blood in the specimen, thereby increasing the diagnostic sensitivity [22]. However, it is also important to reduce the number of adverse events parallel to these attempts to improve the diagnostic ability of EUS-FNA. EUS-FNA is considered a safe tissue sampling method with an adverse events rate of <1% [23], and the frequency of the occurrence of bleeding and pancreatitis is at 0.23% and 0.28% [24,25], respectively, but it should be noted that the incidence can sometimes be severe [26]. In particular, needle tract seeding, which is the formation of seeding nests along the puncture path after EUS-FNA for malignant disease, has been reported to occur in 0.33% of cases, which can have a significant impact on the treatment plan and the prognosis of patients and is one of the topics of a recent study [27]. To reduce these adverse events, we believe that it is desirable to minimize the needle diameter and the number of puncture attempts.
TSCI is a tissue discrimination instrument that uses a specific wavelength of light of 605 nm to facilitate the determination of the presence of target specimens in EUS-FNA samples. The use of this device is expected to improve the pathological diagnostic ability by ensuring the sample quality and reduce the adverse events by preventing unnecessary punctures. In 2021, TSCI was significantly more useful in discriminating target specimens with 95.8% for TSCI observation than normal observation with 75.0% (p = 0.025) [12]. However, the degree of improvement in visibility was unknown because the evaluation was based on two choices: visible or invisible. In our study, the degree to which the target specimen could be discriminated was scored on a five-point scale, albeit subjectively, and the median discrimination score was 1 (IQR: 1–2) for normal observation and 5 (IQR: 4–5) for TSCI observation. The use of TSCI resulted in a large score increase, proving the superiority of TSCI by a significant margin over its usefulness in the previous report (p < 0.001).
One important aspect of this study was that it included 37% of cases in which EUS-FNA was performed using a 25G puncture needle, which has not been examined in previous reports. In this study, the discrimination ability of the 25G needle was not significantly different from that of the 22G needle, suggesting that TSCI is useful even for thin samples collected using a 25G small-diameter needle. Although we sometimes use a large-diameter needle for EUS-FNA to secure sample volume, a small-diameter needle is often used in the case of difficulty in a puncture or operable lesion in which the risk of seeding needs to be minimized [28]. In such a situation, the contribution of TSCI will be even greater because sample evaluation using conventional observation is difficult due to the small sample size. It is also important to note that the TSCI did not consume the collected samples. The usefulness of ROSE as a method to improve the diagnostic ability of EUS-FNA has been widely reported [7,8]. This can eliminate unnecessary punctures, as a cytological evaluation of the sample is immediately performed at the time of the EUS-FNA, while consuming a sample for cytology reduces tissue for histology and may cause a histological evaluation, such as structural atypia, more difficult. Furthermore, as precision medicine based on genetic information is expected to develop further in the future, securing the number of target specimens using EUS-FNA will become increasingly important [29]. TSCI is also expected to be a significant contribution in this regard.
Another important verification in this study is whether sample evaluation using TSCI is possible for everyone. ROSE is diagnosed by cytologists or pathologists trained according to the evaluation criteria established for cytology; therefore, not everyone can perform ROSE. TSCI is required so that everyone can evaluate the presence of target specimens at the same level. In this study, there was no significant difference between novices and experienced raters in cases where the discrimination score of the target sample was four or higher. In other words, with TSCI observation, an equal discriminative ability can be expected for all, if a brief and simple explanation is provided. The simplicity of use and the fact that novices can discriminate the target specimens suggest that this device will be useful for medical institutions in Japan, where there is a shortage of cytologists, so we look forward to its widespread use.
Although we have discussed the usefulness of TSCI, it is necessary to understand its disadvantages. TSCI is effective only in identifying the target specimen, not in differentiating between benign and malignant tumors. In particular, pancreatic cancer, one of the most common carcinomas for which EUS-FNA is performed, is characterized by abundant stroma and fibrosis in the surrounding tissues. Even if a sample containing a large amount of target specimen is obtained, the actual number of cancer cells may be negligible, and no definitive diagnosis may be reached. However, the macroscopic on-site quality evaluation (MOSE) study reported by Iwashita et al. showed that the sensitivity of malignancy was significantly higher when the grossly visible tissue component in the sample obtained using EUS-FNA was 4 mm or longer than when it was 4 mm or shorter (95.5% vs. 57.1%, p < 0.0001) [18]. This finding that a longer gross tissue component improves the malignant diagnostic ability may also be applicable for diagnosis using TSCI. Nevertheless, this point needs further verification based on a pathological diagnosis. Therefore, the TSCI used in our study was a device that contributed to bridging the gap between ROSE and MOSE by reducing the number of EUS-FNA-related adverse events and solving the disadvantages of ROSE, the consumption of specimens, and MOSE, the inability to identify the target specimens contained in a small thin sample.
A limitation of this study is that it was a single-center retrospective study with small sample size. In addition, the discriminative ability of the target specimen was scored subjectively, causing it to be difficult to perform a constant objective evaluation. Moreover, the TSCI images were evaluated immediately after the conventional observation images, which may result in a bias. To further validate its usefulness, it is desirable to create an objective evaluation index in addition to prospectively accumulating the number of cases at multiple centers.
In conclusion, the discrimination ability of the target specimens in samples obtained using EUS-FNA was significantly higher with TSCI observation than with conventional white light observation, regardless of the puncture needle diameter. There was no difference in the discrimination ability between experienced and novice raters, indicating that specimen evaluation using TSCI can be performed for samples of various sizes by any evaluator.
Author Contributions
Conceptualization, H.K. (Hiroki Koda) and K.M.; methodology, Y.T.; software, T.Y.; validation, S.K., Y.T. and H.K. (Hiroki Kurumi); formal analysis, H.K. (Hiroki Koda) and H.N.; investigation, S.K. and T.S.; resources, K.M.; data curation, T.Y., W.H. and Y.S. (Yuri Sakamoto); writing—original draft preparation, H.K. (Hiroki Koda); writing—review and editing, K.M. and T.O.; visualization, N.Y. and Y.S. (Yuta Seki); supervision, H.I. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board of Tottori University Hospital (approval number 2335, approval date 5 July 2014).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical.
Acknowledgments
We express our sincerest gratitude to the staff of our medical office for their expertise and support in all aspects of the research and for their help in preparing the manuscript and the patients who participated in this study. In addition, we also thank the following doctors who were affiliated with the Department of Gastroenterology and Nephrology, Tottori University Hospital at that time: Kenichi Harada, the medical director of Gastroenterology at the National Hospital Organization Yonago Medical Center, Tottori, Japan; Masahiko Koda, director of Hino Hospital, Tottori, Japan; Yoshikazu Murawaki; Masaru Ueki, Division of Medical Education, School of Medicine, Tottori University, Tottori, Japan; and Kohei Hosoda, the medical director of Gastroenterology, Tottori Prefectural Kousei Hospital, Tottori, Japan. Kazuo Yashima, Koichiro Kawaguchi, and Yuichiro Ikebuchi are still affiliated with the Department of Gastroenterology and Nephrology, Tottori University Hospital. Finally, we thank English proofreaders for editing and reviewing this manuscript for English language.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
EUS, endoscopic ultrasonography; EUS-FNA, endoscopic ultrasound-guided fine needle aspiration; SEL, subepithelial lesion; ROSE, rapid on-site evaluation; TSCI, target sample check illuminator; PC, pancreatic cancer; AIP, autoimmune pancreatitis; SPN, solid pseudopapillary neoplasm; LEC, lymphoepithelial cyst; GIST, gastrointestinal stromal tumor; DLBCL, diffuse large B-cell lymphoma; MOSE, macroscopic on-site quality evaluation; PPV, positive predictive value; NPV, negative predictive value; and IQR, interquartile range.
References
- Law, R.; Bronner, M.; Vogt, D.; Stevens, T. Autoimmune pancreatitis: A mimic of pancreatic cancer. Cleve Clin. J. Med. 2009, 76, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Strohm, W.D.; Phillip, J.; Hagenmüller, F.; Classen, M. Ultrasonic tomography by means of an ultrasonic fiberendoscope. Endoscopy 1980, 12, 241–244. [Google Scholar] [CrossRef] [PubMed]
- Vilmann, P.; Jacobsen, G.K.; Henriksen, F.W.; Hancke, S. Endoscopic ultrasonography with guided fine needle aspiration biopsy in pancreatic disease. Gastrointest. Endosc. 1992, 38, 172–173. [Google Scholar] [CrossRef]
- Abraham, S.C.; Wilentz, R.E.; Yeo, C.J.; Sohn, T.A.; Cameron, J.L.; Boitnott, J.K.; Hruban, R.H. Pancreaticoduodenoctomy (Whipple resections) in patients without malignancy: Are they all ‘chronic pancreatitis’? Am. J. Surg. Pathol. 2003, 27, 110–120. [Google Scholar] [CrossRef]
- Banafea, O.; Mghanga, F.P.; Zhao, J.; Zhao, R.; Zhu, L. Endoscopic ultrasonography with fine-needle aspiration for histological diagnosis of solid pancreatic masses: A meta-analysis of diagnostic accuracy studies. BMC Gastroenterol. 2016, 16, 108. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Takeda, Y.; Onoyama, T.; Kawata, S.; Kurumi, H.; Koda, H.; Yamashita, T.; Isomoto, H. Endoscopic ultrasound-guided fine-needle aspiration biopsy–recent topics and technical tips. World J. Clin. Cases 2019, 7, 1775–1783. [Google Scholar] [CrossRef]
- Hébert-Magee, S.; Bae, S.; Varadarajulu, S.; Ramesh, J.; Frost, A.R.; Eloubeidi, M.A.; Eltoum, I.A. The presence of a cytopathologist increases the diagnostic accuracy of endoscopic ultrasound-guided fine needle aspiration cytology for pancreatic adenocarcinoma: A meta-analysis. Cytopathology 2013, 24, 159–171. [Google Scholar] [CrossRef]
- Matynia, A.P.; Schmidt, R.L.; Barraza, G.; Layfield, L.J.; Siddiqui, A.A.; Adler, D.G. Impact of rapid on-site evaluation on the adequacy of endoscopic-ultrasound guided fine-needle aspiration of solid pancreatic lesions: A systematic review and meta-analysis. J. Gastroenterol. Hepatol. 2014, 29, 697–705. [Google Scholar] [CrossRef]
- Chen, G.; Liu, S.; Zhao, Y.; Dai, M.; Zhang, T. Diagnostic accuracy of endoscopic ultrasound-guided fine-needle aspiration for pancreatic cancer: A meta-analysis. Pancreatology 2013, 13, 298–304. [Google Scholar] [CrossRef]
- Micames, C.; Jowell, P.S.; White, R.; Paulson, E.; Nelson, R.; Morse, M.; Hurwitz, H.; Pappas, T.; Tyler, D.; McGrath, K. Lower frequency of peritoneal carcinomatosis in patients with pancreatic cancer diagnosed by EUS-guided FNA vs. percutaneous FNA. Gastrointest. Endosc. 2003, 58, 690–695. [Google Scholar] [CrossRef]
- Matsumoto, K.; Ueki, M.; Takeda, Y.; Harada, K.; Onoyama, T.; Kawata, S.; Ikebuchi, Y.; Imamoto, R.; Horie, Y.; Murawaki, Y. Development of a device for detecting target specimens from EUS-guided FNA samples. Endosc. Int. Open 2015, 3, E662–E664. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Hara, K.; Yasuda, I.; Itoi, T.; Kurumi, H.; Matsumoto, S.; Doi, S.; Honjo, M.; Takeda, Y.; Shibuya, J.; et al. Usefulness of a target sample check illuminator in the detection of target specimens in endoscopic ultrasound-guided fine-needle biopsy samples: Multicenter prospective study. Dig. Endosc. 2021, 33, 970–976. [Google Scholar] [CrossRef] [PubMed]
- Zijlstra, W.G.; Buursma, A.; Meeuwsen-van der Roest, W.P. Absorption spectra of human fetal and adult oxyhemoglobin, de-oxyhemoglobin, carboxyhemoglobin, and methemoglobin. Clin. Chem. 1991, 37, 1633–1638. [Google Scholar] [CrossRef]
- Egawa, S.; Toma, H.; Ohigashi, H.; Okusaka, T.; Nakao, A.; Hatori, T.; Maguchi, H.; Yanagisawa, A.; Tanaka, M. Japan Pancreatic Cancer Registry; 30th year anniversary: Japan Pancreas Society. Pancreas 2012, 41, 985–992. [Google Scholar] [CrossRef] [PubMed]
- Katanuma, A.; Itoi, T.; Baron, T.H.; Yasuda, I.; Kin, T.; Yane, K.; Maguchi, H.; Yamazaki, H.; Sano, I.; Minami, R.; et al. Bench-top testing of suction forces generated through endoscopic ultrasound-guided aspiration needles. J. Hepatobiliary Pancreat. Sci. 2015, 22, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Bang, J.Y.; Magee, S.H.; Ramesh, J.; Trevino, J.M.; Varadarajulu, S. Randomized trial comparing fanning with standard technique for endoscopic ultrasound-guided fine-needle aspiration of solid pancreatic mass lesions. Endoscopy 2013, 45, 445–450. [Google Scholar] [CrossRef]
- Larghi, A.; Ibrahim, M.; Fuccio, L.; Lekkerkerker, S.; Eisendrath, P.; Frazzoni, L.; Fockens, P.; La Marca, M.; van Hooft, J.E.; Deviere, J.; et al. Forward-viewing echoendoscope versus standard echoendoscope for endoscopic ultrasound-guided tissue acquisition of solid lesions: A randomized, multicenter study. Endoscopy 2019, 51, 444–451. [Google Scholar] [CrossRef]
- Iwashita, T.; Yasuda, I.; Mukai, T.; Doi, S.; Nakashima, M.; Uemura, S.; Mabuchi, M.; Shimizu, M.; Hatano, Y.; Hara, A.; et al. Macroscopic on-site quality evaluation of biopsy specimens to improve the diagnostic accuracy during EUS-guided FNA using a 19-gauge needle for solid lesions: A single-center prospective pilot study (MOSE study). Gastrointest. Endosc. 2015, 81, 177–185. [Google Scholar] [CrossRef]
- Song, T.J.; Kim, J.H.; Lee, S.S.; Eum, J.B.; Moon, S.H.; Park, D.Y.; Seo, D.W.; Lee, S.K.; Jang, S.J.; Yun, S.C.; et al. The prospective randomized, controlled trial of endoscopic ultrasound-guided fine-needle aspiratioin using 22G and 19G aspiration needles for solid pancreatic or peripancreatic masses. Am. J. Gastroenterol. 2010, 105, 1739–1745. [Google Scholar] [CrossRef]
- Itonaga, M.; Yasukawa, S.; Fukutake, N.; Ogura, T.; Asada, M.; Shimokawa, T.; Inatomi, O.; Nakai, Y.; Shiomi, H.; Nebiki, H.; et al. Comparison of 22-gauge standard and Franseen needles in EUS-guided tissue acquisitioin for diagnosing solid pancreatic lesions: A multicenter randomized controlled trial. Gastrointest. Endosc. 2022, 96, 57–66. [Google Scholar] [CrossRef]
- Mukai, S.; Itoi, T.; Ashida, R.; Tsuchiya, T.; Ikeuchi, N.; Kamada, K.; Tanaka, R.; Umeda, J.; Tonozuka, R.; Fukutake, N.; et al. Multicenter, prospective, crossover trial comparing the door-knocking method with the conventional method for EUS-FNA of solid pancreatic masses (with videos). Gastrointest. Endosc. 2016, 83, 1210–1217. [Google Scholar] [CrossRef] [PubMed]
- Nakai, Y.; Isayama, H.; Chang, K.J.; Yamamoto, N.; Hamada, T.; Uchino, R.; Mizuno, S.; Miyabayashi, K.; Yamamoto, K.; Kawakubo, K.; et al. Slow pull versus suction in endoscopic ultrasound-guided fine-needle aspiration of pancreatic solid masses. Dig. Dis. Sci. 2014, 59, 1578–1585. [Google Scholar] [CrossRef] [PubMed]
- Yoshinaga, S.; Itoi, T.; Yamao, K.; Yasuda, I.; Irisawa, A.; Imaoka, H.; Tsuchiya, T.; Doi, S.; Yamabe, A.; Murakami, Y.; et al. Safety and efficacy of endoscopic ultrasound-guided fine needle aspiration for pancreatic masses: A prospective multicenter study. Dig. Endosc. 2020, 32, 114–126. [Google Scholar] [CrossRef]
- Eloubeidi, M.A.; Gress, F.G.; Savides, T.J.; Wiersema, M.J.; Kochman, M.L.; Ahmad, N.A.; Ginsberg, G.G.; Erickson, R.A.; Dewitt, J.; Van Dam, J.; et al. Acute pancreatitis after EUS-guided FNA of solid pancreatic masses: A pooled analysis from EUS centers in the United States. Gastrointest Endosc. 2004, 60, 385–389. [Google Scholar] [CrossRef]
- Hamada, T.; Yasunaga, H.; Nakai, Y.; Isayama, H.; Horiguchi, H.; Matsuda, S.; Fushimi, K.; Koike, K. Severe bleeding and perforation are rare complications of endoscopic ultrasound-guided fine needle aspiration for pancreatic masses: An analysis of 3090 patients from 212 hospitals. Gut Liver 2014, 8, 215–218. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Hara, K.; Sawaki, A.; Mizuno, N.; Hijioka, S.; Imamura, H.; Niwa, Y.; Tajika, M.; Kawai, H.; Kondo, S.; et al. Ruptured pseudoaneurysm of the splenic artery complicating endoscopic ultrasound-guided fine-needle aspiration biopsy for pancreatic cancer. Endoscopy 2010, 42 (Suppl. 2), E27–E28. [Google Scholar] [CrossRef] [PubMed]
- Kitano, M.; Yoshida, M.; Ashida, R.; Kita, E.; Katanuma, A.; Itoi, T.; Mikata, R.; Nishikawa, K.; Matsubayashi, H.; Takayama, Y.; et al. Needle tract seeding after endoscopic ultrasound-guided tissue acquisition of pancreatic tumors: A nationwide survey in Japan. Dig. Endosc. 2022, 34, 1442–1455. [Google Scholar] [CrossRef]
- Sakamoto, H.; Kitano, M.; Komaki, T.; Noda, K.; Chikugo, T.; Dote, K.; Takeyama, Y.; Das, K.; Yamao, K.; Kudo, M. Prospective comparative study of the EUS guided 25-gauge FNA needle with the 19-gauge trucut needle and 22-gauge FNA needle in patients with solid pancreatic masses. J. Gastroenterol. Hepatol. 2009, 24, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Kandel, P.; Nassar, A.; Gomez, V.; Raimondo, M.; Woodward, T.A.; Crook, J.E.; Fares, N.S.; Wallace, M.B. Comparison of endoscopic ultrasound-guided fine-needle biopsy versus fine-needle aspiration for genomic profiling and DNA yield in pancreatic cancer: A randomized crossover trial. Endoscopy 2021, 53, 376–382. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).